Abstract
Context: Alternanthera repens (L.) Kuntze (Amaranthaceae) is widely used in Mexican traditional medicine for the treatment of gastrointestinal disorders that are mainly related to diarrhea.
Objective: The aim of the present study was to investigate the spasmolytic effect of hexane (Hx), methanol (Me) and aqueous (Aq) extracts as well as chromatographic Me fractions (F1–F6) of A. repens in rat ileum.
Materials and methods: Dried and powdered aerial parts were used to obtain the extracts. The rat ileum preparations were incubated in Tyrode’s solution gassed (95% O2–5% CO2) at 37 °C. The effect on the contractile response of isolated ileum was evaluated by obtaining cumulative concentration–response curves to CaCl2, KCl, 5-HT and acetylcholine in the absence and presence of different doses of Aq (0.56–2.09 mg/mL), Me (0.24–0.91 mg/mL) and Hx (0.24–0.91 mg/mL) extracts, as well as six Me fractions of 0.66 mg/mL (F1 to F6).
Results: The A. repens Me (0.24 mg/mL) caused an inhibitory response of the Ca2+-induced contractions, with IC50 values of 0.18 ± 0.061 and 0.67 ± 0.061 mM in the presence and absence of the Me, respectively. Me fractions F2 to F4 presented a significant inhibitory effect (F(3,8) = 60.17, p = 0.0001), causing a reduction in the CaCl2-induced contractions and shifting the Ca2+ (0.39 to 1.81 mM) concentration–response curves to the right. With respect to the effect on 5-HT-induced contractions, IC50 values Hx extract (0.24 mg/mL) were 5.44 ± 0.08 × 10−6 M and 3.38 ± 0.07 × 10−6 M in the presence and absence of the Hx, respectively.
Discussion and conclusion: The spasmolytic effects induced by Me and Me fractions of A. repens may involve a serotonergic and Ca2+ influx blockade mechanisms, which may justify the use of A. repens extracts as an effective traditional treatment against diarrhea.
Introduction
Diarrhea is a gastrointestinal disorder that has been traditionally treated with herbal medicines all over the world. Alternanthera repens (L.) Kuntze (Amaranthaceae), known in Mexico as tianguis, tianquiz or tianguispepetla, is claimed to be effective in reducing fever and it is commonly used to ameliorate gastrointestinal ailments such as stomachache, intestinal inflammation and diarrhea (Aguilar et al., Citation1994; Argueta, Citation1994). Zavala et al. (Citation1998) reported that the use of A. repens was effective to reduce the symptoms of diarrhea. This effect seemed to be due to the capability of the herbal remedy to slow down intestinal peristalsis (Astudillo-Vázquez et al., Citation2008). However, the mechanism of action of A. repens is still unclear.
Considering the above, the aim of this work is to elucidate the mechanism by which A. repens diminishes the intestine peristaltic movements. In order to study this, different extracts of A. repens were prepared: aqueous (Aq), hexane (Hx) and methanol (Me), as well as chromatographic fractions of the Me. The anti-peristaltic activity of the extracts was assessed on ileum sections of male Wistar rats.
Materials and methods
Plant material
Leaves of A. repens were collected at the Iztacala Faculty gardens of the Universidad Nacional Autónoma de México (UNAM) from June to September 2009. The leaves were authenticated at the MEXU herbarium of the UNAM Biology Institute by Dr. Hilda Flores, a specialist in the Amaranthaceae family. The plant was registered with the voucher number 11256. The plant material was also deposited at the Izta Herbarium of the Iztacala Faculty (voucher specimen number 849).
Preparation of crude extracts and methanol fractions
Aqueous extract (Aq)
Dried leaves of A. repens (5 g) were ground and mixed into 100 mL of distilled water. Subsequently, the mix was boiled for 15 min. After cooling down to room temperature, the aqueous suspension was filtered through Whatman filter paper No. 1. The filtrate was dried in a conventional oven at 60 °C during 24 h. This way we obtained dry material not semisolid that allowed the easy dilution of the Aq extract in an adequate concentration.
Hexane extracts (Hx)
Dried leaves of A. repens (50 g) were placed in a flask and completely covered with hexane at room temperature while avoiding sunlight exposure. After 48 h, the hexane suspension was filtered through Whatman filter paper No. 1. The filtrate was concentrated at a reduced pressure and stored at room temperature. The residual leaves were dried and preserved for further methanol extraction.
Methanol extracts (Me)
Oil-free leaves obtained in the previous procedure were immersed in methanol at room temperature for 48 h and concentrated using the method described above. The extract was kept at room temperature in tightly closed vials avoiding sunlight exposure.
Fractionation of the methanol extract
The Me which showed the greatest antispasmodic activity in the bioassays and was subjected to chromatographic fractionation. An aliquot of the Me (0.612 g) was dissolved in a small amount of CH2Cl2 and was passed through 1 g of silica gel (SiO2 Merck 60–230 mesh). The Me was then poured onto a chromatographic column (45 g of SiO2) and subsequently eluted with 100% CH2Cl2. The mobile phase consisted of varying ratios of CH2Cl2/MeOH, starting with pure CH2Cl2. A total of six fractions were obtained: F1 (95:05); F2 (90:10); F3 (85:15); F4 (80:20); F5 (75:25); and F6 (70:30). Afterwards, samples of each fraction were loaded onto 5 × 3 cm silica gel thin layer chromatography plates (TLC) and eluted with a mixture of CH2Cl2/MeOH (80:20). The eluates were dried at room temperature, observed under ultraviolet light and revealed with ammonium ceric sulphate.
The extracts and fractions were kept at room temperature in tightly closed vials avoiding sunlight exposure and immediately used in pharmacological experiments. Aq extract was suspended in 0.9% saline while Hx, Me extracts or Me fractions (F1 to F6) were dissolved in DMSO and serial dilutions were then made, the concentration of DMSO never exceeding 0.1% in the organ bath. In all solutions the pH was adjusted to 7.4.
Animals
Adult male Wistar rats weighing 250 to 300 g were obtained from the breeding colony of the UNAM Neurobiology Institute. The animals were kept in air-filtered plastic cages under standard temperature conditions (21 °C), 12 h dark/12 h light photoperiods and with free access to food and water throughout the experiment. All the experimental procedures were approved by the Ethical Committee of the UNAM Neurobiology Institute and abide by the Mexican federal regulations for animal experimentation (NOM-062-ZOO-1999; SAGARPA, Citation2001).
Smooth muscle preparation
Animals were given intraperitoneal injections with 45 mg/kg of pentobarbital and killed by cervical dislocation. The ileum was dissected by cutting the last 10 cm of the intestine from the ileocecal junction. The dissected ileum was placed into a Petri dish containing Tyrodes solution with the following composition (mM): NaCl 136.89, KCl 2.68, MgCl2 1.05, CaCl2 1.8, NaH2PO4 0.42, NaHCO3 11.9 and glucose 5.5. The pH was adjusted to 7 and the plates were kept at 37 °C. The ileum was carefully rinsed to remove fecal matter and then it was cut into 1 cm fragments. These fragments were placed into an isolated organ chamber filled with 25 mL of Tyrodes solution. The chamber was continuously aerated with a mixture of 95% O2 and 5% CO2 (carbogen) and the temperature was kept at 37 °C. The thread of a side was coupled to both a myograph F-60 (Narco Bio-Systems, Houston, TX) and a physiograph (Model DMP-4B). A tension of 0.5 g was applied to the 1 cm ileum fragments. For at least 30 min before addition of any drug or test material the intestinal responses were recorded, equilibrated and stabilized. The contractile effect was assessed by considering the extent of the contractions occurring when the test material was added regarding those produced by the following compounds alone: CaCl2 (20 mM), KCl (50 mM) or 5-HT (10−6 M) and acetylcholine, ACh (10−6 M).
Inhibition of dose–response curves to CaCl2
After an initial incubation period of 60 min in Tyrode's solution, the nutrient solution was replaced by a Ca2+-free Tyrodes solution during 10 min for Ca2+ removal. After equilibration, the ileum strips were incubated for 20 min with different concentrations of the plant extract: Aq (0.56, 1.09 or 2.09 mg/mL), Hx (0.24, 0.47 or 0.91 mg/mL), Me (0.24, 0.47 or 0.91 mg/mL), and F1 to F6 fractions (0.66 mg/mL). Cumulative concentration–response curves to CaCl2 (from 0.39 to 1.81 mM) were obtained by increasing the amount of CaCl2 each time in the absence and presence of different concentrations of the extracts and fractions.
Relaxant effect on K+-induced contractions
In this experiment, the tissue was incubated with a K+-free Tyrodes solution. The same amounts of extracts and fractions than those used above were added to the organ bath. After incubation, the concentration–response curves of K+ were determined by accumulative addition of KCl (from 0.98 to 4.54 mM) in the absence and presence of different concentrations of the extracts and fractions.
Inhibition of dose–response curves to 5-HT
For assessing the nature of the interaction with serotonin receptors, the ileum strips were incubated for 20 min with different treatments: Aq (0.56, 1.09 or 2.09 mg/mL), Hx (0.24, 0.47 or 0.91 mg/mL), Me (0.24, 0.47 or 0.91 mg/mL), and F1 to F6 fractions (0.66 mg/mL). Additionally, 5-HT (0.28 mL) was cumulatively injected into the organ bath with concentrations ranging from 2.23 × 10−6 to 10.25 × 10−6 M and cumulative concentration–response curves to 5-HT were obtained in the presence or absence of the different concentrations of A. repens extracts and fractions.
Inhibition of concentration–response curve to acetylcholine (ACh)
In order to confirm if an additional mechanism is involved in the spasmolitic effect of A. repens Aq, the ileum strips were incubated with the Aq extract (2.09 mg/mL) during 20 min. After ACh (0.28 mL) was cumulatively added to the organ bath (concentrations ranging from 2.11 × 10−6 to 465.38 × 10−6 M) and cumulative concentration–response curves to ACh were obtained in the absence and presence of different concentrations of Aq extract.
Statistical analysis
Relaxation of the intestinal preparation was expressed as a percentage of the control response mediated by an agonist (CaCl2, KCl, 5-HT or ACh). All values were presented as mean ± S.E.M. of n = 4 per each experiment. All the statistical calculations were carried out using the Statistical Package for the Social Sciences SPSS (Ver. 12, IBM, Mexico D.F., Mexico). The cumulative concentration–response curves (CRCs) were analyzed by performing an analysis of variance (ANOVA) of repeated measures and post-hoc Duncańs tests. For constructing the bar graphics, a one-way ANOVA and a post-hoc Duncan test (sample versus control) were performed. A probability value of p < 0.05 was considered to be significant. The half maximal inhibitory concentration (IC50) was determinated by a Probit analysis.
Results
Effect of the A. repens crude extracts and Me fractions on the CaCl2-induced contractions
The spasmolytic effect can interfere with the contraction mechanism of extracellular Ca2+ internalization or release from Ca2+ depots in the sarcoplasmic reticulum (Masiel et al., Citation2004). To know whether the crude extracts and fractions block the input of extracellular Ca2+, it was tested the ability of the treatments to inhibit contractions with the CaCl2 inducer. shows that two crude extracts inhibited the stimulatory effect of CaCl2. The Me (0.24 mg/mL) and Aq (0.56 mg/mL) extracts caused a significant reduction (F(3,8) = 42.8, p = 0.0001) of the %Maximal response to CaCl2 (0.39 to 1.81 mM). Therefore, the A. repens Me extracts had a spasmolytic effect at concentrations ranging from 0.24 to 0.91 mg/mL (data not shown) with half maximal inhibitory concentration (IC50) of 0.18 ± 0.061 mM and 0.67 ± 0.061 mM in the presence (0.24 mg/mL) and absence of the Me extract, respectively.
Figure 1. Contractile response to increasing calcium (Ca2+) concentrations (0.39–1.81 mM) in ileum preparations. The tissues were depolarized with KCl (50 mM) and initially maintained in Ca2+free medium in the absence or in the presence of crude extracts (n = 4). Data are expressed as the % of the maximal contractile response to CaCl2. (*) Indicates a statistically significant difference based on the ANOVA of repeated measures and the post-hoc Duncan test (p ≤ 0.05). Values shown are the mean ± SEM (standard error of the mean) of six determinations. In all the experiments, the inhibitory effects of Aq, Hx and Me were reversed after 0.5 h of successive watch out.
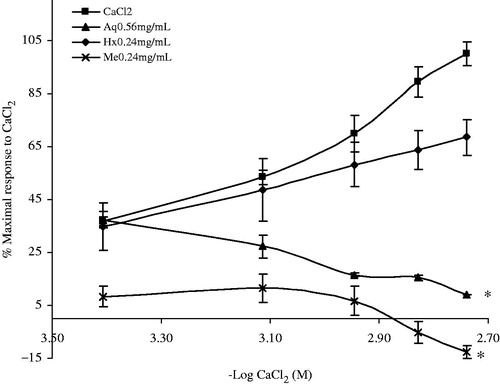
Likewise, Aq extract had a spasmolytic effect in a concentration range from 0.56 to 2.09 mg/mL (data not shown), with IC50 values of 0.25 ± 0.03 mM and 0.60 ± 0.061 mM in the presence (0.56 mg/mL) and absence of the Aq extract, respectively. However, the effect of the Aq extract was lesser than that produced by the Me extract.
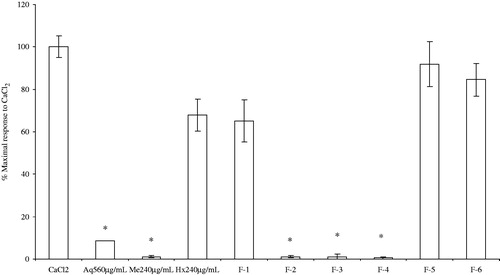
On the other hand, the effect of the A. repens Hx extracts on CaCl2-induced contraction was not significantly different from the treatments with no extract addition. However, fractions F2 to F4 at 0.66 mg/mL caused a significant reduction in the CaCl2-induced contractions and shifted the Ca2+ concentration–response curves (0.39 to 1.81 mM) to the right. Therefore, it is concluded that these fractions had a significant inhibitory effect (F(3,8) = 60.17, p = 0.0001) ().
Figure 2. Antispasmodic effect of the crude extracts and the methanolic fractions of A. repens leaves on the CaCl2 contractions of rat ileum slices. (*) Indicates a statistically significant difference with the one-way ANOVA and the post-hoc Duncan test (p ≤ 0.05). Values shown are the mean ± SEM (standard error of the mean) of four determinations.
Effect of the A. repens crude extracts and Me fractions on the KCl-induced contractions
The antispasmodic effect of the A. repens crude extracts and Me fractions on the contractions produced by KCl were also evaluated. Prior to the addition of the contractile agent, the tissue was incubated with different concentrations of the plant extracts/fractions and then exposed to contractions produced by K+ (0.98–4.54 mM). The extent of inhibition (%) was calculated in terms of the contraction obtained in the absence of the extract. shows that two crude extracts inhibited the stimulatory effect of KCl of the rat ileum. The effect of the A. repens Me (0.24 mg/mL) and Aq (0.56 mg/mL) extracts caused a significant reduction in the %Maximal response to KCl (F(3,12) = 25.62, p = 0.0001), Me had a spasmolytic effect at concentrations ranging from 0.24 to 0.91 mg/mL (data not shown) with IC50 values of 0.043 ± 0.001 mM and 1.57 ± 0.181 mM in the presence (0.24 mg/mL) and absence of the Me, respectively.
Figure 3. Contractile responses to potassium (K+) increasing concentrations (0.98–4.54 mM) in ileum preparations. The tissues were prepared with K+-free Tyrodes solution in the absence (n = 4) or in the presence of crude extracts. Data are expressed as the % of the maximal contractile response to KCl. (*) Indicates statistically significant difference with the ANOVA of repeated measures and the post-hoc Duncan test (p ≤ 0.05). Values shown are the mean ± SEM. In all the experiments, the inhibitory effects of Aq, Hx and Me were reversed after 0.5 h of successive watch out.
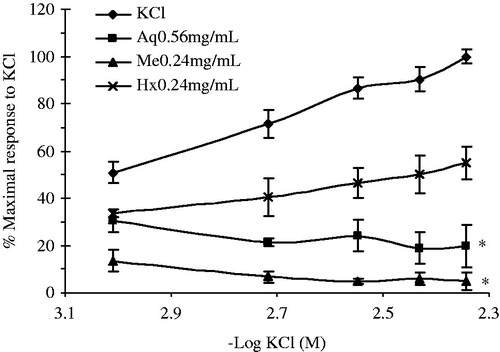
The Aq extracts had a spasmolytic activity within a concentration range of 0.56–2.09 mg/mL (data not shown), with IC50 values of 0.82 ± 0.01 mM and 1.04 ± 0.08 mM in the presence (0.56 mg/mL) and absence of the Aq extract, respectively. On the other hand, the effect of the A. repens Hx extracts on KCl-induced contractions was not significantly different with respect to that of the control. Nevertheless, fractions F2 to F4 at 0.66 mg/mL had a significant inhibitory effect (F(3,12) = 23.44, p = 0.0001) with respect to the control response. The rest of the fractions did not have a significant effect on KCl stimulation ().
Figure 4. Spasmolytic effect of the crude extracts and the methanolic fractions of A. repens on the KCl contractions of rat ileum slices. (*) Indicates a statistically significant difference with one-way ANOVA and the post-hoc Duncan test (p ≤ 0.05). Values shown are the mean ± SEM (standard error of the mean) of four determinations.
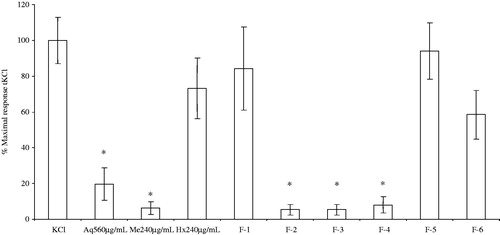
Effect of the crude extracts and Me fractions of A. repens on the spasmodic activity of 5-HT
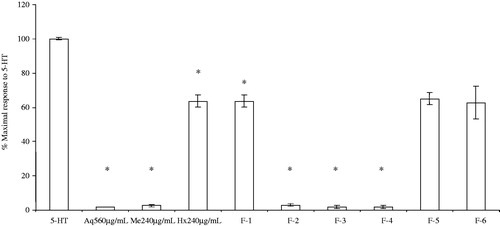
The contraction processes mediated by some endogenous neurotransmitters such as 5-HT induce the activation of a G protein-coupled receptor which then activates phospholipase C to produce inositol triphosphate (IP3) and diacylglycerol (DAG). These molecules mediate the release/entry of Ca2+ into smooth muscle cells (Billington & Penn, Citation2003). Considering this, the antagonist effect of the crude extracts and Me fractions of A. repens on the 5-HT-stimulated response was explored to determine the role of the treatments in the spasmolytic mechanism. Cumulative CRCs for the treatments induced by 5-HT (2.23 × 10 −6 to 10.25 × 10−6 M) were performed with and without extracts. The effect of the A. repens crude extracts on the contractions induced by 5-HT is shown in . The Aq, Me and Hx extracts significantly shifted downward the cumulative concentration–response curves with respect to 5-HT concentration–response curve (F(3,12) = 508.15, p = 0.0001).
Figure 5. Contractile responses to increasing 5-HT concentrations (2.23 × 10−6–10.25 × 10−6 M) in ileum preparations. The tissues were prepared with Tyrodes solution in the absence or in the presence of crude extracts (n = 4). Data are expressed as the % of the maximal contractile response to 5-HT. (*) Indicates a statistically significant difference with the ANOVA of repeated measures and the post-hoc Duncan test (p ≤ 0.05). Values shown are the mean ± SEM. In all the experiments, the inhibitory effect of Aq, Hx and Me were was reversed after 0.5 h of successive watch out.
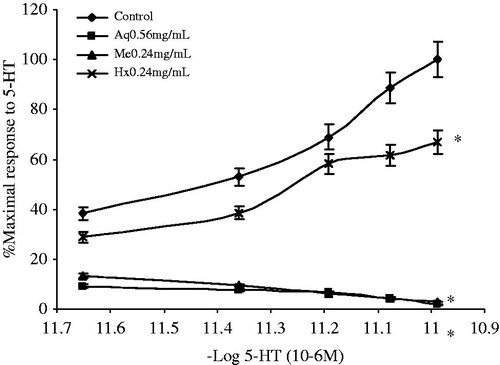
The A. repens Aq extract caused a spasmolytic effect at concentrations ranging from 0.56 to 2.09 mg/mL (data not shown), with IC50 values of 7.19 ± 0.04 × 10−8 M and 3.38 ± 0.07 × 10−6 M in the presence (0.56 mg/mL) and absence of the Aq extract, respectively. The A. repens Me extracts had a spasmolytic activity in a concentration range of 0.24–0.91 mg/mL (data not shown), with IC50 values of 2.24 ± 0.06 × 10−7 M and 3.38 ± 0.07 × 10−6 M in the presence (0.24 mg/mL) and absence of the Me extract, respectively. The A. repens Hx extract also inhibited 5HT-induced ileum contractions, presenting a spasmolytic effect within the concentration range of 0.24–0.90 mg/mL (data not shown), with IC50 values of 5.44 ± 0.08 × 10−6 M and 3.38 ± 0.07 × 10−6 M in the presence (0.24 mg/mL) and absence of the Hx extract, respectively. Interestingly, the A. repens Hx extracts had an inhibitory effect only on the ileum contraction induced by 5HT. On the other hand, the F1 to F4 fractions (0.66 mg/mL) had a significant inhibitory effect ().
Effect of Aq of A. repens on spasmodic activity of ACh
The Aq extract caused a 58.6% inhibition of the stimulatory effect of ACh with respect to the effect obtained with ACh alone. The A. repens Aq had a spasmolytic effect at a concentration of 2.09 mg/mL (data not shown), with IC50 values of 72.68 ± 0.08 × 10−6 M and 124.04 ± 0.05 × 10−6 M in the presence and absence of the Aq extract, respectively.
Discussion
Antidiarrheal activity of some medicinal plants can be due to antibacterial (Alanís et al., Citation2005), antisecretory (Velázquez et al., Citation2006), or spasmolytic mechanisms of action (Estrada-Soto et al., Citation2010). The A. repens extracts and Me fractions were effective in diminishing ileum peristalsis in a similar manner to the results reported elsewhere for Mentha pulegium L. (Lamiaceae) (Estrada-Soto et al., Citation2010). These results can help to provide a better understanding of the mechanism by which this plant ameliorates diarrhea. In addition, the use of this herbal medicine can be justified for cases in which it is necessary to reduce intestinal motility, as in the case of non-infectious diarrhea. More studies should be conducted to isolate, purify and chemically characterize the active components of A. repens aiming at elucidating the mechanisms underlying the plant antidiarrheal activity.
The gastrointestinal motor tone is regulated through multiple physiological mediators, such as acetylcholine, histamine, substance P, cholecystokinins, prostaglandins and 5-HT (Hoogerwerf & Pasricha, Citation2006). The release of these chemicals in the gut causes stimulatory effects, mediated through an ultimate increase in cytosolic Ca2+ (Burks, Citation1987). Ca2+ antagonists are compounds with the ability to block any of the above pathways or which have a non-specific inhibitory action. Such antagonists have been effectively used to treat hyperactive gut disorders. A substance which causes the inhibition of high K+-induced contractions is a blocker of the Ca2+ influx through the L-type Ca2+ channels (Godfraind et al., Citation1986). With this consideration, high K+ concentrations were used (50 mM) to assess the Ca2+ channel blocking activity of the A. repens extracts. This activity consists of the depolarization of the ileum preparations and the production of myo-contractions by opening the voltage-dependent Ca2+ channels, thus allowing the influx of extracellular Ca2+ and causing a contractile effect (Karaki et al., Citation1997).
The spasmolytic activity of the extracts/fractions of A. repens was confirmed, as indicated by the contraction of ileum slices obtained from male Wistar rats. The results show that the A. repens extracts effectively inhibit the peristaltic movement of the rat ileum. These findings can be complemented with the work of Zavala et al. (Citation1998), who reported the antidiarrheal activity of A. repens extracts. These results are in agreement with those of Astudillo-Vázquez et al. (Citation2008), who reported that the plant extracts reduced the intestinal transit of food in rats. In contrast with those studies, in our work the methanol extracts had a greater spasmolytic activity than the aqueous samples. These differences may be due to the fact that the other authors worked directly with animals, rather than with ileum sections; additionally, in those studies, diarrhea was induced with different compounds.
All the extracts and three of the methanol fractions reduced the contractions induced by 5-HT. Considering that 5-HT in the intestine causes contractions (Villalón et al., Citation1995) and that smooth muscle contraction is calcium dependent (Hellstrand, Citation1998), it is possible that the methanol extract and its fractions contain a calcium antagonist or an inhibitor of the 5-HT receptors. In addition to this, considering that three fractions of the Me extract were effective in diminishing ileum contractions, further studies may aim at determining the compounds involved in the spasmolytic activity of the Me fractions.
Acetylcholine (ACh) is a neurotransmitter released by the parasympathetic nervous system. Its action in the gastrointestinal tract (GIT) involves the stimulation of nicotinic acetylcholine and muscarinic acetylcholine receptors. The M2 and M3 receptor subtypes play an essential physiological role in the smooth muscle contraction/relaxation of the GIT (Masiel et al., Citation2004). The following mechanisms help to explain the increased GIT contractility induced by synthetic drugs or medicinal plant extracts: (1) stimulation of the ACh release from the cholinergic nerve endings; (2) stimulation/inhibition of the acetyl cholinesterase enzyme (AChE) at the neuro-effector junction; and (3) direct activation/inactivation of the muscarinic receptors of the smooth muscles, including those of the GIT (Brown & Taylor, Citation1996).
Although the A. repens aqueous extract caused a 58.6% reduction in the IC50 value, the ACh-induced contraction of the rat ileum does not displaced the CRC to the right. This indicates that non-competitive inhibition occurred between the Aq extract and the cholinergic receptors of the rat ileum smooth muscle. Therefore, considering the results above together with those obtained for the Aq extracts with serotonin, it can be claimed that the Aq extracts are capable of mediating spasmolytic effects on isolated rat ileum through the inhibition of a wide range of contractile stimuli, such as neurotransmitters (ACh and 5-HT) and high potassium. This suggests that the Aq extract relaxant effect on the ileum is not caused by a specific receptor but rather by either a general receptor inactivation or a depolarization of the membrane.
Conclusions
This study shows that the spasmolytic activity induced by Aq, Me, Hx and Me fractions of A. repens may involve the serotonergic and blockade of Ca2+ influx mechanisms, thus providing a pharmacological rationale for their use in the treatment of diarrhea. However, more studies should be conducted to further understand the antidiarrheal, antisecretory and antispasmodic mechanisms of the plant extracts. In summary, this study provides scientific support for the medicinal use of A. repens to treat gastrointestinal disorders such as diarrhea.
Declaration of interest
The authors declare that there is no conflict of interest with respect to the publication of this work.
This study was supported by PAPCA 2007-2008, FES-Iztacala, UNAM, PAPIIT-DGAPA, UNAM IN212906-3, SIP-IPN 20131354, ICYTDF PICSO-12-096 and CONACYT CB-2008-105683 research.
Acknowledgements
We would like to thank Mrs. Juliana A. De los Santos Vera for her English assistance in the preparation of this manuscript.
References
- Aguilar CA, Camacho JR, Chino S, et al. (1994). Herbario Medicinal del Instituto Mexicano del Seguro Social. Información Etnobotánica. México: Edición del Instituto Mexicano del Seguro Social
- Alanís AD, Calzada F, Cervantes JA, et al. (2005). Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol 100:153–7
- Argueta VA. (1994). Atlas de las Plantas de la Medicina Tradicional Mexicana III. México: Instituto Nacional Indigenista
- Astudillo-Vázquez A, Dávalos Valle H, De Jesús L, et al. (2008). Investigation of Alternanthera repens and Bidens odorata on gastrointestinal disease. Fitoterapia 79:577–80
- Billington CK, Penn RB. (2003). Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Resp Res 4:1–23
- Brown JH, Taylor P. (1996). Muscarinic receptor agonists and antagonists. In: Gilman AG, Hardman JG, Limbird LE, et al., eds. Goodman & Gillman’s: The Pharmacological Basis of Therapeutics. New York: McGraw-Hill, 141–59
- Burks TF. (1987). Actions of drugs on gastrointestinal motility. In: Johnson LR, ed. Physiology of the Gastrointestinal Tract. New York: Ravan Press, 723–43
- Estrada-Soto S, González-Maldonado D, Castillo-España P, et al. (2010). Spasmolytic effect of Mentha pulegium L. involves ionic flux regulation in rat ileum strips. J Smooth Muscle Res 46:107–17
- Godfraind T, Miller R, Wibo M. (1986). Calcium antagonism and calcium entry blockade. Pharmacol Rev 38:321–416
- Hellstrand P. (1998). Long-term effects of intracellular calcium and growth factors on excitation and contraction in smooth muscle. Acta Physiol Scand 164:637–44
- Hoogerwerf WA, Pasricha PJ. (2006). Pharmacotherapy of gastric acidity, peptic ulcer and gastroesophagial reflux disease. In: Brunton LL, Lazo JS, Parker KL, eds. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill, 967–81
- Karaki H, Ozaki H, Hori M, et al. (1997). Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 49:157–230
- Masiel SS, Dias KLG, Medeiros IA. (2004). Calcium mobilization as the endothelium-independent mechanism of action involved in the vasorelaxant response induced by the aqueous fraction of the ethanol extract of Albizia inopinata G.P. Lewis (AFL) in the rat aorta. Phytomedicine 11:130–4
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA). (2001). Norma Oficial Mexicana NOM-062-ZOO-1999: Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Diario Oficial de la Federación. 22 de Agosto. México
- Velázquez C, Calzada F, Torres J, et al. (2006). Antisecretory activity of plants used to treat gastrointestinal disorders in Mexico. J Ethnopharmacol 103:66–70
- Villalón CM, Terrón JA, Ramírez-San Juan E, Saxena PR. (1995). 5-Hydroxytryptamine: Considerations about discovery, receptor classification and relevance to medical research. Arch Med Res 26:331–44
- Zavala MA, Pérez S, Pérez C, et al. (1998). Antidiarrheal activity of Waltheria americana, Commelina coelestis and Alternanthera repens. J Ethnopharmacol 61:41–7