Abstract
Context: Glinus oppositifolius (L.) Aug. DC. (Molluginaceae), a perennial subshrubs herb, grows at low altitudes in the southern part of Taiwan, and is used in traditional Chinese medicine for herpes zoster and herpangina.
Objective: This study describes nutritional and therapeutic potential of Glinus oppositifolius and summarizes scientific evidence that supports traditional claims; recent progress in research for this plant is reviewed herein.
Materials and methods: The literature has been retrieved from the web-based online systems including PubMed, Medline, and Google Scholar. The articles related to phytochemistry, pharmaceutical biology and ethnopharmacology have been excluded.
Results and discussion: In clinical practice, the plant has been extensively investigated in a broad range of studies to provide scientific evidence for folklore claims or to find new therapeutic uses. The present review may arouse related research and make a more valid display for Taiwanese native medicinal plants.
Introduction
Glinus oppositifolius (L.) Aug. DC. (Molluginaceae), (Chinese name: Jia fan lu), a Taiwan native plant, is bitter, slightly sweet in taste, and cold in nature. Literature records concerning the carpet weed are limited. The plant is a slender spreading or ascending annual herb with stems up to 40 cm long. The leaves are opposed two by two, but they seem verticillate by three to five according to the development of axial leaves. The plant is widely distributed in tropical Asia, tropical Africa and Australia, and grows at low altitudes in the southern part of Taiwan (Huang & Wu, Citation1998). lists the regions, traditional uses and the main pharmacological activities of G. oppositifolius used by the local people and ethnic groups in different parts of the world. In context, G. oppositifolius in Taiwan is used in treating inflammation with chronic illnesses, including cancer. In Thailand, the leaves are used as a vegetable for cooking purposes, as well as an expectorant and antipyretic agent (Sahakitpichan et al., Citation2010). In Mali, West Africa, aerial parts of G. oppositifolius are used for treating abdominal pain and jaundice (Inngjerdingen et al., Citation2005). A decoction of a fine powder of the aerial parts is used as a chemotherapeutic against malaria (Diallo et al., Citation1999). Glinus oppositifolius has also been reported for treating joint pains, inflammation, diarrhea, intestinal parasites, fever, furuncles, skin disorders and the plant-macerate is also used as a wound healing remedy (Debes, Citation1998; Diallo, Citation2000). In India, G. oppositifolius is reputed to have antiseptic and antidermatitic properties and is used traditionally in the treatment of earache, itch, skin diseases, and acts as stomachic, uterine stimulant, aperients, and lochia (Asok Kumar et al., Citation2009; Hariharan & Rangaswami, Citation1971). For phytochemical data and biological activity tested in vitro and in vivo, we conducted an electronic literature search of MEDLINE, PubMed, Scirus, Google Scholar and Web of Science from 1998 and updated to Jan 2013. The keywords used for the electronic literature search for this review were scientific name and its synonym/s, medical uses, chemical studies, pharmacological activity and natural products.
Table 1. Ethnobotanical uses of G. oppositifolius found worldwide.
Materials and methods
Chemical constituents
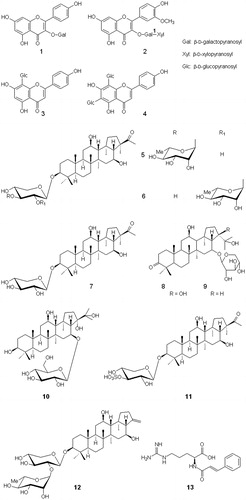
The plant extract gave positive reaction for carbohydrates, alkaloids, tannins, flavonoids, saponins, steroids etc.; the structures of some of the compounds isolated are given in and the chemical constituents identified in G. oppositifolius have been characterized as:
Aromatics: benzoic acid, 4-hydroxybenzoic acid, 4-hydroxybenzaldehyde, 4-hydroxy-acetophenone, methyl 4-hydroxybenzoate, p-anisic acid, vanillin, 4-hydroxy-3-methoxyacetophenone, acetosyringone, 4-hydroxy-3,5-dimethoxy benzaldehyde, 4-hydroxybenzyl alcohol, 2-(4-hydroxyphenyl)ethanol, cinnamic acid, 3-(4″-hydroxyphenyl)-(E)-propenoic acid methyl ester, (E)-methyl-3-(4-hydroxy-3-methoxy phenyl)acrylate, trans-ferulic acid (Chen, Citation2011).
Flavonoid glycosides: kaempferol 3-O-galacto pyranoside (1), isorhamnetin 3-O-β-d-xylopyranosyl-(1→2)-β-d-galactopyranoside (2), vitexin (apigenin 8-C-β-d-glucopyranoside) (3), and vicenin-2 (apigenin 6,8-di-C-β-d-glucopyranoside) (4) (Sahakitipichan et al., Citation2010), 5,7,4′-trihydroxyflavonol, 6,8-dimethyl-5,7,4′-trihydroxyflavone, 5,7-dihydroxy-6,8-dimethylflavone, 5,4′-dihydroxy-7-methoxy-6,8-dimethylflavone, 7-hydroxy-5-methoxy-6,8-dimethylflavanone, 3-hydroxy-5,7-dimethoxy-6,8-dimethylflavone, 7-hydroxy-5-methoxy-6,8-dimethylflavone, 7-hydroxy-5-methoxy-6-methylflavanone, 5-hydroxy-8-hydroxymethyl-7-methoxy-6-methylflavone, 5,7-dihydroxy-4′-methoxy-6,8-dimethylflavone (Chen, Citation2011).
Triterpenoid saponins: spergulacin (5), spergulacin-A (6), 3-O-(β-d-xylo-pyranosyl)-spergulagenin-A (7) (Kumar et al., Citation2013), spergulagenin-A, spergulagenin-B, spergulagenin-C, spergulagenin-D (Chen, Citation2011), linoside A {16-O-(β-arabinopyranosyl)-3-oxo-12,16β,21β,22-tetrahydroxyhopane} (8), glinoside B {16-O-(β-arabinopyranosyl)-3-oxo-12,16β,22-trihydroxyhopane} (9) (Traore et al., Citation2000), glinoside C {16-O-(β-d-glucopyranosyl)-3β,12β,16β,21α,22-pentahydroxyhopane} (10), spergulin A {3-O-(β-d-xylopyranosyl 4-sulphate)-spergulagenin A} (11) and spergulin B {3-O-[α-rhamnopyranosyl (1→2)-β-d-xylopyranosyl]-spergulatriol} (12) (Kumar et al., Citation2013).
Steroids: spinasterol, β-sitosterol, stigmasterol (Chen, Citation2011).
Pectin type polysaccharides: GOA1 {arabinose, galactose, arabinogalactans type I (AG-I) and type II (AG-II)}, and GOA2 (galacturonic acid, rhamnose, and arabinose and galactose) (Inngjerdingen et al., Citation2005).
Nucleoside: adenosine (Sahakitipichan et al., Citation2010).
Acylamino acid derivative: l-(−)-(N-trans-cinnamoyl)-arginine (13) (Sahakitpichan et al., Citation2010) and others: 132(R)-pheophytin a (Chen, Citation2011), etc.
Pharmacological activities
Acute oral toxicity
Methanol and aqueous extracts of the plant have not shown any toxic effect up to 4000 mg/kg; as per the ranking system European Economic Community (EEC) for acute oral toxicity, the LD50 dose of 2000 mg/kg and above is categorized as unclassified (EC Directive 83/467/EEC, 183) (Behera et al., Citation2010). The acute toxicity of the ethanol extract of G. oppositifolius was determined as per the Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA) guideline no. 420 (fixed dose method), and was observed that the extracts shows no mortality even at 2000 mg/kg dose (Sahu et al., Citation2012). Panigrahi et al. (Citation2012) have reported that the administration of stepwise doses of methanol extracts were carried out as per the OECD guidelines 423, from 100 mg/kg up to the dose 2000 mg/kg to female albino mice, and observed the signs of toxicity up to 72 h in the tested animals that did not show mortality.
Antifungal activity
Whole plant extracts prepared in dichloromethane have demonstrated antifungal activity against Candida albicans that was applied on glass-backed silica plates, incubated overnight at 30 °C and sprayed with methylthiazolyltetrazolium chloride (MTT). Active compounds appeared as clear spots against a purple-colored background (Diallo et al., Citation2001).
Antimicrobial activity
The methanol extracts have exhibited significant antimicrobial activity against four Gram positive strains of Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Aspergillus niger by cup-plate agar diffusion method, comparable to the standard drug norfloxacin (Pattanayak et al., Citation2011).
Antiplasmodial activity
The antiplasmodial activity was assessed against two strains of Plasmodium falciparum: the chloroquine-sensitive strain 3D7 and the chloroquine-resistant strain W2, and the results presented evidence that fractions (glinoside A and B) had a better antiplasmodial activity (IC50 = 31.80 µg/ml) than pure glinoside A (IC50 = 42.30 µg/ml) (Traore et al., Citation2000).
Larvicidal activity
Both the dichloromethane and methanol extracts of the whole plants gave 100% mortality after 24 h at a concentration of 500 mg/L, were considered as active against Culex quinquefasciatus and Anopheles gambiae larvae (Diallo et al., Citation2001).
Molluscicidal activity
Activity was expressed as the concentration (mg/l) of the plant extract which gave 100% mortality of the snails after 24 h. Extracts of the whole plants, prepared in dichloromethane and methanol have been tested against two types of Biomphalaria pfeifferi, Biomphalaria truncates were molluscicidal (Diallo et al., Citation2001).
Anthelmintic activity
The methanol extracts have shown anthelmintic activity on adult India earthworms “Pheretima posithuma” in a dose-dependent manner giving shortest time of paralysis and death with 20 mg/ml concentration and the activities were comparable with the standard drug albendazole (Pattanayak et al., Citation2011).
Analgesic and anti-inflammatory activity
The analgesic activity was tested using acetic acid-induced writhing and tail immersion test while the anti-inflammatory activity by the carrageenan-induced paw edema test. A significant peripheral and central analgesic effect was shown by the methanol extract at both 200 and 400 mg/kg doses; and the extract (500 mg/kg) also reduced the paw inflammation of mice induced by carrageenan (Hoque et al., 2011a).
Antioxidant and radical scavenging activities
The whole plant was extracted with ethanol (70%) and used for the evaluation of various in vitro antioxidant assays which includes H-donor activity, nitric oxide scavenging, superoxide anion scavenging, reducing ability, hydroxyl radical, hydrogen peroxide scavenging, total phenolic content, total flavonoid content, total antioxidant activity by thiocyanate and phosphomolybdenum method, metal chelating, β-carotene bleaching, total peroxy radical assays, and the activity of the extracts was found to be correlated to their total contents of ascorbic acid, butylated hydroxyl toluene (BHT), α-tocopherol, curcumin, quercetin and Trolox. The generation of free radicals, namely, , OH•, H2O2, NO• and peroxyl radicals, were effectively scavenged by the ethanol extract of G. oppositifolius; and the antioxidant activity depends on the concentration and increases with increasing amounts of the extract (Asok Kumar et al., Citation2009). The methanol extracts, prepared by overnight maceration of the whole plant, have shown antioxidant activity in the β-carotene linoleate model (Diallo, Citation2001). Leaf methanol extract of the plant has exhibited antioxidant activity by 1,1-diphenyl-2-picrylhydrazyl(DPPH) radical scavenging assay and nitric oxide scavenging assay; the amount of total flavonoid was 25.46 mg/g and total antioxidant activity was 79.48 mg/g equivalent to quercetin and ascorbic acid, respectively (Hoque et al., 2011b). Aqueous and methanol extracts of the whole plants have been investigated for antioxidant activity using two in vitro models, linoleic acid model and DPPH model, and these studies have shown that both extracts have good antioxidant activity, but the activity had shown that the methanol fraction possesses more significance compare to the standard (Behera et al., Citation2010).
Immunomodulating
Two pectin-type polysaccharides, GOA1 and GOA2, isolated from the water extract of the aerial parts of G. oppositifolius at 50°C were investigated for their activity towards the complement system and leukocyte subsets because of the assumed effects on conditions related to the immune system (Inngjerdingen et al., Citation2005). Inngjerdingen et al. (Citation2007a) have reported that both GOA1 and GOA2 were shown to exhibit potent dose-dependent complement fixating activities, and induced chemotaxis of macrophages, T cells and NK cells. The immunomodulating properties of GOA1 were further shown to induce proliferation of B cells, the secretion of IL-1β by macrophages, in addition to a marked increase of mRNA for IFN-γ in NK-cells. The ability of GOA2 to induce secretion of proinflammatory cytokines was examined and marked up-regulations in mRNA for IL-1β from rat macrophages and IFN-γ from NK-cells were found.
Antihyperglycemic activity
Methanol extracts of the whole plant in single oral doses 200 and 400 mg/kg have exhibited significant antihyperglycemic activity in glucose-overloaded hyperglycemic mice (Hoque et al., 2011b) and rats (Behera et al., Citation2010; Panigrahi et al., Citation2012) compared to the standard drug metformin and glibenclamide, respectively. Ethanol extract (200 and 400 mg/kg, p.o.) of the aerial parts of G. oppositifolius produced significant decrease in the blood glucose level compared with the controls in alloxan-induced hyperglycemic, normoglycemic and oral glucose tolerance test in Wistar Albino rats and was comparable with the standard drug glibenclamide (2.5 mg/kg, p.o.) (Sahu et al., Citation2012).
Antihyperlipidemic activity
Aqueous and methanol extracts of G. oppositifolius were found to have significant antihyperlipidemic activity in STZ-induced diabetic rats in 14- and 28-d orally treated with two doses, 200 and 400 mg/kg (Behera et al., Citation2010). The methanol extract of the plant was evaluated for antihyperlipidemic activity in Triton-induced hyperlipidemic rats. Treatment with 200 and 400 mg/kg extract of G. oppositifolius exhibited a significant reduction in serum lipid profile like total cholesterol, triglycerides, low-density lipoprotein (LDL), very low-density lipoprotein (VLDL) and increase in high-density lipoprotein (HDL) in hyperlipidemic rats compared to hyperlipidemic control (Panigrahi et al., Citation2012).
Hepatoprotective
Hepatoprotective effect of the methanol extract of G. oppositifolius root against liver damage induced by carbon tetrachloride (CCl4) was studied in albino rats. Natarajan et al. (Citation2010) have reported that oral administration of the methanol extract showed significant protective action made evident by its effect on the level of serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), total bilirubin and direct bilirubin. The results showed that treatment with G. oppositifolius extracts appears to enhance the recovery from hepatic damage induced by CCl4.
Discussion and conclusion
Traditional medicine and diet has served mankind through the ages for the prevention and treatment of most chronic diseases. Chemical constituents present in G. oppositifolius are reported to be a good source of saponins, flavonoids, carbohydrates, polysaccharides, steroids, alkaloids and several other aromatic compounds. In previous studies, triterpenoidal saponins were reported to act as antitropozoal agents (Traore et al., Citation2000) and possessed significant hypoglycemic and hypolipidemic effects (Behera et al., Citation2010; Hoque et al., 2011b; Panigrahi et al., Citation2012; Sahu et al., Citation2012). Theoretically, α-glucosidase is an intestinal enzyme that catalyses the final step in the process of carbohydrate hydrolysis, it is commonly known that inhibition of this enzyme plays an important role in the management of post-prandial hyperglycaemia, as it delays the digestion of carbohydrates. Recently, hopanoid triterpene saponins were observed to exert great α-glucosidase inhibitiory activity from G. oppositifolius (Kumar et al., Citation2013). Besides, pectic polysaccharides extracted from the plants were investigated for their activity towards the complement system and leukocyte subsets, and reported to act as immunomodulating agents (Inngjerdingen et al., Citation2005, Citation2007a,Citationb). Research findings have also shown that the polysaccharides enhanced immunity by improving the activity of immunocyte, activising the secretion of cell factor, inducing the production of antibody and activising the complementary system (Wang et al., Citation2006). Furthermore, the plant extracts showed free radical scavenging and antioxidant activities (Asok Kumar et al., Citation2009; Diallo et al., Citation2001), it is important because free radicals are implicated in a number of pathological conditions such as inflammatory diseases, atherosclerosis, cerebral ischemia, AIDS and cancer (Thomas & Kalyanaraman, Citation1997). Some of the investigations indicated that flavonoids vitexin is a class of nature lignan compounds and vitexin-induced antitumor effect and cytotoxic activity is exerted through proapoptotic process, which is mediated by a decreased Bcl-2/Bax ratio and activation of caspases (Zhou et al., Citation2009). Lately, G. oppositifolius has been shown to possess suppression effect on avian leucosis virus (ALV) (Hung et al., Citation2008).
The present review provides a summarized literature regarding phytochemical, pharmacological activities, and ethnopharmacological uses of G. oppositifolius, which showed a rich source of bioactive compounds and are worthy of further study. It may be helpful to researchers, intending to investigate the plant in future studies, and natural pharmaceutical industry for preparing evidence-based formulations. Clinical evaluation will throw more light on clinical usefulness, safety and efficacy of these plant extracts.
Declaration of interest
The authors report no conflicts of interest.
References
- Asok Kumar K, Uma Maheswari M, Sivashanmugam AT, et al. (2009). Free radical scavenging and antioxidant activities of Glinus oppositifolius (carpet weed) using different in vitro assay systems. Pharm Biol 47:474–82
- Behera GM, Satish Kumar BN, Malay Baidya M, Panigrahi G. (2010). Antihyperglycemic, antihyperlipidemic and antioxidant activity of Glinus oppositifolius (L.) Aug. DC. Pharmacologyonline 3:915–36
- Chen YH. (2011). Studies on the chemical constituents from Glinus oppositifolius (L.) Aug. DC [Master’s thesis]. China Medical University, Taichung City, Taiwan
- Debes SC. (1998) En medisinplante fra Mali, Glinus oppositifolius. [Master’s Thesis]. School of Pharmacy, University of Oslo, Norway
- Diallo D, Hveem B, Mahmoud MA, et al. (1999). An ethnobotanical survey of herbal drugs of Gourma district. Mali Pharmaceutical Biol 37:80–91
- Diallo D. (2000) Ethnopharmacological survey of medicinal plants in Mali and phytochemical study of four of them: Glinus oppositifolius (Aizoaceae), Diospyros abyssinica (Ebenaceae), Entada Africana (Mimosaceae), Trichilia emetica (Meliaceae). These de Doctorat. Faculte des Sciences, Universite de Lausanne, Switzerland
- Diallo D, Marston A, Terreaux C, et al. (2001). Screening of Malian plants for antifungal, latvicidal, molusscicidal, antioxidant and radical scavenging activities. Phytother Res 15:401–6
- Hariharan V, Rangaswami S. (1971). Steroids and triterpenoids from the roots of Mollugo spergula. Phytochemistry 10:621–4
- Hoque N, Habib R, Imam MZ, et al. (2011a). Analgesic and anti-inflammatory potential of methanol extract of Glinus oppositifolius L. Aust J Basic Appl Sci 5:729–33
- Hoque N, Imam MZ, Akter S, et al. (2011b). Antioxidant and antihyperglycemic activities of methanol extract of Glinus oppositifolius leaves. J Appl Pharm Sci 1:50–3
- Huang TC, Wu JT. (1998). Flora of Taiwan, 2nd. Edition 4:326. Taipei, Taiwan: Editorial Committee of the Flora of Taiwan
- Hung HW, Wang CH, Huang DF, et al. (2008). The suppression effect of Glinus oppositifolius on avian leucosis virus (ALV). 2008 Anniversary Conference Proceeding of the Chinese Society of Veterinary Science. Oral No. 29. Tansui, Taiwan, p. 49
- Inngjerdingen KT, Debes SC, Inngjerdingen M, et al. (2005). Bioactive pectic polysaccharides from Glinus oppositifolius (L.) Aug. DC., a Malian medicinal plant, isolation and partial characterization. J Ethnopharmacol 101:204–14
- Inngjerdingen KT, Kiyohara H, Matsumoto T, et al. (2007a). An immunomodulating pectic polymer from Glinus oppositifolius. Phytochemistry 68:1046–58
- Inngjerdingen KT, Patel TR, Chen X, et al. (2007b). Immunological and structural properties of a pectic polymer from Glinus oppositifolius. Glycobiology 17:1299–310
- Kumar D, Shah V, Ghosh R, Pal BC. (2013). A new triterpenoid saponin from Glinus oppositifolius with α-glucosidase inhibitory activity. Nat Prod Res 27:624–9
- Natarajan P, Thanga Thirupathi A, Raja Sekharan T, et al. (2010). Hepatoprotective effect of Glinus oppositifolius Linn. Res J Pharmacol Pharmacodyn 2:289–92
- Panigrahi G, Mishra US, Mahapatra S, et al. (2012). Hypoglycemic and hypolipidemic activities of methanol extract of Glinus oppositifolius. Int J Pharm 2:491–7
- Pattanayak S, Nayak SS, Dinda SC, et al. (2011). Antimicrobial and anthelmintic potential of Glinus oppositifolius (Linn) family: Molluginaceae. Pharmacologyonline 1:165–9
- Sahakitpichan P, Disadee W, Ruchirawat S, Kanchanapoom T. (2010). l-(−)-(N-trans-Cinnamoyl)-arginine, an acylamino acid from Glinus oppositifolius (L.) Aug. DC. Molecules 15:6186–92
- Sahu SK, Das D, Tripathy NK, et al. (2012). Evaluation of hypoglycemic activity of Mollugo pentaphylla and Glinus oppositifolius (L). Rasayan J Chem 5:57–62
- Traore F, Faure R, Ollivier E, et al. (2000). Structure and antiprotozoal activity of triterpenoid saponins from Glinus oppositifolius. Planta Med 66:368–71
- Thomas CE, Kalyanaraman B. (1997). Oxygen Radicals and the Disease Process. Amsterdam: Harwood Academic Publishers
- Wang TY, Zhao B, Wang C. (2006). Research advances in immunomodulation and antitumor activity of polysaccharides. Chin J Process Eng 6:674–82
- Zhou Y, Liu YE, Cao J, et al. (2009). Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin Cancer Res 15:5161–9