Abstract
Context: Schistosomiasis is a parasitic disease that results in severe organ damage. Snail control is the best measure to control schistosomiasis. Plant-derived molluscicides have gained increasing attention for the control of schistosomiasis because they have low toxicity towards non-target organisms. Tannins are particularly suitable for snail control because they are less toxic than saponins to non-target organisms.
Objective: To identify the most toxic components of two plants belonging to the family Myrtaceae, namely Eucalyptus globulus Labill. and Melaleuca styphelioides Sm against the different developmental stages of Schistosoma mansoni and its snail host.
Materials and methods: The 80% MeOH leaf extracts of the tested plants and their isolated compounds were screened for their molluscicidal activity (expressed as LC50 and LC90 after 24 h exposure) against the snail Biomphalaria alexandrina. The anti-schistosomal activity of the tested samples was determined at 20 ppm (after 1 or 2 h exposure) against the different developmental stages of S. mansoni, including the miracidia, cercariae and worms. Biochemical parameters were measured to determine the toxicity mechanisms of the treated snails. The structures of the isolated compounds were elucidated based on NMR, UV and HRESI-MS/MS data.
Results: Potent molluscicidal activity was observed for the ellagitannin dimer eucalbanin B (12), with an LC50 value of 55 ppm. Treatment of the snails with the LC25 of eucalbanin B (30.8 ppm) resulted in a significant decrease in the protein level by 22.7% and 25.8% in the snail tissues and hemolymph, respectively. The decreased protein content was attributed to destruction of the snail tissue and impairment in protein synthesis under stress conditions of intoxication with eucalbanin B. Alterations in the activities of the transaminases and phosphatases in the treated snails indicated destruction and intoxication of the snail tissues. A significant increase in the levels of the transaminases alanine aminotransferase (ALT) (57.8%) and aspartate aminotransferase (AST) (113.2%) in the snail hemolymph and a significant decrease in their tissue levels to 7.4 and 48.6%, respectively, were attributed to their release from the damaged tissue into the hemolymph. Alkaline phosphatase (ALP) was significantly increased by 38.5 and 181.4% in the hemolymph and tissues, respectively. Acid phosphatase (ACP) was also significantly increased by 48.4 and 21.2% in the hemolymph and tissues, respectively. The 80% MeOH extract of E. globulus together with mallophenol B (3), 2,2,8-trimethyl-6-formyl-chrom-3-ene-7-O-β-d-glucopyranoside (5) and benzyl alcohol 7-O-(3′,4′,6′-tri-O-galloyl)-β-d-glucopyranoside (10) exhibited miracidicidal activity with almost 100% toxicity at 20 ppm for the three compounds and 80% toxicity for the extract. Moreover, E. globulus extract showed cercaricidal and schistosomicidal activity with 100 and 40% mortality, respectively.
Conclusion: E. globulus is a potential source for biocidal compounds against S. mansoni and its snail host. This is the first study to test the biocidal activity of the isolated compounds.
Introduction
Schistosomiasis is a parasitic disease that results in anemia, impaired growth and severe organ damage. It is estimated that 41 000 people die from the disease each year, and approximately 90% of all cases occur in Africa (WHO, Citation2010). Intestinal schistosomiasis caused by Schistosoma mansoni is endemic in Egypt. The snail host Biomphalaria alexandrina is essential for the transmission of the disease (WHO, Citation2010). Chemotherapy (such as praziquantel treatment), health education and snail control are among the available measures to control schistosomiasis (Xiaonong et al., Citation2002). The disadvantages of chemotherapy are that it does not eliminate the infection completely, there is a risk of re-infection and resistance is a major problem (Singh et al., Citation2010). The minimum amount of fund required to provide praziquantel to endemic communities in sub-Saharan Africa has been calculated to be US $200 million annually (WHO, Citation2010). An improved method of controlling schistosomiasis is to destroy the snail host (Singh et al., Citation2010). Plants offer a wide range of bioactive compounds with molluscicidal activity, including saponins, alkaloids, diterpenes and tannins (Marston & Hostettmann, Citation1985; Singh et al., Citation2010). Plant-derived molluscicides are inexpensive, biodegradable and have low toxicity to non-target organisms compared to synthetic molluscicides, such as niclosamide, which cause serious environmental pollution. In addition, many classes of plant-derived molluscicides are water-soluble, which makes them more suitable for wide application in endemic areas for control of the snail vector (Marston & Hostettmann, Citation1985; Singh et al., Citation2010). Previous studies showed that tannin-rich plants exhibited strong molluscicidal activity against the snail Biomphalaria glabrata (Marston & Hostettmann, Citation1985). Tannins are particularly suitable for snail control because tannin-containing plants are widely distributed, and the isolation of tannins from these plants is relatively easy (Marston & Hostettmann, Citation1985). These attributes, together with lower toxicity than saponins towards non-target organisms, prompted us to investigate the molluscicidal activity of two ellagitannin-rich plants belonging to the family Myrtaceae, namely, Eucalyptus globulus Labill. and Melaleuca styphelioides Sm.
The genus Eucalyptus is a rich source of bioactive natural products, including ellagitannins (Al-Sayed et al., Citation2010), flavonoids (Tian et al., Citation2009), phloroglucinol derivatives (Al-Sayed et al., Citation2010) and terpenoids (Tian et al., Citation2009). Certain Eucalyptus species are reported to have strong molluscicidal effects on Oncomelania snails, including Eucalyptus camaldulensis Dehnh and Eucalyptus cordata Labill (Hong et al., Citation2001; Xiaonong et al., Citation2002). The bark of Eucalyptus exserta F. Muell also has molluscicidal activity against Pomacea snails (Li et al., Citation2012). Eucalyptus globulus Labill. (Myrtaceae) is the most cultivated species of the genus (Coppen, Citation2002). Melaleuca styphelioides Sm. (Myrtaceae) is a medium-sized ornamental tree. Previous studies have reported that various Melaleuca species are rich sources of bioactive compounds, such as volatile oils (Farag et al., Citation2004), ellagitannins (Yoshida et al., Citation2008) and flavonoids (Yoshimura et al., Citation2008). To the best of our knowledge, the chemical composition of M. styphelioides has not been extensively investigated.
In this study, we report the molluscicidal activity of the total methanol extracts and isolated compounds from E. globulus and M. styphelioides on B. alexandrina snails. Because the need for new antischistosomal drugs is quite apparent, the antischistosomal activity of these plants and their isolated pure compounds was also determined against the different developmental stages of S. mansoni, including the larval stages (miracidia and cercariae) and the worms. Moreover, the biochemical parameters of the snail tissues treated with the tested materials were measured to determine the physiologic activity and possible toxicity mechanisms in the treated snails. The objective of this study is to identify the most toxic components of these plant extracts against the different developmental stages of S. mansoni and its intermediate snail host.
Materials and methods
General experimental procedures
NMR spectra were measured by Bruker Avance 500 spectrometer (Bruker BioSpin Inc., Fällanden, Switzerland). CD3OD was used as a solvent. Proton and carbon spectra were referenced internally to TMS signal. DQF-COSY, HSQC, HMBC and 1D-TOCSY were measured by the pulse programs installed by Bruker. The LC-HRESIMS was performed on Bruker micrOTOF-Q Daltonics (API) Time-of-Flight Mass Spectrometer (Bremen, Germany), coupled to 1200 series HPLC system (Agilent Technologies, Waldbronn, Germany). Chromatographic separation was performed on a Superspher 100 RP-18 (75 × 4 mm i.d.; 4 μm) column (Merck, Darmstadt, Germany). The mobile phase consisted of acetonitrile (A) and 0.4 % formic acid (B). The ionization technique was pneumatically assisted electrospray. The mass spectrometer was operated in negative mode. Capillary voltage, 4000 V; end plate offset, −500 V. For collision-induced dissociation MS/MS measurements, 20–70 eV were used. Argon was used as collision gas. Preparative HPLC was conducted using Waters 600E multisolvent delivery system, the column used was a Lichrospher® 100 RP-18 (250 × 10 mm i.d.; 10 μm) (Merck Darmstadt, Germany). Silica gel 60 (200–300 mesh) was obtained from Merck, Darmstadt, Germany. Sephadex LH-20 was obtained from Amersham Biosciences, Sweden. RP-C18 was obtained from Sigma-Aldrich GmbH, Darmstadt, Germany and precoated silica gel TLC GF254 was obtained from Riedel-De Häen-AG, Seelze, Germany.
Plant material
The leaves of E. globulus were collected in August 2009 from the botanical garden of the zoo at Giza in Egypt. The leaves of M. styphelioides were collected in July 2009 from Anshas Botanical Garden, El-sharkya, Egypt. The plants were botanically identified by Therese Labib, the taxonomy specialist at the herbarium of El-Orman Botanical Garden, Giza, Egypt. Voucher specimens of E. globulus and M. styphelioides were deposited at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt (ASU EGM2009 and ASU MSM2009, respectively).
Extraction and isolation
Air-dried powdered leaves of E. globulus (500 g) were extracted three times with 80% MeOH to obtain 50 g of the total extract. Column fractionation was performed with a portion of the extract (40 g) using a Sephadex LH-20 column (5 × 50 cm). Elution was performed with H2O followed by H2O–MeOH mixtures of decreasing polarities to yield four fractions (I–IV). Fraction I (6 g, eluted with water) was subjected to column chromatography over a Sephadex LH-20 column (5 × 50 cm) using H2O–MeOH mixtures for elution to obtain three major fractions. Fraction 1 (1.8 g) was subjected to CC over silica gel 60 using CHCl3:MeOH mixtures for elution followed by preparative HPLC purification to yield pure samples of compounds 1 (15 mg), 2 (15 mg) and 3 (30 mg). Compounds 4 (20 mg) and 5 (60 mg) were obtained from major fraction 2 (2.7 g) by repeated column fractionation over Sephadex LH-20 followed by purification on RP-18 column (2 × 20 cm) eluted with H2O–MeOH mixtures to yield compounds 4 and 5. A portion of fraction II (1 g, eluted with 30% MeOH) was subjected to repeated CC over a Sephadex LH-20 column (5 × 50 cm) with elution performed using a CH2Cl2:MeOH gradient of increasing polarity to yield compound 6 (200 mg). Part of fraction III (5 g, eluted with 60% MeOH) was subjected to CC over a Sephadex LH-20 column (5 × 50 cm) using a CH2Cl2:MeOH gradient for elution followed by repeated Sephadex LH-20 CC of the fractions using H2O–MeOH gradient solvent systems to yield compounds 7 (500 mg), 8 (50 mg), 9 (30 mg) and 10 (20 mg). Fraction IV (2.5 g, eluted with MeOH) was subjected to CC over Sephadex LH-20 using a CH2Cl2:MeOH gradient for elution to yield compounds 11 (50 mg) and 12 (100 mg).
For M. styphelioides, air-dried powdered leaves (500 g) were extracted three times with 80% MeOH. The MeOH soluble portion was concentrated and freeze-dried to obtain 80 g of the extract. Column fractionation was performed with 30 g of the MeOH-soluble portion using a Sephadex LH-20 column (5 × 50 cm) by eluting with H2O followed by H2O–MeOH mixtures to yield seven major fractions (I–VII). Fr II (1.5 g, eluted with 20% MeOH) was re-chromatographed over a Sephadex LH-20 column (5 × 50 cm) to obtain three sub-fractions. Sub-fraction 1 (900 mg) was fractionated over RP-18 column (1 × 20 cm) with elution performed using H2O followed by H2O–MeOH mixtures to yield compound 13 (73 mg). Fr III (2.0 g, eluted with 30% MeOH) was re-chromatographed over a Sephadex LH-20 column (5 × 50 cm) to obtain four sub-fractions. Compound 6 (which was also isolated from E. globulus) was purified by repeated CC of sub-fraction 1 (700 mg) over a Sephadex LH-20 column (3 × 30 cm) using mixtures of H2O and MeOH to yield pure sample of compound 6 (220 mg). Fr V (2.5 g, eluted with 50% MeOH) was re-chromatographed over a Sephadex LH-20 column (5 × 50 cm) to obtain eight sub-fractions. Sub-fraction 1 (1.4 g) was fractionated by Sephadex LH-20 CC (3 × 30 cm) using 80% MeOH for elution to yield compound 14 (1 g).
Snails
In this study, adult B. alexandrina snails from the Laboratory of Medical Malacology Department, Theodor Bilharz Research Institute (TBRI), Giza, Egypt, were used. The snails were maintained at 25 °C in dechlorinated water before use in the bioassay. Active snails with a shell diameter of 8–10 mm were chosen for the study.
S. mansoni miracidia, cercariae and worms
The miracidia, cercariae and worms of S. mansoni were obtained from the Schistosome Biological Supply Center at TBRI. The parasite eggs were extracted from the intestines of hamsters infected 45 d earlier. Clean eggs were hatched in small amounts of dechlorinated water at 25 °C. The worms were extracted from the infected hamsters by perfusion. Male and female adult worms were equally used in this study. The worms were maintained in an incubator adjusted at 37 ± 0.5 °C. The cercariae were obtained from experimentally infected B. alexandrina snails. The miracidia and cercariae were maintained at 25 ± 0.5 °C.
Molluscicidal activity
The efficacy of each plant extract or compound against the adult snails was determined using standard methods (WHO, Citation1965). The test samples were dissolved in DMSO and diluted to the desired concentrations with dechlorinated water. The final DMSO concentration was less than 0.1%. For each concentration, three replicates of 10 snails were used. Niclosamide was used as the positive control. The exposure and recovery periods were 24 h for each test. Three replicates of control snails were maintained under the same experimental conditions. The efficacy of the extracts and compounds was expressed as the LC50 and LC90 values (Litchfield & Wilcoxon, Citation1949). The sub-lethal concentrations LC10 and LC25 were calculated using the IPM SPSS Statistics program, version 20 for Windows with probit analysis (Finney, Citation1971).
Miracidicidal, cercaricidal and schistosomicidal activity
Ten milliliters of dechlorinated water containing approximately 100 freshly hatched miracidia and freshly shed cercariae were mixed with 10 mL of 40 ppm extract or compound solution to obtain a concentration of 20 ppm according to the previous method (Mostafa & Gawish, Citation2009) with some modifications. Similarly, 100 live worms were maintained in 10 mL of phosphate buffer (pH 7.4) and then mixed with 10 mL of the tested sample (40 ppm) to obtain a concentration of 20 ppm and then incubated at 37 ± 0.5 °C. All the exposure periods were according to the previous methods (Braguine et al., Citation2012; Eissa et al., Citation2011) with some modifications. Praziquantel at 20 ppm was used as a positive control for the schistosomicidal activity. Approximately equal numbers of miracidia, cercariae in 20 mL of dechlorinated water or live worms in 20 mL of phosphate buffer were used as the negative control. The exposure period was 1 or 2 h for each tested sample. Three replicates were used for each test. Microscopic observation of the movement and mortality of the miracidia, cercariae and worms was performed every 15 min. The organisms were considered dead when their motion ceased completely for 1 min. The dead organisms were then counted (Mostafa & Gawish, Citation2009).
Biochemical assay
Approximately 50 snails were exposed to a concentration corresponding to the LC25 of compound 12 for three weeks (WHO, Citation1965). The control group was left unexposed under the same conditions. The drug was renewed twice weekly. Hemolymph samples were collected according to a previous method (Abdul-Salam & Michelson, Citation1983). Tissue homogenates were prepared by dissecting the soft parts of the snail from the shells. Next, 1 g from each experimental group was homogenized in 5 mL of distilled water, and the resulting fresh supernatant was used for the assays. All biochemical parameters were determined using reagent kits from the bio-Merieux Company (Marcy l'Etoile, France). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed according to a previously described method (Reitman & Frankel, Citation1957). Alkaline phosphatase (ALP) and acid phosphatase (ACP) activities were tested as described previously (Kind & King, Citation1954). All enzymes are expressed as units per gram of tissue. The total protein level (µg/mg tissue) was estimated using the Folin-phenol method (Lowry et al., Citation1951). The results of the enzyme activity assays are expressed as the means ± SE of the three replicates following analysis with the IPM SPSS Statistics program version 20 for Windows (SPSS Inc., Chicago, IL).
Statistical analysis
The data are presented as the mean ± SE. Student's t test was used to determine the significant differences between the means of the treated and control groups. The molluscicidal activity of the tested plant was calculated by probit analysis (Finney, Citation1971; Litchfield & Willcoxon, Citation1949).
Results and discussion
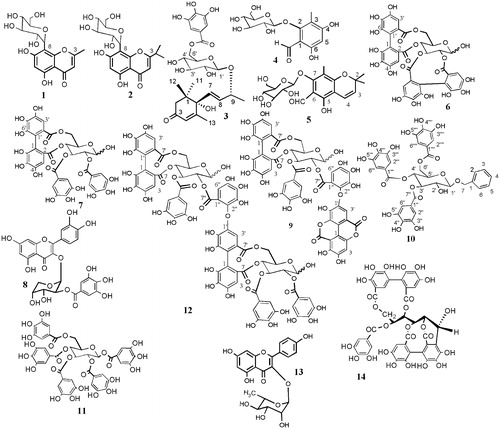
Fractionation of the 80% methanol extract of the leaves of E. globulus yielded compounds 1–12. This is the first report of the isolation of compounds 1, 2, and 3 from the genus Eucalyptus. Compounds 4 and 10 were previously isolated from Eucalyptus gomphocephala (Al-Sayed et al., Citation2010). Compound 12 was isolated from Eucalyptus alba (Yoshida et al., Citation1992). The other compounds were isolated from different species of the genus. Chromatographic fractionation of the methanol-soluble portion of the 80% extract of M. styphelioides leaves yielded compounds 6, 13 and 14. Notably, this is the first report of the phytochemical characterization of these compounds from M. styphelioides. The structures of the compounds () were elucidated on the basis of 1D and 2D NMR (1H, 13C, 1D-TOCSY, DQF-COSY, HSQC, and HMBC), Ultra-Violet spectroscopy, and HRESI-MS/MS data and by comparing these data with those previously reported in the literature ().
Table 1. Identification of the isolated compounds 1–14 using UV and HRESI/MS/MS.
In this study, the biocidal effect of E. globulus and M. styphelioides methanol extracts and their isolated compounds against B. alexandrina snails and different stages of the life cycle of S. mansoni was determined with the objective of developing a new, safe and readily available antischistosomal drug. The molluscicidal activity of the extracts and isolated compounds is listed in . The most active samples were E. globulus total extract, tellimagrandin I (7), pentagalloyl glucose (11) and eucalbanin B (12). The latter exhibited the most potent molluscicidal activity (LC50 = 55 ppm). The molluscicidal activity of the ellagitannin dimer eucalbanin B was in the range of the WHO standard for toxicity, which requires an LC50 of less than 100 ppm for plant molluscicides (Marston & Hostettmann, Citation1985; WHO, Citation1965). The probit mortality analysis showed a linear relationship between the molluscicidal activity and the concentrations (ppm) of the tested samples. The slope values were steep, indicating a large increase in snail mortality with a small increase in the concentration of the tested samples. The LC50 of compounds 1, 2, 4 and 10 were not determined, because only small amounts of these compounds were isolated from E. globulus extract.
Table 2. Molluscicidial activity of extracts and isolated compounds on B. alexandrina snails.
The potent molluscicidal activity of eucalbanin B (12) was supported by the decreased protein content and alterations in the activities of vital enzymes in the snail hemolymph and tissues (), which indicated damage to the snail tissue and disturbance of the physiological activities required for parasite development and cercarial production (Bakry et al., Citation2011). In this study, treatment of the snails with a concentration of eucalbanin B (12) corresponding to the LC25 of the compound resulted in a significant decrease in the concentration of the total proteins in the snail tissues and hemolymph by 22.7 and 25.8%, respectively, compared to the control snails. The decreased protein content may be attributed either to the destruction of the snail tissue and impairment in protein synthesis or to the stimulation of gluconeogenesis to use proteins as a source of energy under stress conditions of intoxication with eucalbanin B (Bakry et al., Citation2011). The results of the present study also indicated that there was a significant increase in the levels of the transaminases ALT (57.8%) and AST (113.2%) in the hemolymph at the end of the third week of exposure to eucalbanin B compared with the levels found in the control, whereas there was a significant decrease in their tissue levels to 7.4 and 48.6% of the control, respectively. The increased levels of transaminases in the hemolymph may be due to their release from the damaged tissue into the hemolymph (Bakry et al., Citation2011), and this would also explain the decreased levels found in the snail tissue. AST and ALT are important enzymes in amino acid metabolism and the generation of energy from amino acids (Tunholi et al., Citation2011). Therefore, the increase in transaminases may also result from the high energy demand of the snail under intoxication conditions. Alterations in the levels of ALP and ACP were also observed. ALP plays a critical role in shell formation, and ACP plays an important role in catabolism and pathological necrosis (Tripathi et al., Citation2004). ALP was significantly increased by 38.5 and 181.4% in the hemolymph and tissues, respectively. ACP was also significantly increased by 48.4 and 21.2% in the hemolymph and tissues, respectively. It is clear from the results of this study that eucalbanin B at a sub-lethal concentration (LC25) disturbs certain enzymes that are necessary for the physiological activity of the snail; therefore, eucalbanin B (12) can be used as a potent molluscicidal agent that targets these enzymes. This supports previous reports regarding the use of snail enzymes as targets for the development of antischistosomal drugs (El-Ansary & Daihan, Citation2006).
Table 3. Changes in the biochemical parameters in hemolymph and soft tissues of B. alexandrina snails after 3 weeks exposure by the LC25 of eucalbanin B (12).
With regard to structural requirements, the present study indicated that high molecular weight ellagitannins, including the dimer eucalbanin B (12), have considerable molluscicidal activity compared to other classes of phenolic compounds, including the ellagitannin monomers (6), (7), (9) and (14), the galloyl ester derivatives (3) and (11), the flavonoids (8) and (13) and the polyoxygenated chromene derivative (5). Therefore, it can be concluded that the high molecular weight of the ellagitannin molecule is important for its molluscicidal activity.
Based on the results of this study, other compounds were shown to be more toxic than the ellagitannins to the miracidia and resulted in almost 100% mortality after a 2 h exposure (). It was clear that mallophenol B (3), 2,2,8-trimethyl-6-formyl-chrom-3-ene-7-O-β-d-glucopyranoside (5) and benzyl alcohol 7-O-(3′,4′,6′-tri-O-galloyl)-β-d-glucopyranoside (10) at a concentration of 20 ppm exhibited significant and potent miracidicidal activities. The 80% MeOH extract of E. globulus also showed strong miracidicidal activity (80% inhibition) after a 2 h exposure. Moreover, E. globulus extract had the highest cercaricidal and schistosomicidal activities among the tested samples, and caused 100% and 40% mortality after a 2 h exposure, respectively. The schistosomicidal activity of E. globulus leaf extract was close to that of the standard praziquantal (43% mortality after a 2 h exposure).
Table 4. Effect of extracts and isolated compounds on Schistosoma mansoni miracidia, cercariae and worms after 1 and 2 h exposure.
The results of this study indicated that E. globulus and its isolated compounds are particularly suitable for using as biocidal agents to control schistosomiasis. E. globulus is widely distributed and the plant is used in folk medicine and food products, so it is generally considered safe (Amakura et al., Citation2002). Moreover, plant collection and the extraction of ellagitannins are easily performed. These attributes make E. globulus a potential source for bioactive compounds suitable for biological application as plant molluscicides to control the intermediate snail host and as biocidal agents against cercariae and miracidia in endemic areas. The toxicity and costs of synthetic molluscicides have resulted in an increased interest in compounds derived from locally growing plants that can be used as molluscicides. Therefore, the isolation and identification of the most active components of molluscicidal plants is of great importance to further synthetic techniques and for the possibility of discovering novel derivatives of the original natural lead compounds that exhibit improved pharmacological activity (Marston & Hostettmann, Citation1985).
It can be concluded from the present study that the ellagitannin dimer eucalbanin B (12) is a candidate molluscicide of plant origin, while mallophenol B (3), 2,2,8-trimethyl-6-formyl-chrom-3-ene-7-O-β-d-glucopyranoside (5) and benzyl alcohol 7-O-(3′,4′,6′-tri-O-galloyl)-β-d-glucopyranoside (10) are potential miracidicidal agents. The present study also demonstrates the possibility of using E. globulus extract as a potent cercaricidal agent for environmental management of affected areas. Notably, this is the first report of the molluscicidal, miracidicidal, cercaricidal and schistosomicidal activities of the extracts and the compounds 3–14.
Declaration of interest
The authors have declared no conflicts of interest.
Acknowledgements
We thank Professor Juha-Pekka Salminen, Department of Chemistry, University of Turku Finland, for the use of the HPLC–MS instrument during this study.
References
- Abdul-Salam JM, Michelson EH. (1983). Schistosoma mansoni: Immunofluorescent detection of its antigen reacting with Biomphalaria glabrata amoebocytes. Exp Parasitol 55:132–7
- Al-Sayed E, Martiskainen O, Bobrowska-Hägerstrand M, et al. (2010). Phenolic compounds from Eucalyptus gomphocephala with potential cytotoxic and antioxidant activities. Nat Prod Commun 5:1639–42
- Amakura Y, Umino Y, Tsuji S, et al. (2002). Constituents and their antioxidative effects in Eucalyptus leaf extract used as a natural food additive. Food Chem 77:47–56
- Bakry FA, Mohamed RT, El-Hommossany K. (2011). Biological and biochemical responses of Biomphalaria alexandrina to some extracts of the plants Solanum siniacum and Artemisia judaica L. Pestic Biochem Physiol 99:174–80
- Braguine CG, Bertanha CS, Gonçalves UO, et al. (2012). Schistosomicidal evaluation of flavonoids from two species of Styrax against Schistosoma mansoni adult worms. Pharm Biol 50:925–9
- Coppen JW. (2002). Eucalyptus: The Genus Eucalyptus, 1st ed. London: Taylor & Francis
- Duan D, Li Z, Luo H, et al. (2004). Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg Med Chem Lett 14:6041–4
- Eissa MM, El Bardicy S, Tadros M. (2011). Bioactivity of miltefosine against aquatic stages of Schistosoma mansoni, Schistosoma haematobium and their snail hosts, supported by scanning electron microscopy. Parasit Vectors 4:73–83
- El-Ansary A, Al-Daihan S. (2006). Important aspects of Biomphalaria snail–schistosome interactions as targets for antischistosome drug. Med Sci Monit 12:282–92
- Farag RS, Shalaby AS, El-Baroty GA, et al. (2004). Chemical and biological evaluation of the essential oils of different Melaleuca species. Phytother Res 18:30–5
- Finney DJ. (1971). Probit Analysis, 3rd ed. United Kingdom: Cambridge University Press, 68–72
- Hatano T, Yoshida T, Shingu T, Okuda T. (1988). 13C Nuclear magnetic resonance spectra of hydrolyzable tannins. II: Tannins forming anomer mixtures. Chem Pharm Bull 36:2925–33
- Hong QB, Zhou XN, Han Y, et al. (2001). Molluscicidal effects of extracts of Eucalyptus camaldulensis on Oncomelania hupensis. Chin J Schisto Cont 13:18–20
- Ito H, Koreishi M, Tokuda H, et al. (2000). Cypellocarpins A–C, phenol glycosides esterified with oleuropeic acid, from Eucalyptus cypellocarpa. J Nat Prod 63:1253–7
- Kind PRN, King EJ. (1954). Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol 7:322–6
- Li J, Xu H, Tang W, Song Z. (2012). Two new triterpenoids from the bark of Eucalyptus exserta and their molluscicidal and cytotoxic activities. Fitoterapia 83:383–7
- Litchfield JT, Wilcoxon FA. (1949). A simplified method of evaluating dose-effect experiment. J Pharm Exp Therap 96:99–113
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75
- Mabry TJ, Markham KR, Thomas MB. (1970). The Systematic Identification of Flavonoids. New York, Heidelberg, Berlin: Springer-Verlag
- Marston A, Hostettmann K. (1985). Review article number 6: Plant molluscicides. Phytochemistry 24:639–52
- Matsunami K, Takamor I, Shinzato T, et al. (2006). Radical-scavenging activities of new megastigmane glucosides from Macaranga tanarius (L.) MULL.-ARG. Chem Pharm Bull 54:1403–7
- Mostafa SSM, Gawish FA. (2009). Towards to control Biomphalaria alexandrina snails and the free living larval stages of Schistosoma mansoni using the microalga Spirulina platensis. Aust J Basic Appl Sci 3:4112–19
- Okamura H, Mimura A, Niwano M, et al. (1993). Two acylated flavonol glycosides from Eucalyptus rostrata. Phytochemistry 33:512–14
- Okuda T, Hatano T, Ogawa N, et al. (1984). Cornusiin A, a dimeric ellagitannin forming four tautomers, and accompanying new tannins in Cornus officinalis. Chem Pharm Bull 32:4662–5
- Reitman S, Frankel S. (1957). A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic trasaminases. Am J Clin Path 28:56–63
- Singh SK, Yadav RP, Singh A. (2010). Molluscicides from some common medicinal plants of eastern Uttar Pradesh, India. J Appl Toxicol 30:1–7
- Tanaka T, Orii Y, Nonaka G, Nishioka I. (1993). Tannins and related compounds. CXXIII. Chromone, acetophenone and phenylpropanoid glycosides and their galloyl and/or hexahydroxydiphenoyl esters from the leaves of Syzygium aromaticum MERR. et PERRY. Chem Pharm Bull 41:1232–7
- Tian LW, Zhang YJ, Wang YF, et al. (2009). Eucalmaidins A–E, (+)-oleuropeic acid derivatives from the fresh leaves of Eucalyptus maideni. J Nat Prod 72:1608–11
- Tripathi SM, Singh VK, Singh S, Singh DK. (2004). Enzyme inhibition by the molluscicidal agent Punica granatum Linn. bark and Canna indica Linn. root. Phytother Res 18:501–6
- Tunholi VM, Lustrino D, Tunholi-Alves VM, et al. (2011). Biochemical profile of Biomphalaria glabrata (Mollusca: Gastropoda) after infection by Echinostoma paraensei (Trematoda: Echinostomatidae). Parasitol Res 109:885–91
- WHO. (1965). Molluscicide screening and evaluation. Bull World Health Org 33:567–81
- WHO. (2010). First report on neglected tropical diseases. Working to overcome the global impact of neglected tropical diseases. WHO Library Cataloguing-in-Publication Data [Online]. Available from: http://www.who.int/neglected_diseases/2010report/en/ [last accessed 5 May 2012]
- Wu QL, Wang SP, Du LJ, et al. (1998). Chromone glycosides and flavonoids from Hypericum japonicum. Phytochemistry 38:1417–20
- Xiaonong Z, Minggang C, McManus D, Bergquist R. (2002). Schistosomiasis control in the 21st century. Proceedings of the international symposium on Schistosomiasis, Shanghai. Acta Trop 82:95–114
- Yoshida T, Maruyama T, Nitta A, Okuda T. (1992). Eucalbanins A, B and C, monomeric and dimeric hydrolyzable tannins from Eucalyptus alba REINW. Chem Pharm Bull 40:1750–4
- Yoshida T, Ito H, Yoshimura M, et al. (2008). C-Glucosidic ellagitannin oligomers from Melaleuca squarrosa Donn ex Sm., Myrtaceae. Phytochemistry 69:3070–9
- Yoshimura M, Ito H, Miyashita K, et al. (2008). Flavonol glucuronides and C-glucosidic ellagitannins from Melaleuca squarrosa. Phytochemistry 69:3062–9