Abstract
Context: A classic traditional Chinese medicine, Zanthoxylum nitidum (Roxb.) DC. widely used in China, exhibits anticancer, anti-inflammatory and antianalgesic activities. Alkaloids are one of the main bioactive components. It is urgent to develop a simple and reliable method to determine the main alkaloids in Z. nitidum roots.
Objective: To determine the three alkaloids in Z. nitidum roots, a reversed-phase liquid chromatographic (RP-LC) method combined with an optimum extraction condition was established.
Materials and methods: A method involving four-factor-three-level orthogonal array design including the extracting solvent and the RP-LC condition was assayed. Twenty batches were collected from different areas of the Guangxi Province at different harvesting times. The determined alkaloids were nitidine chloride (NC, 1), ethoxychelerythrine (2) and liriodenine (3). The stable mobile phase was a C18 packing, and the mobile phase was acetonitril-aqueous phosphoric acid-triethylamine-buffer solution.
Results: The optimum extraction and detection conditions have been determined in the process of quantification of Z. nitidum root alkaloids. The three alkaloids were detected simultaneously in the 20 batches of samples. The results clearly showed that alkaloid concentrations differed significantly among Z. nitidum collected from various collection areas.
Discussion and conclusion: We have established an optimum extraction and detection conditions in the process of quantification the three alkaloids in Z. nitidum roots. From this research, the most influenced factor on Z. nitidum roots was the collecting location, and the next factor was the harvesting time. The collecting location and the harvesting time should be considered as the high-quality medicinal herbs factors.
Introduction
Zanthoxylum nitidum (Roxb.) DC. (Rutaceae), also named “DI QIN NIU”, “MAN JIAO” and “SHUANG MIAN ZHEN”, is a classic traditional Chinese medicinal (TCM) plant distributed chiefly in Guangxi, and also widely distributed in Guangdong, Fujian, Sichuan and Zhejiang provinces of China. The root of the plant is widely used in China to treat rheumatism, toothache, neuralgia and swelling of the throat. Its main bioactive components are alkaloids, coumarins and lignanoids (Yao & Hu, Citation2004). Recently, one of the authors has reported the standard operating procedures (SOP) for this plant (Lai et al., Citation2011). Pharmacological studies show that Z. nitidum roots have marked pharmacological antibacterial (Huang et al., Citation2013; Shi et al., Citation2005), antigastric ulcer, anticancer (Liu & Liu, Citation2010), anti-inflammation (Feng et al., Citation2011; Xu et al., Citation2010; Zhou et al., Citation2012) and antioxidation activities (Pang et al., Citation2007; Xie, 2000), protects liver and improves liver function (Pang et al., Citation2006), and so on. Apart from extensively use in the pharmaceutical industry, the roots are widely applied in light industry. Therefore, extractive conditions and quantitative determination methods in the roots were urgent.
Several qualitative and/or quantitative analytic methods have been reported for alkaloids (Liang & Zhang, Citation2005), neoherculin (Liu et al., Citation2005) and lignanoids (Zhang et al., Citation2002), such as HPLC fingerprinting analysis (Yan et al., Citation2006). However, many current techniques are time-consuming or unable to provide comprehensive chemical assessment of Z. nitidum roots. In this article, we were interested in developing a reversed-phase liquid chromatographic (RP-LC) method for rapid and accurate quantification of specific alkaloids in Z. nitidum roots from different harvest of medicinal plants. Satisfactory results were obtained. The simple procedure afforded efficient separation and quantification of compounds NC (1), ethoxychelerythrine (2) and liriodenine (3) in Z. nitidum roots extract. The contents of compounds 1–3 in Z. nitidum roots have been determined using a simple, stable chromatography and brief run times for the first time.
Materials and methods
Plant material
The 20 batches of Z. nitidum roots were collected from different producing areas and at different harvesting times of Guangxi Province of China (). Plant samples were stored at room temperature (25 °C) until needed for analysis. The species were identified by Prof. Mao-xiang Lai, one of the authors in this paper, and the voucher specimens were deposited at the School of Pharmaceutical Sciences, Guangxi Medical University, China.
Table 1. The specific information of the 20 samples of Z. nitidum roots.
Chemicals, reagents, and standard solutions
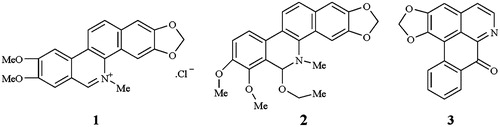
Methanol (MeOH) and acetonitrile (MeCN) were of LC grade (Fisher Company, Dallas, TX), phosphoric acid and triethylamine were of analytical grade (Beijing Chemical Corporation, Beijing, China). Deionized water was used for all analysis and was prepared using a Millipore (Billerica, MA) water purification system. All solvents and solutions were filtered through a Millipore filter (0.45 µm) before used. Reference standards of 1 and 2 were all purchased from the National Institutes for Food and Drug Control (Beijing, China). Compound 3 was kindly provided by the Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, Ministry of Education of China, Guangxi Normal University, whose structure was fully characterized by nuclear magnetic resonance (NMR) and MS spectroscopy. Their structures are shown in . LC analysis indicated approximately 98.0% purity for all three compounds. Stock solutions of 1–3, 241, 391, 153 µg/mL, respectively, were prepared by accurate weighing and dissolution in MeOH in 10 mL volumetric flasks, and then stored at 4 °C. Standard solutions and calibration solutions were serially diluted with MeOH to prepare the appropriate concentrations prior to use.
Preparation of sample solutions
Twenty samples of dried Z. nitidum roots were collected as described in . The roots were pulverized into powder, passed through a 0.9 mm sieve, and then stored in a desiccator until required for assay. An accurately weighed amount (1.0 g) of each sample was extracted with 25 mL of MeOH solution twice under reflux for 2.0 h total (25-fold excess, v/w, 1.0 h each). The extract solution was filtered till hot. The filtrates were combined and concentrated in vacuo to afford a dried residue, which was carefully quantitatively transferred into a 25 mL volumetric flask with MeOH and a portion of the resultant solution was injected into an LC system for analysis of alkaloids. All resulting solutions were filtered through a 0.45 µm Millipore filter before injecting 10 µL for LC analysis.
Apparatus and LC conditions
The LC system was carried out using a Shimadzu LC-20 A HPLC system (Kyoto, Japan), which consisted of a binary gradient pump (model LC-20 A), a SPD-20MA diode array detector, an SiL-20 A auto sampler, a DGU-20A3 degasser and CTO-20 A column oven. The apparatus was interfaced to a Dell PC compatible computer using LC solution software. The RP-LC was carried out on an analytical Diamonsil™ ODS C18 column (250 × 4.6 mm i.d.; 5 μm particles, China) with a Dikma Easy Guard C18 insert (20 × 4.6 mm i.d.). The isocratic mobile phase consisted of a gradient of MeCN (component A) and 0.2% (v/v) aqueous phosphoric acid-triethylamine-buffer solution (PTBS, pH: 2.07, component B) at a flow rate of 1.0 mL/min. The mobile phase consisted of A:B (25:75, v/v). An aliquot (10 μL) of sample was injected into the LC and an analysis was carried out at 35 °C.
Preparation of standard solutions and calibration
Quantitative analysis was carried out using an external standard method. Standards of the alkaloids 1–3 were accurately weighed and dissolved in 10 mL volumetric flasks with MeOH as stock solution with final concentrations of 241, 391, 153 µg/mL, respectively. The stock solutions were used to prepare standard solutions, which were stored at 4 °C and remained stable for at least two months (verified by re-assaying the standard solutions). Calibration curves were established based on six points for each alkaloid with concentrations of 7.53, 15.06, 30.12, 60.25, 120.50 and 241.00 μg/mL for 1; 12.21, 24.43, 48.87, 97.75, 195.50 and 391.00 μg/mL for 2; and 4.78, 9.56, 19.12, 38.25, 76.50 and 153.00 μg/mL for 3, respectively.
Results
Optimization of the extraction conditions
The experimental extraction conditions, for example extraction method, solvent and time, can easily influence extraction efficiency. In fact, as a result, it is necessary to estimate and optimize the factors affecting extraction to achieve maximum recovery. Owing to the excellent extraction of the three alkaloids under reflux, a method involving a four-factor-three-level orthogonal array design [L9 (34) OAD] including the components of extracting solvent, solvent volume, extraction duration and times of reflux was developed for the optimization of the extraction of the alkaloids from Z. nitidum roots [used as sample 20: dried Z. nitidum roots], followed by LC analysis. In this study, the main factors considered in the alkaloids optimization process are displayed in . Nine pulverized crude dried drug powder samples (a same batch sample 20, 1.0 g) were very carefully weighed and extracted according to the L9 (34) orthogonal array design. The variables used in this optimization were: (1) extraction solvents (factor A); (2) extraction number (factor B); (3) extraction method (factor C); and (4) volume of extraction solvents (factor D) {20 mL [20-fold excess, volume of added extraction solvent/crude drug weight (v/w)], 25 mL (25-fold excess, v/w) and 30 mL (30-fold excess, v/w), respectively}. All the levels and factors are shown in .
Table 2. Main factors and their level settings used for optimization of the extraction of the alkaloids.
shows the experimental design of the conditions for optimization of the extraction of the alkaloids and the results obtained from each experimental trial. An L9 (34) matrix with nine treatments (described in detail above) was used in this table to assign the variables and the corresponding level settings considered, and the variables were varied with the level settings values in different experimental trials. The total peak area of the three alkaloids was calculated. It could be used as a response function because it can take into consideration the effect of changes in the variables on the extraction efficiency of this technique. After the nine experimental trials (n = 3) were implemented, the corresponding total peak area of the three alkaloids for the variables set from each experimental trial was calculated and then tabulated (). In addition, the responses of each factor at different levels (1, 2, 3) were given in too. In , the R showed that the effects of the four factors on the extraction efficiencies in the order was A > C > D > B, the best combination of levels was A1 B1 C2 D2. The conclusion was further confirmed by variance analysis in .
Table 3. L9 (34) Matrix as the experimental design for optimization of the extraction of the three alkaloids.
Table 4. Analysis of variance.
Thus, the optimum conditions for extraction of the three alkaloids from the powdered Z. nitidum roots were as follows: dried and pulverized sample 20 (1.0 g) was extracted in 25 mL MeOH solution by refluxing twice for 2.0 h (25-fold excess, v/w, 1.0 h each).
Validation of the method
Calibration and linearity
The linearity of the plot of concentration (x, μg/mL) for each alkaloid against the peak area (y) was investigated. The results were expressed as the value of the correlation coefficient (r) ().
Table 5. Regression equations and the respective correlation coefficients for quantitative determination of the three alkaloids by LC.
Determination of the limits of detection and quantification
Based on a detector signal-to-noise ratio of 10:1, the average limit of quantification (LOQ) calculated from chromatograms of the blank feed standard compounds was 3.00 µg/mL for 1, 5.00 µg/mL for 2 and 2.00 µg/mL for 3. Similarly, based on a detector signal-to-noise ratio of 3:1, the average limit of detection (LOD) was 1.50 µg/mL for 1, 2.00 µg/mL for 2 and 1.00 µg/mL for 3, respectively.
Determination of precision, accuracy and stability
The precision of the method was validated by both intra- and inter-day precision. Five experiments were carried out by injecting the same standard solutions into the LC five times during one day, and one assay was performed each day for three consecutive days to determine intra-day and inter-days precision, respectively. The results were shown in , indicating that the accuracy and precision of the method was sufficient for determination of the three alkaloids in Z. nitidum roots (sample 20 in ).
Table 6. Intra- and inter-day precision of the LC method for determination of the alkaloids.
As for the stability and repeatability assay, the same sample (sample 20) was analyzed at different intervals (0, 12, 24, 36 and 48 h) at room temperature (25 °C), and the sample solution was found to be stable (RSD values of the peak area were lower than 2%). These results are listed in . The repeatability of analysis of the three alkaloids () was determined by analysis of five samples (sample 20) extracted and processed, as described above, in parallel.
Table 7. Stability and repeatability of the LC method for determination of the three alkaloids.
Spiked recovery
To determine the recoveries of the three alkaloids (), the sample powder (sample 20) was spiked with standards at three different concentration levels and then extracted by the procedure represented above. One crude drug sample, for which the contents of 1–3 had already been determined, was spiked by adding known amounts of standards of 1–3 at low (one half of the known amounts), medium (same as the known amounts) and high (one and half of the known amounts) levels. Then, the spiked samples were extracted, processed and quantified in accordance with the methods represented above. All the process was repeated three times for each level and recovery was calculated with the following equation:
Table 8. Recoveries of the LC method for determination of the alkaloids.
Quantitative determination of three alkaloids in Z. nitidum roots
The contents of 1–3 in 20 batches of the Z. nitidum roots were measured with the method developed above. The representative LC chromatograms of three standard alkaloids and the sample of Z. nitidum roots (sample 20) are shown in and , respectively. The amounts of 1–3 were calculated from the regression equations obtained from calibration curves, and the results were expressed as contents of alkaloids in the medicinal material (mg/g crude drug), which are listed in .
Figure 2. LC profile obtained from a mixture of the three alkaloids: 1–3 = same as the structures in .
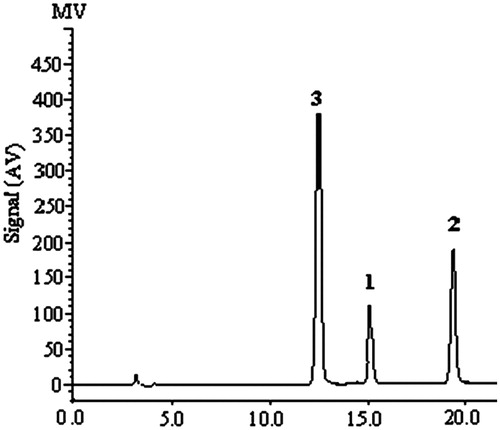
Figure 3. Typical LC profile of an extract from the Z. nitidum roots (sample: 20): 1–3 = same as the structures in .
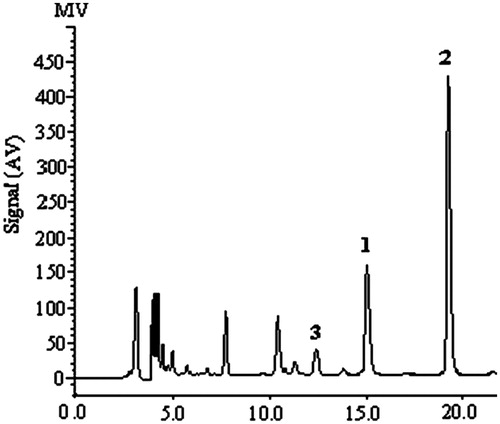
Table 9. Alkaloids content of the Z. nitidum roots.
The results clearly show there are substantial differences on the contents of the individual alkaloids in different harvesting area of Z. nitidum roots. For example, sample 11 had the greatest alkaloid concentration, with sample 4 having the second greatest amount. Among all the samples, the average content of compound 2 (ethoxychelerythrine) was highest and attained 5.06 ± 2.099 mg/g crude drug. These variations, too, might arise from differences in collection areas.
Discussion
The alkaloids are the bioactive compounds in Z. nitidum roots (Shi et al., Citation2005, 2010; Xu et al., Citation2010; Yao & Hu, Citation2004). As the major constituents of Z. nitidum, alkaloids have noteworthy biological activities such as anticancer, anti-inflammation, antihypertension, analgetic and antiemetic effects. Compounds 1, 2 and 3 have anticancer activity (Huang & Li, Citation1980; Suffness & Cordell, Citation1985; Xu et al., Citation2010; Ying et al., Citation2005), compound 3 has strongly antifungal activity (Nissanka et al., Citation2001), and so on. Therefore, the RP-LC method was established to determine the contents of alkaloids 1–3 in Z. nitidum roots.
The solvents, solvent volumes, method of extraction and extraction time seemed to be the most important conditions affecting the efficiency for the extraction of the three alkaloids. In this research, the optimum conditions for extraction of the three alkaloids from Z. nitidum roots were as follows: dried and pulverized Z. nitidum roots (1.0 g) were extracted with 25 mL of MeOH solution (25-fold excess, v/w) by refluxing twice (1.0 h each).
Some RP-LC methods have been developed for quantification of compounds 1 and compound 2 in Z. nitidum roots (Liang & Zhang, Citation2005), and of compounds 1 and 3, N-norchelerythrine, skimmianine and magnoflorine using a Waters Nova-Pak C18 column (150 × 3.9 nm, 5 µm particles) (Shi et al., Citation2006). Other investigators (Qin et al., Citation2006) developed an RP-HPLC method to determine compound 1 in 10 different areas with a Kromasil C18 column (250 × 4.6 nm, 5 µm particles). In this research, satisfactory results were obtained. The interference is always a problem in the identification and determination of target compounds in crude drugs, especially in the determination of trace compounds. As C18 columns are widely used and offer excellent performance for alkaloids, a Diamonsil™ ODS C18 column with a Dikma Easy Guard C18 insert was chosen as the stationary phase. When the stationary phase was fixed, the composition of the mobile phase was a main factor for separating target compounds from interference. Initially, different proportions of the mobile phase system such as MeCN–H2O, MeOH–H2O, MeOH–H2O–HCOOH, MeOH–MeCN–H2O, MeOH–H2O–glacial acetic acid and MeOH–H2O–phosphoric acid were used as an isocratic mobile phase, but no significant resolution of compounds 1–3 could be achieved. Finally, the developed LC method employed an elution system of a gradient of MeCN-aqueous phosphoric acid (0.2%)-triethylamine-buffer solution (pH = 2.07) as the mobile phase. Reasonable retention time symmetrical peaks with good separation were obtained at a flow rate of 1.0 mL/min and a 285 nm as detection wavelength. The three alkaloids showed satisfactory separation from interfering compounds within 20 min. When peak purity was determined by acquiring UV spectra of all peaks of interest, no evidence of impurities was found. The recovery test of the method indicated that the treatment of the samples did not result in loss of the three alkaloids. The method also meets the requirements of convenience and time efficiency for evaluating the three alkaloids content with large quantities of Z. nitidum roots.
Conclusions
In summary, we have established an optimum extraction and detection conditions to quantify the three alkaloids in 20 samples of Z. nitidum roots. This method utilized simple, stable chromatography and short time run condition for the first time. From the contents of the alkaloids in Z. nitidum roots, it was shown clearly that there were essential differences on the contents of the individual alkaloids in different areas of Z. nitidum. Therefore, our studies suggest that the collection of high-quality medicinal herbs depends on the collecting location and harvesting time.
Declaration of interest
The authors explicitly declare no conflict of interest within this manuscript. This project was partly supported by the National Natural Science Foundation of China (30760302), the Chinese Herbal Medicine Support Fund Project by the Chinese National Development and Reform Commission (2007) 2706, the Key Scientific and Technological Projects of Guangxi, China (0992003A-21), and the Doctor Foundation of Guangxi Medical University.
References
- Feng J, Zhou JF, Qin FJ, et al. (2011). Studies on the anti-inflammatory and analgesic activities of different polarities from the roots and stems of Zanthoxylum nitidum (Roxb.) DC. Pharmacol Clin Chin Mater Med 27:60–3
- Huang YL, Feng J, Wang HH, Lai MX. (2013). Studies on the anti-bacterial activity of different polarities from the roots and stems of Zanthoxylum nitidum (Roxb.) DC. Pharmacol Clin Chin Mater Med 29:103–5
- Huang ZX, Li ZH. (1980). Studies on the antitumor constituents of Zanthoxylum nitidum (Roxb.) DC. Acta Chim Sinica 38:535–42
- Lai MX, Lin ZH, Hu D, et al. (2011). The standardization production standard operating procedures (SOP) for Zanthoxylum nitidum (Roxb.) DC. Res Pract Chin Med 25:3–5
- Liang GH, Zhang JH. (2005). Determination of ethoxychelerythrine and nitidine chloride in Zanthoxylum nitidum (Roxb.) DC. J Chin Med Mater 28:898–9
- Liu LM, Liu HG. (2010). Anti-hepatoma activity of nitidine chloride and its effect on topoisomerase. Chin Pharmacol Bull 26:497–500
- Liu SH, Qin QY, Tang XL, et al. (2005). Extraction of neoherculin from Zanthoxylum nitidum (Roxb.) DC. Nat Prod Res Dev 17:337–9
- Nissanka APK, Karunaratne V, Bandara BMR, et al. (2001). Antimicrobial alkaloids from Zanthoxylum tetraspermum and caudatum. Phytochemistry 56:857–61
- Pang H, He H, Jian LJ, et al. (2007). Effect of total alkaloids from Zanthoxylum nitidum on gastric ulcer in experimental models. Pharmacol Clin Chin Mater Med 23:38–9
- Pang H, Tang GF, He H, et al. (2006). Protective effects of the extract from Zanthoxylum nitidum (Roxb.) DC. on experimental liver injury. Guangxi Med J 28:1606–8
- Qin LF, Lai MX, Liang W. (2006). Determination of nitidine chloride in Zanthoxylum nitidum (Roxb.) DC. from different areas by RP-HPLC. Guangxi Sci 13:297–9
- Shi Y, Li DX, Min ZD. (2005). Activity of Zanthoxylum species and their compounds against oral pathogens. Chin J Nat Med 3:248–51
- Shi Y, Li DX, Li GP, He ZD. (2006). Determination of five alkaloids in Zanthoxylum nitidum (Roxb.) DC. by RP-HLPC. Chin Tradit Herb Drugs 37:129–31
- Shi XP, Guan RQ, Zhang MD, Zhang WM. (2010). Progress in the study of Zanthoxylum alkaloids. Chin Wild Plant Res 29:1–7
- Suffness M, Cordell GA. (1985). Antitumor alkaloids, the alkaloids: Chemistry and pharmacology. In: Suffness M, ed. Chapter 1 Antitumor Alkaloids. Academic Press Inc., 178–89
- Xie YF. (2000). Antioxidative effect of extracts from Zanthoxylum nitidum (Roxb.) DC. Lishizhen Med Mater Med Res 11:1–2
- Xu L, Huang Y, Dong Z, Yi D. (2010). Experimental research of total alkaloids from Zanthoxylum nitidum on anti-inflammation in rats with ulcerative colitis. J Emerg Tradit Chin Med 19:480, 506
- Yan YZ, Xie PS, Tian RT, Lin RM. (2006). HPLC fingerprinting analysis and quality assessment of herbal drug radix Zanthoxyli nitidii. Tradit Chin Drug Res Clin Pharmacol 17:440–3
- Yao RC, Hu J. (2004). Review of the study on the constituents and pharmacology of Zanthoxylum nitidum. J Pharmaceutical Practice 22:264–7
- Ying H, Hu HS, Chang TT, Chen CH. (2005). Liriodenine inhibits the proliferation of human hepatoma cell lines by blocking cell cycle progression and nitric oxide-mediated activation of p53 expression. Food Chem Toxicol 43:1117–26
- Zhang SY, Zhou BJ, Wang Y. (2002). Determination of l-sesamin and l-asarinin in Zanthoxylum (Roxb.) DC. by high performance liquid chromatography. J First Mil Med Univ 22:654–5
- Zhou JF, Qin FJ, Feng J, Wei LT. (2012). Anti-inflammatory and analgesic activities of essential oil from the roots of Zanthoxylum nitidum (Roxb.) DC. Lishizhen Med Mater Med Res 23:19–20