Abstract
Context: Centaurea L. (Asteraceae) species used as herbal remedies in Turkish traditional medicine have shown several biological properties.
Objective: Extracts obtained from the aerial parts of Centaurea aphrodisea Boiss., Centaurea athoa DC., Centaurea hyalolepis Boiss., Centaurea iberica Trev. and Centaurea polyclada DC. were evaluated for their antioxidant, cytotoxic and anti-inflammatory activities.
Materials and methods: Extracts of Centaurea species were tested for their antioxidant activity in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) screening assays and for in vitro anti-inflammatory activity by Nf-κB and iNOS inhibition assays. The extracts were tested for their in vitro cytotoxicities against a panel of human solid tumor cell lines (SK-MEL: malignant melanoma, KB: oral epidermal carcinoma, BT-549: breast ductal carcinoma and SK-OV-3: ovary carcinoma) as well as non-cancerous kidney fibroblast (Vero) and kidney epithelial cells (LLC-PK1) by Neutral Red assay. In vivo anti-inflammatory activity of C. athoa was evaluated by the carrageenan-induced paw edema test in rats.
Results: Antioxidant activities were observed for methanol extracts of plants. C. polyclada had the strongest effect on BT-549, KB and SK-OV-3 cell lines (30, 33 and 47 µg/ml, respectively). Nf-κB inhibition of chloroform extract of C. athoa was determined equivalent to positive control parthenolide (IC50: 6 µg/ml). This extract also showed anti-inflammatory activity by the carrageenan-induced paw edema test in rats, in all hours at a dose of 50 mg/kg compared to the control group.
Discussion and conclusion: C. athoa is suggested to be a potential source of lead compounds for inflammatory diseases due to the significant in vitro and in vivo anti-inflammatory results.
Introduction
Several diseases such as cancer, diabetes, heart diseases and arteriosclerosis are considered to be associated with oxidative damage. Antioxidant products are important in the prevention of oxidative stress-related diseases. In recent years, due to the possible toxic side effects of synthetic antioxidants, interest in utilizing natural antioxidants has increased substantially. Phenols, flavonoids and other non-nutrient compounds of fruits and vegetables have been recognized as potential factors that can be beneficial to human health because of their antioxidant, anticarcinogenic and anti-inflammatory activities (Singh et al., Citation2008).
Plants have a long history of use in the treatment of cancer. Over 60% of currently used anticancer agents are derived from natural sources (Cragg & Newman, Citation2005). A major group of these products are powerful antioxidants, others are phenolics in nature and the remainder includes reactive groups that confer protective properties (Reddy et al., Citation2003).
Many of the inflammatory diseases are becoming common in aging society throughout the world. In view of the growing need for effective anti-inflammatory agents, the potential for natural products to serve as safe and effective therapeutic agents has gained increasing attention by researchers (Gosslau et al., Citation2011). NO production and iNOS expression are considered to be related to the pathogenesis of several diseases such as inflammation and carcinogenesis. Regulation of iNOS in tissues might be important for the treatment of inflammation and tumorigenesis. Also nuclear factor kappa B (NF-кB) plays a critical role in the expression of many genes involved in immune and inflammatory response (Jung et al., Citation2007).
The genus Centaurea L. (Asteraceae) is represented by 192 taxons in Turkish flora and with an endemism ratio of 61% (Uysal, Citation2008; Wagenitz, Citation1975). The genus has been subjected to several phytochemical studies leading to the isolation of sesquiterpene lactones and flavonoids as main secondary metabolites of its species (Kaij-a Kamb et al., Citation1992; Karamenderes et al., Citation2007a,Citationb). Various species of Centaurea are used as herbal remedies for their digestive, tonic, expectorant, antipyretic and antidiarrheal effects in Anatolian traditional medicine (Baytop, Citation1999). Bioactivity studies on some Centaurea species have reported anti-inflammatory, antioxidant, antimicrobial, antiulserogenic and cytotoxic properties (Arif et al., Citation2004).
As a continuation of our studies on Centaurea species, now we report in vitro antioxidant, cytotoxic and anti-inflammatory properties of various extracts of Centaurea aphrodisea, Centaurea athoa, Centaurea hyalolepis, Centaurea iberica and Centaurea polyclada and in vivo anti-inflammatory activity of C. athoa.
Materials and methods
Plant material
Centaurea species were collected in June 2010 from South Western and South Eastern parts of Turkey and identified by Dr. Serdar Senol from Section of Botany, Department of Biology, Faculty of Science, Ege University. Voucher specimens have been deposited in the Herbarium of Ege University (IZEF), Faculty of Pharmacy, Izmir, Turkey. Details of the collection localities are presented in .
Table 1. Collection sites, abbreviations and yields of the extracts of Centaurea L. species.
Reagents
All cells were obtained from ATCC (Rockville, MD). DCFH-DA, RPMI-1640 and DMEM/F12 media were from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) and bovine calf serum were from Atlanta Biologicals (Lawrenceville, GA). The NF-κB reporter construct contained two copies of the element from immunoglobulin K promoter (p BIIXLUC) and was a gift from Dr. Riccardo Dalla-Favera. The Sp-1 reporter plasmid (pGL3-promoter) was obtained from Promega (Madison, WI). All other chemicals and reagents were of the highest purity available and obtained from Sigma (St. Louis, MO).
Preparation of extracts
Dried and ground aerial parts of the plants were extracted with n-hexane, chloroform and methanol at room temperature in an ultrasonic bath (3 × 2 L, 24 h for each). The combined extracts were evaporated separately under reduced pressure to dryness at 40 °C. Yields of the extracts are given in .
Assay for DPPH radical-scavenging activity
Free radical scavenging activity (FRSA) of the compounds on stable was determined spectrophotometrically and calculated as a percentage of 2,2-diphenyl-1-picrylhydrazyl (DPPH) discolouration. The DPPH assay was performed as previously described with simple modifications (Wang et al., Citation2006). Briefly, extracts in methanol (100 µl) at different concentrations (50–1000 µg/ml) were added to 200 µM DPPH (100 µl) in methanol and mixed in a 96-well microplate. After a gentle mixing and standing 30 min at room temperature, the absorbance of the resulting solution was measured at 517 nm in microplate reader (Molecular Devices, Versamax, Sunnyvale, CA). Ascorbic acid was used as a positive control.
The percent DPPH scavenged by each pure compound was calculated using the following equation:
where AB is the absorbance of the control (t: 0 min), AA is the absorbance of the sample (t: 15 min). Ascorbic acid concentrations were 2, 4, 8, 12, 16 and 20 µg/ml (prepared by dilution from 1 mg/ml stock solution).
Assay for Trolox equivalent antioxidant capacity
Antioxidant activity was measured by using the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) method as described (Re et al., Citation1999). ABTS (7 mM) was mixed with 2.45 mM potassium persulphate in order to produce ABTS+ radical. The absorbance of this radical was left in 30 °C and dark room for 2 d to get stable absorbance at 734 nm.
Extracts (100–1000 µg/ml) were diluted in ethanol. Ethanol was used as blank. ABTS+ solution diluted with 5 mM phosphate buffer until to have to an absorbance of 0.70 ± 0.02 at 734 nm. About 1 ml of this stock solution was taken and mixed with 10 µl sample solution and measured at 734 nm, 1 min after initial mixing and up to 6 min. All measurements were carried out triplicate. The percentage of inhibition were calculated as follows:
AABTS+ is the absorbance of ABTS+ at 734 nm (0.700 ± 0.02), A6.min is the 6 min absorbance after the addition of sample to ABTS+. A standard curve of Trolox was obtained using Trolox standard solution at various concentrations (2.5–15 mM) in ethanol. The absorbance of the samples was compared to that of the standard curve and the antioxidant properties were expressed as mM Trolox equivalent/mg for extracts.
Determination of total phenolic content
Determination of total phenolic content was performed by the method of Mc Donald et al. (Citation2001), using the Folin Ciocalteu reagent. Briefly, 0.5 ml of each extract (0.1 mg/ml) was mixed with 5 ml of Folin Ciocalteu reagent (1 : 10 with distilled water). About 4 ml (1 M) Na2CO3 was added to this mixture and heated in water bath (45 °C) for 15 min. The absorbance of the mixtures was measured at 765 nm. A standard curve was prepared by using gallic acid in various concentrations (50, 100, 150, 200 and 250 mg/L). All measurements were carried out triplicate and the results were expressed as gallic acid equivalents (GAE) (mg/L).
Determination of total flavonoid content
Total flavonoid contents of methanol extracts were determined by the aluminum chloride colorimetric method with some modifications (Chang et al., Citation2002). About 0.5 ml of each extract (1 g/L) was mixed with 1.5 ml methanol, 0.1 ml of 10% alluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water and incubated at room temperature for 30 min. Standard curve was prepared with quercetin (12.5, 25, 50, 75 and 100 μg/ml) with the same method. The absorbance of the mixtures was measured at 415 nm. All measurements were carried out triplicate. Results were expressed as quercetin equivalents (QE) (μg/ml).
Assay for in vitro cytotoxicity
The extracts were tested for their in vitro cytotoxicities against a panel of human solid tumor cell lines (SK-MEL: malignant melanoma, KB: oral epidermal carcinoma, BT-549: breast ductal carcinoma and SK-OV-3: ovary carcinoma) as well as non-cancerous kidney fibroblast (Vero) and kidney epithelial cells (LLC-PK1). Cells (25 000 cells/well) were seeded in the wells of a 96-well plate and incubated for 24 h. Samples were added followed by an incubation for 48 h. The number of viable cells was determined using the supravital dye Neutral Red (Borenfreund et al., Citation1990). Briefly, the cells were washed with saline and incubated for 3 h with a solution of neutral red. The cells were washed again to remove extracellular dye. A solution of acidified ethanol was added to liberate the incorporated dye from viable cells and the absorbance was read at 450 nm. Doxorubucin was used as positive control.
Assay for inhibition of NF-κB
The SW1353 human chondrosarcoma cells were cultured in 1 : 1 mixture of DMEM/F12 supplemented with 10% FBS, 100 U/ml penicillin G sodium and 100 µg/ml streptomycin at 37 °C in an atmosphere of 5% CO2 and 95% humidity. Cells (1.2 × 107) were washed once in an antibiotic and FBS-free DMEM/F12, and then resuspended in 500 µl of antibiotic-free DMEM/F12 containing 2.5% FBS. NF-κB luciferase plasmid construct was added to the cell suspension at a concentration of 50 µg/ml and incubated for 5 min at room temperature. The cells were electroporated at 160 V and one 70-ms pulse using BTX disposable cuvettes model 640 (4-mm gap) in a BTX Electro Square Porator T 820 (BTX I, San Diego, CA). After electroporation, 1.25 × 105 cells per well were plated in 200 µL of DMEM/F12 containing 10% FBS and antibiotics. After 24 h, cells were treated with different concentrations of test compounds for 30 min and then induced with PMA (70 ng/ml) for 8 h. Cells were lyzed by adding 40 µL of a 1 : 1 mixture of LucLite reagent and PBS containing 1 mM calcium and magnesium (Packard Instrument Company, Meriden, CT). Light output was detected on a TopCount (Packard) in a single-photon counting mode. Relative luciferase units (RLU) were 12–15-fold higher in PMA-induced cells versus controls. Parthenolide was used as the positive control (Subbaramaiah et al., Citation2001).
Assay for inhibition of iNOS activity
Mouse macrophages (RAW264.7) are cultured in phenol red free RPMI medium with 10% bovine calf serum. For the assay, cells are seeded in 96-well plates (4 × 105 cells/well) and incubated for 3 h. After inducing with LPS, test samples were added and cells are further incubated for 24 h. The level of nitrite in the medium was measured by using Griess reagent. Percent inhibition of nitrite production by sample was calculated in comparison to vehicle control. IC50 values were obtained from dose curves (Quang et al., Citation2006).
Animals
The protocol was approved by the Ege University, Local Ethical Committee of Animal Experiment (Date: 23 December 2011, No.: 2011/210). Experiments were performed on male Wistar rats (weighing 150–200 g, each) and they were purchased from the Experimental Animal Center of Ege University (Izmir, Turkey). Animals were housed in a room maintained at 22 ± 1 °C with an alternating 12 h light-dark cycle. Food and water were available ad libitum. The animals were transported to a quiet laboratory at least 1 h before the experiment. All experiments conformed to ethical guidelines for investigation of experimental pain in conscious animals (Zimmermann, Citation1983). The number of animals and the intensity of noxious stimuli were the minimum possible with which to demonstrate reliable effects of the agents tested. Each animal was used once only and was humanely sacrificed immediately after completion of testing.
Assay for in vivo anti-inflammatory activity
The anti-inflammatory activity was evaluated by the carrageenan-induced paw-edema test in the rat (Winter et al., Citation1962). Rats were deprived of food overnight and treated orally with saline (as the control group) and C. athoa chloroform extract (6.75, 12.5, 25 and 50 mg/kg doses of dissolved in 2% Tween 20), 30 min before 0.1 ml 1% carrageenan in isotonic saline was injected subplantarly into the left hindpaw. The contra lateral paw was injected with 0.1 ml saline and used as the control. The volume difference between the inflammatory agents and saline injected paws was used to evaluate the inflammatory response. Paw volume (V) was measured with a water plethysmometer (Lettica, LE 7500, Barcelona, Spain) before and 1, 2, 3, 4, 5 and 6 h after the injection of carrageenan into the plantar region of the left hindpaw (n = 6 for each group). The anti-inflammatory test was repeated with 10 mg/kg indomethacin administration. The percent inhibition of edema induced by carrageenan was calculated for each group using the equation below. Indomethacine (10 mg/kg) and was used as reference drug
Acute toxicity
In this study, the acute oral toxicity of C. athoa chloroform extract was assessed using the limit test in the rat (Derelanko et al., Citation1995). In the test, male and female Wistar albino rats weighing 150–200 g each were used (n = 2 for each group). The limit dose (2000 mg/kg body wt.) for acute oral toxicity according to EPA/OECD was used.
Statistical analysis
Results are reported as means ± SEM (n), with n indicating the number of animals. IC50 calculations were performed with Graph Pad Prism (San Diego, CA). Data were analyzed using Student's t-test, ANOVA or non-parametric tests. Differences between extract or drug treated and control groups were also evaluated using Dunnett's t-test. The mean and SD of n = 6 were calculated. A probability value of p ≤ 0.05 was considered statistically significant.
Results
Yield of extracts of C. aphrodisea, C. athoa, C. hyalolepis, C. iberica and C. polyclada are given in .
FRSAs of methanol extracts were measured by the DPPH assay. The highest activity was observed for C. hyalolepis extract with a IC50 of 147.8 µg/ml. When compared to the standard antioxidant ascorbic acid (6.68 µg/ml), all other extracts showed weak activity (200.1–273.4 µg/ml). Antioxidant capacities of methanol extracts were evaluated by the ABTS+ method and the results are given as Trolox equivalents in . Each extract exhibited concentration-dependent antioxidant capacities. Among all tested extracts, C. aphrodisea showed strongest activity at 1000 µM concentration (5.923 ± 0.47). It is followed by C. polyclada with a value of 4.673 ± 0.04. C. iberica exhibited a significant Trolox equivalent value (3.005 ± 0.01) at 500 µM concentration.
Table 2. Trolox equivalent antioxidant capacities of Centaurea L. methanol extracts.
As can be seen from , the phenolic content of C. hyalolepis was superior to other extracts (11.439 mg GAE/L) among all species. This is followed by C. polyclada extract (8.558 mg GAE/L) and C. iberica had the lowest phenolic content (5.728 ± 0.682 mg GAE/L). When comparing flavonoid contents, all extracts had similar values in the range 16.172–34.866 µg QUE/ml. The highest flavonoid content was determined with the C. hyalolepis extract (34.866 µg QUE/ml).
Table 3. Total phenolic and total flavonoid contents of Centaurea L. species methanol extracts.
The n-hexane, chloroform and methanol extracts were tested for their in vitro cytotoxicity against SK-MEL (malignant melanoma), KB (oral epidermal carcinoma), BT-549 (breast ductal carcinoma), SK-OV-3 (ovary carcinoma), Vero (non-cancerous kidney fibroblast) and LLC-PK1 (kidney epithelial) cell lines. As can be seen in , only the chloroform extracts of tested plants exhibited an inhibition on cells. Among all tested extracts, C. polyclada chloroform extract had the strongest effect on BT-549, KB and SK-OV-3 cell lines (30, 33 and 47 µg/ml, respectively). C. athoa chloroform extract had cytototoxic activity against all tested tumor cells (40–56 µg/ml) but it also inhibited the non-cancerous cells LLC-PK1 and Vero (20 and 52 µg/ml, respectively). Chloroform extracts of C. aphrodisea, C. hyalolepis and C. iberica had moderate effects on BT-549, KB and SK-OV-3 cell lines. We could not determine any significant activity with n-hexane and methanol extracts.
Table 4. Cytotoxic activities of various extracts of Centaurea L. species on diffirent cell lines.
In vitro anti-inflammatory capacities were measured by Nf-κB and iNOS inhibition assays. Results are shown in . Among the extracts, the strongest anti-inflammatory activity was observed by the chloroform extract of C. athoa with both assays (6 µg/ml for Nf-κB and 16 µg/ml for iNOS assay). Inhibition of Nf-κB activation of this extract was equal to synthetic anti-inflammatory agent parthenolide used as a positive control. The chloroform extracts of all tested plants had more significant activities than n-hexane and methanol extracts for both assays. Only methanol extract of C. athoa (28 µg/ml) and n-hexane extract of C. hyalolepis (33 µg/ml) showed an effect on iNOS inhibition. None of the extracts inhibited SP-1-dependent luciferase expression. Measurement of SP-1-mediated luciferase expression is useful for detecting agents that non-specifically inhibit luciferase expression due to cytotoxicity, inhibition of luciferase enzyme activity or light output.
Table 5. Inhibition of NF-кB and iNOS by various extracts of Centaurea L. species.
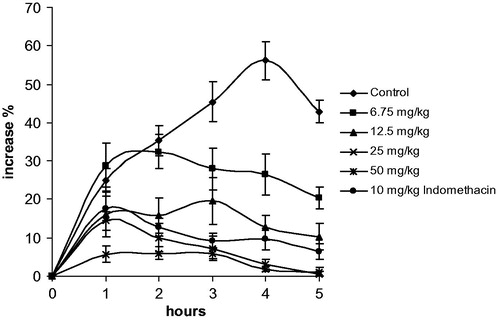
In light of the in vitro anti-inflammatory studies, we evaluated in vivo anti-inflammatory capacity of the chloroform extract of C. athoa by the carrageenan-induced paw edema test in rats. Results are shown in . The intraplanatar injection of carrageenan caused a time-dependent paw edema in rat, although saline injection caused no swelling (data not shown). Peroral administration of 50 mg/kg extract showed considerable inhibition of paw swelling in all hours compared to the control group (p < 0.05). In contrast, administration of 25 mg/kg extract inhibited at 2, 3, 4, 5 and 6 h while 12.5 and 6.75 mg/kg extracts inhibited paw swelling at 3, 4, 5 and 6 h (p < 0.05). Similarly, a significant inhibition after 2, 3, 4, 5 and 6 h (p < 0.05) was obtained by indomethacin administration. Percent increment in paw swelling was calculated by using the values before carrageenan injection. No lethality was observed among rats treated with oral doses of the chloroform extract of C. athoa.
Figure 1. Inhibition of inflammatory effect of carrageenan (0.1 ml 1% carrageenan/rat) by C. athoa extract (6.75, 12.5, 25 and 50 mg/kg) and indomethacin (10 mg/kg). Data are represented as means ± SEM of duplicate measurements of six rats. Also the percentage inhibitions of edema compared with the negative control formed by carrageenan are shown.
Discussion
As we know the antioxidant compound groups (phenolics, flavonoids, etc.) are more dominant in polar fractions, we tested antioxidant capacities over methanol extracts. The DPPH assay is an easy and straightforward method for determining the free radical scavenging property of a compound. While the presence of the highest number of phenolic hydroxyl groups in the molecule will increase the antioxidant properties, highest activity obtained for C. hyalolepis (IC50: 147.8 µg/ml) mainly can be associated with its highest phenolic and flavonoid contents (11.44 mg GAE/L and 34.87 µg QE/ml). In a previous study, Granger et al. (Citation2009) reported the radical scavenging activity of the methanol extract of C. polyclada with an IC50 value 0.02 mg/ml. This is consistent with our result for C. polyclada. ABTS is also a general method used for testing antioxidant capacity of compounds but there are no so much studies on Centaurea species concerning this method. To our knowledge, this is the first antioxidant evaluation of Centaurea taxons mentioned above, except C. polyclada.
In vitro cytotoxicity investigations on plant extracts are commonly the first steps of research for anticancer compounds from natural sources. In recent years, the Centaurea genus has attracted great interest of researchers from this area due to its wide distribution and chemical properties. In our study, cytotoxicities of all extracts were determined by Neutral-Red assay which is one of the most used cytotoxicity tests based on the ability of live cells to uptake and bind neutral red.
The results reported here show that only the chloroform extracts exhibited an inhibition on all tested cell lines. This activity could be due to the apolar molecules especially sesquiterpene aglicons that are well-known cytotoxic compounds via alkylating functional groups in the molecule structures. C. polyclada had the strongest effect on BT-549, KB and SK-OV-3 cell lines (30, 33 and 47 µg/ml, respectively) and suggested to be a potential for further chemical and detailed activity researches.
In a literature survey, we could not find cytotoxic activity reports for these Centaurea species on the mentioned cell lines. In contrast, most previous studies dealing with the cytotoxic activities of Centaurea species have involved pure compounds isolated from various extracts of plants. Especially, isolated sesquiterpenes were found to be responsible for cytotoxic properties. The cytotoxic activity of sesquiterpene lactones isolated from the aerial parts of various species of the genus Centaurea against five human cell lines (DLD1, SF268, MCF7, H460 and OVCAR3) and the compound 8α-O-(3-hydroxy-2-methylenepropanoyl) dehydromelitensin exhibited a growth inhibiting effect against OVCAR3 (IC50: 7.4 µM) as well as other cell lines (Koukoulitsa et al., Citation2002). Moreover, we have reported the cytotoxic activity of cnicin from C. calolepis towards pig kidney epithelial (LLC-PK11), human malignant melanoma (SK-MEL) and human ductal carcinoma (BT-549) cells with IC50 values of 23.3, 14.0 and 18.3 μM, respectively (Baykan Erel et al., Citation2011). These reports also support the idea that the activity of chloroform extracts observed in our study may be due to the sesquiterpene contents of them.
Based to our knowledge, this is also the first in vitro anti-inflammatory report on C. aphrodisea, C. athoa, C. hyalolepis, C. iberica and C. polyclada. The chloroform extracts of all plants showed significant inhibitions on NF-кB and iNOS expression with IC50 values between 6–37 and 16–29 µg/ml, respectively. C. athoa was the most effective one even though the inhibition of Nf-κB activation of this extract was equal to synthetic anti-inflammatory agent parthenolide (6 µg/ml). Therefore, we decided to carry out the further experiments with this extract and in vitro finding were well correlated with the in vivo effects of C. athoa. Peroral administration of extract showed considerable inhibition in all hours at a dose of 50 mg/kg and treatment with 25 mg/kg extract produced a significant inhibition after 2 h as well as the standard anti-inflammatory agent indomethasine (10 mg/kg).
The carrageenan-induced paw edema test is a suitable test for the evaluation of anti-inflammatory activity. This method has frequently been used to assess the antiedematous effects of natural products. The development of edema in the rat hindpaw following the injection of carrageenan has been described as a biphasic event. The initial phase of edema (0–1 h) has been attributed to the release of histamine, 5-hydroxytryptamine (5-HT) and bradykinin and the second accelerating phase of swelling (1–6 h) has been correlated with the elevated production of prostaglandins and the induction of inducible cyclo oxygenase (COX-2) in the hindpaw (Koca et al., Citation2009a; Siebert et al., Citation1994). Moreover, in a previous investigation, the inhibition of the sustained phase of the paw edema following the intraplantar injection of carrageenan is suggested as suitable for the in vivo assessment of the anti-inflammatory actions of novel inhibitors of the iNOS (Salvemini et al., Citation1996). As the 50 mg/kg doses of C. athoa extract demonstrated effects in both phases of acute inflammation in a dose-dependent manner, its effects may be mediated by inhibiting the proinflammatory mediators such as iNOS and COX-2 through blocking NF-κB activation.
Generally members of the Centaurea genus have been the subject of anti-inflammatory research depending on their traditional uses (Karamenderes et al., Citation2007a,Citationb; Koca et al., Citation2009a). C. iberica has been investigated by using in vivo inhibition of acetic acid induced increase in capillary permeability for anti-inflammatory activity. A dose-dependent inhibitory activity was observed only for methanol extract at the dose of 200 mg/kg (Koca et al., Citation2009b). In our study, the methanol extract of C. iberica did not demonstrate any effect and the chloroform extract showed moderate inhibition on iNOS and NF-κB with IC50 values of 25 and 37 µg/ml, respectively.
In a previous study, we have demonstrated the in vitro anti-inflammatory effects of C. hierapolitana, C. calolepis and C. cadmea and the chloroform extracts of these plants exhibited strong anti-inflammatory activities by inhibiting their effects on the activation NF-кB (Karamenderes, et al., Citation2007). Our previous chemical investigations on C. calolepis resulted with the isolation of the sesquiterpene cnicin, and it was found to have strong inhibition on NF-кB (IC50: 1.8 µM) and iNOS activation (IC50: 6.5 µM) (Baykan Erel et al., Citation2011) so it was suggested as a potent anti-inflammatory agent. Significant results observed for C. athoa in this study in both in vitro and in vivo experiments can be also attributed to sesquiterpenes. However, further phytochemical investigations are needed to support this suggestion. As a part of our ongoing program of research on Centaurea species, C. athoa and C. polyclada, isolation and purification studies are in progress to find the compounds responsible for the activities.
Declarations of interest
The authors report no declarations of interest.
Acknowledgements
We thank Serdar Senol (Section of Botany, Department of Biology, Faculty of Science, Ege University, Izmir, Turkey) and Dr. Esat Cetin (Department of Biology, Faculty of Science, Harran University, Sanliurfa, Turkey) for their help in the collection and identification of the plant materials.
References
- Arif R, Küpeli E, Ergun F. (2004). The biological activity of Centaurea L. species. Gazi Uni J Sci 17:149–64
- Baykan Erel S, Karaalp C, Bedir E, et al. (2011). Secondary metabolites of Centaurea calolepis and evaluation of cnicin for anti-inflammatory, antioxidant, and cytotoxic activities. Pharm Biol 49:840–9
- Baytop T. (1999). Türkiye’de Bitkiler ile Tedavi. Istanbul, Turkey: Nobel Tıp Kitabevleri
- Borenfreund E, Babich H, Martin-Alguacil N. (1990). Rapid chemosensitivity assay with human normal and tumor cells in vitro. In vitro Cel Dev Biol 26:1030–4
- Chang CC, Yang MH, Wen HM. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–82
- Cragg GM, Newman DJ. (2005). Plants as a source of anti-cancer agents. J Ethnopharmacol 100:72–9
- Derelanko MJ, Hollinger MA, eds. (1995). CRC Handbook of Toxicology. New York, London, Tokyo: CRC Press, Boca Raton
- Gosslau A, Li S, Ho CT, et al. (2011). The importance of natural product characterization in studies of their anti-inflammatory activity. Mol Nut Food Res 55:74–82
- Granger M, Samson E, Sauvage S, et al. (2009). Bioactivity of extracts of Centaurea polyclada DC. (Asteraceae). Arch Biol Sci Belgrade 61:447–52
- Jung KH, Ha E, Kim MJ, et al. (2007). Suppressive effects of nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression by Citrus reticulata extract in RAW 264.7 macrophage cells. Food Chem Toxicol 45:1545–50
- Kaij-a Kamb M, Amaros M, Gire L. (1992). Chemistry and biological activity of the genus Centaurea. Pharm Acta Helv 67:178–88
- Karamenderes C, Bedir E, Pawar R, et al. (2007a). Elemanolide sesquiterpenes and eudesmane sesquiterpene glycosides from Centaurea hierapolitana. Phytochemistry 68:609–15
- Karamenderes C, Konyalıoğlu S, Khan S, Khan IA. (2007b). Total phenolic contents, free radical scavenging activities and inhibitory effects on the activation of NF-kappa B of eight Centaurea L. species. Phytother Res 21:488–91
- Koca U, Toker G, Küpeli-Akkol E. (2009a). Assessment of the extracts of Centaurea tchihatcheffii Fischer for anti-inflammatory and analgesic activities in animal models. Trop J Pharm Res 8:193–200
- Koca U, Pesin-Süntar I, Keles H, et al. (2009b). In vivo anti-inflammatory and wound healing activities of Centaurea iberica Trev. ex Spreng. J Ethnopharmacol 126:551–6
- Koukoulitsa E, Skaltsa H, Karioti A, et al. (2002). Bioactive sequiterpene lactones from Centaurea species and their cytotoxic/cytostatic activity against human cell lines in vitro. Planta Med 68:649–52
- Mc Donald S, Prenzler PD, Antolovich M, Robards K. (2001). Phenolic content and antioxidant activities of olive extracts. Food Chem 73:73–84
- Quang DN, Harinantenaina L, Nishizawa T, et al. (2006). Inhibition of nitric oxide production in RAW 264.7 cells by azaphilones from xylariaceous fungi. Biol Pharm Bull 29:34–7
- Re R, Pellegrini N, Proteggente A, et al. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–7
- Reddy L, Odhav B, Bhoola KD. (2003). Natural products for cancer prevention: A global perspective. Pharmacol Ther 99:1–13
- Salvemini D, Wang ZQ, Wyatt PS, et al. (1996). Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharm 118:829–38
- Siebert K, Zhang Y, Leahy K, et al. (1994). Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain Proc Natl Acad Sci USA 91:12013–17
- Singh R, Singh B, Singh S, et al. (2008). Anti-free radical activities of kaempferol from Acacia nilotica (L.) Willd. Ex. Del. Toxicol In Vitro 22:1965–70
- Subbaramaiah K, Bulic P, Lin Y, et al. (2001). Development and use of a gene promoter-based screen to identify novel inhibitors of cyclooxygenase-2 transcription. J Biomol Screen 6:101–10
- Uysal T. (2008). Centaurea ertugruliana (Asteraceae), a new species from Turkey. Ann Bot Fennici 45:137–40
- Wagenitz G. (1975). Centaurea L. In: Davis PH, ed. Flora of Turkey and the East Aegean Islands, Vol. 5, Edinburgh: Edinburgh University Press, 465–585
- Wang KJ, Zhang YJ, Yang CR. (2006). New phenolic constituents from Balanophora polyandra with radical scavenging activity. Chem Biodivers 3:1317–24
- Winter CA, Risley EA, Nuss CW. (1962). Carrageenan-induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111:544–7
- Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–10
