Abstract
Context: Desmostachya bipinnata (L.) Stapf [Gramineae (Poaceae)] has been traditionally used to treat various disorders such as asthma, kidney stone, diarrhea, wound healing, etc.
Objective: The present study involves pharmacognostic, botanical, and preliminary phytochemical examination of various plant parts and powders of D. bipinnata.
Materials and methods: Leaves, stem, roots, underground and aerial part powders were microscopically examined. Pharmacognostic standardization parameters were determined as per the WHO guidelines. Parameters, including extractive value in different solvents, ash values, and loss on drying, were determined. Preliminary phytochemical studies, fluorescence analysis, and chromatographic profiling were performed for the correct identification of this crude drug and some of its phytoconstituents.
Results: Microscopical studies showed various characteristic features including, collateral vascular bundle, bundle sheath cells, and bulliform cells in leaf, conjoint, collateral and closed vascular bundles, and sclerenchymatous sheath in stem; and silica bodies in root. Phytochemical screening and chromatographic profile of aerial plant parts revealed the presence of alkaloids, tannins, flavonoids, steroids, glycosides, and coumarins. Underground plants parts indicated the presence of glycosides, steroids, flavonoids, coumarins, and alkaloids.
Discussion and conclusion: The results of the performed studies are helpful in correct identification, characterisation of D. bipinnata. Preliminary phytochemical studies and chromatographic profiling may be helpful in further isolation and purification of lead compounds from different extracts.
Introduction
Desmostachya bipinnata (L.) Stapf (syn: Eragrostis cynosuroides) Gramineae (Poaceae), is commonly known as sacrificial grass, kusha (Khare, Citation2007), drabh (Qureshi et al., Citation2010), and dab (Praveen et al., Citation2007). D. bipinnata leaf paste is used to cure cuts and wounds (Katewa & Jain, Citation2006). D. bipinnata roots are used to treat rheumatism (Ahmad et al., Citation2010), carbuncles, piles, cholera, dysuria, (Qureshi et al., Citation2010) diuretic, galactagogue, astringent (Khare, Citation2007), dysentery, leucorrhea, and wounds (Praveen et al., Citation2007).
Flavonoids, viz., kaempferol, quercetin, quercetin-3-glucoside, trycin, and trycin-7-glucoside, have been isolated from D. bipinnata (Awaad et al., Citation2008). Trycin and trycin-7-glucoside showed promising antiulcerogenic activity (Awaad et al., Citation2008). D. bipinnata oil contains camphene (16.79%), isobornyl acetate (9.92%), tricyclene (4.30%), (±) trans-2,6-γ-irone (2.21%), caryophyllene diepoxide (12.29%), β-eudesmol (11.16%) eseroline (25.15%), and calarene (3.48%) (Kumar et al., Citation2010). Diphenyliodinium bromide, 1-limenone, 2-cyclohexene-1-one and 8-nitro-12-tridecanolide were also found in small amounts in D. bipinnata oil (Kumar et al., Citation2010). D. bipinnata oil showed good activity against different test microorganisms. Quercetin, isolated from D. bipinnata, is a useful chemoprotective agent in Helicobacter pylori infections (Ramadan & Safwat, Citation2009). Analgesic, antipyretic, and anti-inflammatory effects of various whole plant extracts in solvents, viz., petroleum ether, benzene chloroform, ethanol, and water, were investigated in albino rats (Panda et al., Citation2009). The alcoholic and aqueous root extracts of D. bipinnata were found effective in treating castor oil and charcoal meal–induced diarrhea (Hegde et al., Citation2010).
The present study deals with determination of several pharmacognostic parameters, preliminary phytochemical tests, and chromatographic profiles of the D. bipinnata.
Materials and methods
Plant material: collection, authentication
Whole plants of D. bipinnata were collected in September 2009 from Chirawa, district Jhunjhunu, Rajasthan, India. The collected plant was authenticated by Dr. R. P. Pandey from Botanical Survey of India, Jodhpur, India. A voucher specimen, JNU/PH/2010/Db D2, was deposited in the herbarium of Jodhpur National University, Jodhpur, India.
Microscopy, powder analysis and standardization parameters
Hand sections of fresh root, stem and leaves were used for microscopical characterization. Sections were treated with chloral hydrate to remove chlorophyll. Saffranin, phloroglucinol HCl, iodine, ruthenium, sudan red, sulphuric acid, etc., were used to stain different sections (Khandelwal, Citation2005).
Different plant parts, viz., roots, stems and leaves, were dried in shade for 1 month. Dried plant parts were crushed and ground using an electric mixer-grinder and screened using BSS standard sieve no. 22 of average aperture size 710 μm. Shade-dried, coarsely powdered drug was used for powder analysis, fluorescence studies (Mukherjee, Citation2003), and preparation of various extracts. Total ash value, acid- insoluble ash value, sulphated ash value, water soluble ash value, loss on drying, and extractable matter in petroleum ether, chloroform, ethyl acetate, ethanol, hydro alcoholic (1:1) and water were estimated, as per standard procedures given in World Health Organization (WHO) guidelines (WHO, Citation1998).
Preparation of plant extracts
The powdered crude drug, aerial and underground parts (10 g) were extracted in a Soxhlet extractor with petroleum ether, chloroform, ethanol, ethanol (50%) and water separately to extract non-polar and polar compounds. The obtained extracts were filtered through Whatman filter paper, concentrated by evaporation on water bath, and residual moisture was removed in dessiccator.
Preliminary phytochemical studies
Phytochemical analysis, from aerial and underground parts, was performed for various phytoconstituents, like alkaloids, glycosides, terpenes, coumarins, flavonoids, carbohydrates, proteins, volatile oils, saponins, etc., with the help of standard reagents and solutions (Khandelwal, Citation2005). Chromatographic profiles were generated with several solvent systems for different phytoconstituents (Egon, Citation2007; Wagner & Blat, Citation2007).
Results and discussion
Microscopy
Leaf
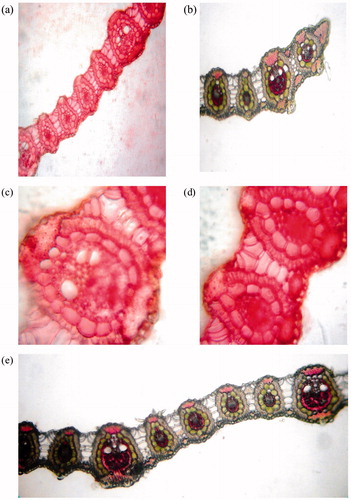
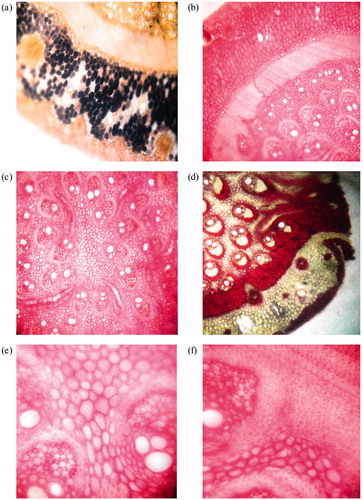
Microscopic characters of leaves are presented in . Mid-rib collateral vascular bundle consists of two metaxylem and a phloem in between them (). This type of vascular bundle is also present in Cynodon dactylon L. Pers. (Poaceae) leaves. Vascular bundle is covered with continuous layer of sclerenchymatous cells (). Thick wall, small cells are present on lower portion of the leaf, while cells on upper portion are relatively larger with thin wall ().
Leaf lamina section and marginal section
Lower epidermis of leaf lamina is smooth and comprises circular cells with thick cuticle, while upper epidermis is not so smooth and comprises circular cells with papillate walls (). In marginal section of leaf, both epidermal layers are smooth and cells are square shaped with thickening (). Vascular bundles are somewhat smaller than mid rib vascular bundles (). Leaf has furrows, where epidermal cells and mesophyll cells forms bulliform cells (). Xylem and pholem are less distinct and covered with 9 to 10 chloroplast bearing bundle sheath cells () but vascular bundle is covered with 5 to 6 chloroplast bearing bundle sheath cells in Cynodon dactylon leaves.
Stem
A continuous layer of epidermis is covered with cuticle. Ground tissue, present on the inner side, consists of parenchymatous cells. Epidermis and parenchymatous cells (2–3 layers) are lignified followed by parenchymatous cells, which shows the presence of starch grains and oil glands (). Vascular bundles are conjoint, collateral, and closed (). Different size vascular bundles are scattered in ground tissue (), covered with sclerenchymatous sheath (), and shows lignin deposition (). Same type of vascular bundle scattered in ground disuse and lignin depositon is also observed in Saccharum spontaneum Linn (Poaceae) stem. Roots have been found emerging from stem node (). Saccharum spontaneum and D. bipinnata belongs to the family Poaceae (Suresh Kumar et al., Citation2009).
Root
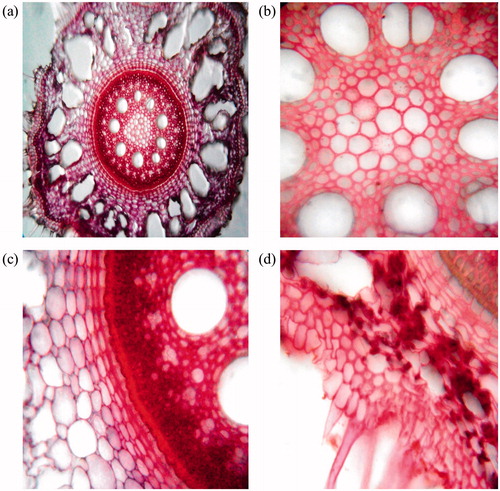
A continuous layer of epidermis was observed with thin wall, semicircular shape epidermal cells, which have projections on outer walls (). Outer cortex consists of three to four layers of sclerenchymatous cells (). Inner cortex comprises of aerenchymatous cells, consisting of radially elongated wide air chambers with thin partition filaments (). These aerenchymatous cells with elongated wide air chambers are also present in Imperata cylindrica Linn (Poaceae). Spherical cells have been observed in these partition filaments (). The stele is covered by endodermis and deposition of silica bodies at inner side (). Circular, thin wall meta xylem varies in number from 9 to 10 (). These thin walled meta xylem present in Imperata cylindrica varies in number from 5 to 7. In between, two meta xylem, 2 to 3 phloem are present. Pith tissue consists of sclerenchyamatous cells also present in Imperata cylindrica.
Figure 3. Microscopical characters of Desmostachya bipinnata root. (a) T.S. of root, (b) T.S. showing steler region having sclerenchymatous cells, (c) endodermis, metaxylem and phloem and (d) epidermis, trichomes and aerenchymatous cells.
Microchemical tests, applied to leaves, rhizomes, and roots, for examining the presence of starch, lignin, calcium oxalate, etc. are given in . Starch and lignin were present in leaf and stem, while root contained only lignin. Additionally, oil glands were observed in stem.
Table 1. Microchemical tests performed with various plant parts of Desmostachya bipinnata (L.) Stapf.
Powder analysis
Aerial plant parts powder showed the presence of parenchymatous cells (58.8 µm length and 14.7 µm width) (), vessels (223.65 µm length and 50.4 µm width) both simple and lignified (), trichomes (186.69 µm length and 14.7 µm width), starch grains (27.93 µm length and 22.86 µm width), phloem (), and fibers (306.02 µm length and 14.7 µm width) (). Underground plant part powder analysis revealed the presence of trichomes (235.08 µm length and 17.35 µm width) (), vessels (241.68 µm length and 35.35 µm width) (), fibers (339.96 µm length and 17.27 µm width) (), starch grains (37.57 µm length and 32.46 µm width) (), sclerenchymatous cells (32.19 µm length and 15.15 µm width) (), and endodermal cells (). Measument of these powder characteristics are summarized in .
Figure 4. Powder characteristics of aerial parts of Desmostachya bipinnata. (a) Lignified vessel, (b) phloem, (c) fiber, parenchymatous cells and (d) fibers, epidermal cells, parenchymatous cells and vessels.
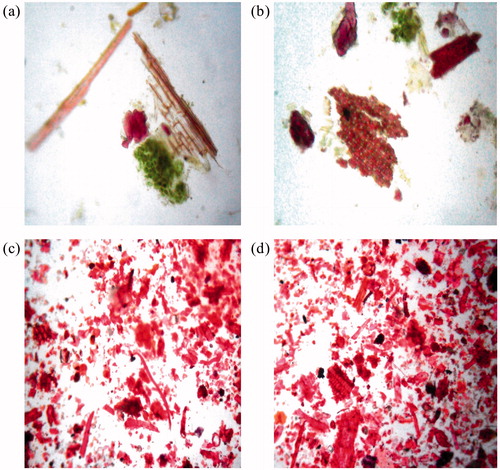
Figure 5. Powder characteristics of underground parts of Desmostachya bipinnata. (a) Endodermal cells, trichomes, (b) sclerenchymatous cells, (c) fiber and (d) starch grains, vessels.
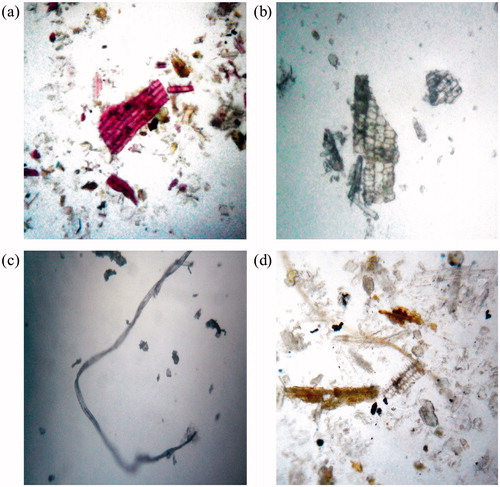
Table 2. Microscopic measurements for several powder characteristics in Desmostachya bipinnata (L.) Stapf.
Fluorescence studies with different solvents and powder (aerial and underground parts) were conducted (). In aerial parts, at long UV (365 nm), greenish white fluorescence was observed with water, sulphuric acid, concentrated nitric acid, acetic acid, and ammonia; whitish fluorescence was observed with methanol, ethanol, ethanolic sodium hydroxide, methanolic sodium hydroxide, concentrated nitric acid, chloroform, petroleum ether, sodium hydroxide, and potassium hydroxide. At short UV (254 nm), greenish fluorescence was observed with ethanolic potassium hydroxide, methanolic potassium hydroxide, acetic acid, and ammonia. In underground plant parts, at long UV (365 nm), greenish fluorescence was observed with water and concentrated hydrochloric acid; whitish fluorescence was observed with methanol, ethanol, acetic acid, ammonia, chloroform, petroleum ether, sodium hydroxide, and potassium hydroxide. At short UV (254 nm), greenish fluorescence was observed with ethanolic sodium hydroxide, ethanolic potassium hydroxide, methanolic potassium hydroxide, ethanolic potassium hydroxide, concentrated hydrochloric acid, petroleum ether, sodium hydroxide, potassium hydroxide, acetic acid, and ammonia.
Table 3. Fluorescence studies with different plant parts of Desmostachya bipinnata (L.) Stapf.
W.H.O standardization parameters
Different ash values, viz., total ash value, an acid-insoluble ash value, a water-soluble ash value, and sulphated ash value were determined to find the inorganic content in each sample and results are presented in . Crude fiber content, foreign organic matter, swelling index, foaming index, etc., were also estimated for aerial and underground parts (). Solvents of different polarity ranges were used to determine the extractive value and extract yield in the petroleum ether, ethyl acetate, chloroform, ethanol, ethanol (50%), and water for aerial and underground parts ( and ). Further phytochemical screening (Table 6) and chromatographic profile (Table 7) of aerial plant parts revealed the presence of alkaloids, tannins, flavonoids, steroids, glycosides, and coumarins in hydro-alcoholic and alcoholic extracts; flavonoids and glycosides in water extract; alkaloids, flavonoids, and steroids in chloroform extract; while, steroids were found in ethyl acetate extract. Phytochemical screening of underground plants parts indicated the presence of glycosides and alkaloids in water extract; glycosides, flavonoids, coumarins, and alkaloids in hydroalcoholic extract; glycosides, flavonoids, steroids, coumarins, and alkaloids in alcoholic extract; coumarins and alkaloids in chloroform extract; flavonoids, coumarins, and alkaloids in ethyl acetate extract.
Table 4. Estimated standardization parameters of Desmostachya bipinnata (L.) Stapf as per WHO guidelines.
Table 5. Extractive yields estimated for various plant parts of Desmostachya bipinnata (L.) Stapf.
Table 6. Phytochemical tests performed with various plant parts of Desmostachya bipinnata (L.) Stapf.
Table 7. Chromatographic profile of various plant parts of Desmostachya bipinnata.
Conclusion
Pharmacognostical and preliminary phytochemical investigations were performed on various plant parts of D. bipinnata. The results of these investigations are expected to help in correct identification, characterization and other relevant findings. Standard profile of D. bipinnata may be prepared from identified characters and characterized parameters. The outcomes of preliminary phytochemical investigations may guide isolation and purification of lead compounds responsible for traditional therapeutic claims.
Declaration of interest
The authors declare no conflict of interests. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors express their thanks to Dr. R. P. Pandey from Botanical Survey of India, Jodhpur, India, for authentication of plant material.
References
- Ahmad F, Khan M, Ahmad M, et al. (2010). Ethnomedicinal uses of grasses in salt range region of northern Pakistan. J Med Plant Res 4:362–9
- Awaad A, Mohamed N, Maitland D, Soliman G. (2008). Anti-ulcerogenic activity of extract and some isolated flavonoids from Desmostachya bipinnata (L.) Stapf. Rec Nat Prod 2:76–82
- Egon S. (2007). Thin Layer Chromatography. New Delhi: Springer Private Limited
- Hegde M, Lakshman K, Girija K, et al. (2010). Assessment of antidiarrhoeal activity of Desmostachya bipinnata L. (Poaceae) root extracts. Bol Latinoam Caribe Plant Med Arom 9:312–18
- Katewa S, Jain A. (2006). Traditional Folk Herbal Medicines. Udaipur, Jaipur: Apex Publishing House
- Khandelwal K. (2005). Practical Pharmacognosy. Pune: Nirali prakashan
- Khare CE. (2007). Indian Medicinal Plants An Illustrated Dictionary. Berlin/Heidelberg: Springer-Verlag
- Kumar K, Sharvanee, Patel J, Choudhary R. (2010). Chemical composition and antimicrobial activity of the essential oil of Desmostachya bipinnata Linn. Int J Phytomed 2:436–9
- Mukherjee P. (2003). Quality Control of Herbal Drugs: An Approach to Evaluation of Botanicals. New Delhi: Business Horizon Pharmaceutical Publishers
- Panda S, Choudhury N, Patro V, et al. (2009). Analgesic, antipyretic and anti-inflammatory effect of the whole plant extract of Desmostachya bipinnata Stapf (Poaceae) in albino rats. Drug Invent Today 1:150–3
- Praveen A, Upadhay B, Roy S, Kumar A. (2007). Traditional uses of medicinal plants among the rural communities of churu district in the Thar Desert, India. J Ethnopharmacol 113:387–99
- Qureshi R, Bhatt G, Memon R. (2010). Ethnomedicinal uses of herbs from northern part of Nara desert, Pakistan. Pak J Bot 42:839–51
- Ramadan M, Safwat N. (2009). Antihelicobacter activity of a flavonoid compound isolated from Desmostachya bipinnata. Australian J Basic Appl Sci 3:2270–7
- Suresh Kumar CA, Varadharajan R, Muthumani P, et al. (2009). Pharmacognostic and preliminary phytochemical investigations on the stem of Saccharum spontaneum. J Pharm Sci Res 1:129–36
- Wagner H, Blat S. (2007). Plant Drug Analysis: A Thin Layer Chromatography Atlas. Munchen, Germany: Springer
- WHO. (1998). Quality Control Methods for Medicinal Plant Materials. Geneva: World Health Organization