Abstract
Context: The genus Cyclamen L. (Primulaceae) is rich in saponins known to have interesting biological activities.
Objective: To isolate saxifragifolin B and cyclamin, two triterpene saponins, from Cyclamen libanoticum Hildebr and Cyclamen persicum Mill, and to assess their cytotoxic, clastogenic/aneugenic, and anticlastogenic effects, as well as antioxidant potential.
Materials and methods: Saxifragifolin B and cyclamin were tested for their cytotoxicity against SK-BR-3, HT-29, HepG2/3A, NCI-H1299, BXPC-3, 22RV1, and normal DMEM cell lines using WST-1 assay. Their clastogenic/aneugenic activities and anticlastogenic effects against the anticancer drug mitomycin C were assessed by the in vitro micronucleus assay in CHO cells. Their antioxidant capacities were determined using Fe2+-chelating and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assays.
Results: Both saponins were described for the first time in Cyclamen libanoticum. They showed strong cytotoxic activities against the tested cancer cell lines. Saxifragifolin B was found to be 56- and 37-times more active than mitomycin C against breast adenocarcinoma (SK-BR-3) and lung carcinoma (NCI-H1299), respectively. Also, saxifragifolin B did not induce micronuclei formation and prevented cells from mitomycin C clastogenic effect. Cyclamin induced a significant increase of micronucleated cells after metabolic activation with S9 mix, and did not possess any anticlastogenic activity. Both molecules exhibited low antioxidant activities as compared to reference compounds.
Discussion and conclusions: This study showed the remarkable cytotoxic activity of saxifragifolin B, especially against breast adenocarcinoma and lung carcinoma and its chemoprotective activity against mitomycin C. Thus, saxifragifolin B could be suggested as a potential cytotoxic drug with a preventive effect against possible exposures to genotoxic agents.
Introduction
In spite of progress in combinatorial chemistry, plants remain an important source of therapeutic compounds. Plants secondary metabolites, such as terpenes, sterols, alkaloids, and phenols, are known to be implicated in their defense mechanisms against potential predators and parasites, or against other plants competing for space. Some compounds were found to be highly cytotoxic toward a wide range of proliferating cells, and more specifically against tumor cells. The discovery of such active molecules led to a remarkable improvement in the anticancer chemotherapy treatments. Compounds such as podophyllotoxin derived from Podophyllum emodi, paclitaxel isolated from Taxus brevifolia or camptothecin derived from Camptotheca acuminata were found to be very potent (Cragg et al., Citation1997; Schwartsmann et al., Citation2002).
Several studies reported that some plant extracts or isolated compounds could protect cells against mutagenic agents (Wall, Citation1992). These natural products include antioxidant and antimutagenic molecules, which have been empirically used for centuries as protective drugs in traditional medicine (Wall, Citation1992). These metabolites have been supposed to be implicated in plant defense mechanisms against environmental stresses, including temperature, pH or hygrometry variations, as well as DNA damage induced by the constant exposure to UV radiations. The structure and function elucidation of such molecules contributed to the development of efficient chemopreventive agents against the genotoxic xenobiotics and environmental carcinogens (Huang et al., Citation2010).
The recent discovery of plant metabolites with both anticancer and antimutagenic properties is an important breakthrough in anticancer research. These compounds, such as berberine isolated from Berberis vulgaris (Imanshahidi et al., Citation2008), have been shown to have multitarget effects on various mechanisms of human carcinogenesis, from cell initiation to metastasis development. Moreover, due to their synergistic effects with conventional chemotherapy or radiotherapy, they are considered as very promising chemotherapeutic agents, which could be used to improve anticancer treatments and reduce adverse effects.
The genus Cyclamen L. (Primulaceae) is represented by around 20 wild and cultivated species (Speroni et al., Citation2007). Three of them, Cyclamen persicum Mill, Cyclamen coum Mill, and Cyclamen libanoticum Hildebr, can be found in Lebanese soils, but only C. libanoticum is endemic of the Lebanese flora. Cyclamen tubers have been used in the folk medicine for their sedative, purgative, antihelmintic, laxative, and abortive properties (Speroni et al., Citation2007). Different extracts of Cyclamen tubers were shown to possess cytotoxic, antimicrobial, spermicidal, analgesic, and anti-inflammatory activities (Çaliş et al., Citation1997; Dall'Acqua et al., Citation2010; Kupchan et al., Citation1967; Mahasneh & El-Oqlah, Citation1999; Primorac et al., Citation1985).
In a constant attempt of screening for new anticancer chemotherapeutic agents, C. persicum and C. libanoticum were studied for their saponin composition. Two major known triterpenoid saponins, saxifragifolin B (Park et al., Citation2010) and cyclamin (Reznicek et al., Citation1989), were isolated from C. libanoticum and C. persicum. Their structures were established using NMR and MS analyses. Both saponins were described for the first time in C. libanoticum. The cytotoxic activities of the two saponins were assessed against six tumor cell lines. Also, their clastogenic and anticlastogenic activities against the anticancer drug mitomycin C were evaluated by the micronucleus assay on Chinese hamster ovary cells (CHO-K1).
Materials and methods
Plant material
Tubers of C. persicum were collected in May 2011 from Deir El Kamar area in Mount Lebanon (altitude 900–1000 m). Tubers of C. libanoticum were collected in April 2012 from Yahchouch area in Mount Lebanon (altitude 750–800 m). Both plants were identified by Pr. G. Tohmé (CNRS, Lebanon). The voucher specimens were deposited in the herbarium of the Faculty of Pharmacy, Lebanese University, Beirut, Lebanon, under the codes CPTD10 and CLC12, respectively, for C. persicum and C. libanoticum.
Chemicals
3-[2-Pyridyl]-5,6-diphenyl,2,4-triazine-4,4′-disulfonic acid monosodium salt hydrate (ferrozine), 2,2-diphenyl-1-picrylhydrazyl (DPPH), (+)-catechin, ascorbic acid, ethylene diamine tetra acetic acid (EDTA), and mitomycin C were purchased from Sigma-Aldrich Co (Steinheim, Germany). FeSO4·7H2O was purchased from Merck (Darmstadt, Germany). All reagents and organic solvents were of analytical grade with more than 99.5% purity.
Extraction of saponins
The air-dried tubers of C. persicum and C. libanoticum (20 g) were extracted three times with MeOH:H2O (70:30) (200 mL) under reflux for 30 min. The extracts were concentrated under reduced pressure to obtain crude extracts: 11.8 g for C. persicum and 10.4 g for C. libanoticum. Crude extracts (5 g) were subjected to column chromatography on polyamide CC6 (100 g, particle size <0.07 mm, Macherey-Nagel, Dueren, Germany) using H2O (600 mL) and MeOH (600 mL) as mobile phases; fractions of 25 mL were collected. Methanol fractions (namely fractions 5–25) containing major saponins were combined to give 1.10 and 0.95 g for C. persicum and C. libanoticum, respectively. About 0.35 g of the methanolic fraction were chromatographed on 35 g silica gel eluting with 400 mL CH2Cl2:MeOHH2O (64:32:2; solvent 1) and 200 mL CH2Cl2:MeOH:H2O (64:32:5; solvent 2); fractions of 10 mL were collected. Saponin 1, 10.0 mg (C. persicum; namely fractions F9–F14) and 7.0 mg (C. libanoticum; namely fractions F11–F17), was isolated from solvent 1. Saponin 2, 11.0 mg (C. persicum; F21–F32) and 4.0 mg (C. libanoticum; F24–F30), was obtained from solvent 2. Fractions and pure saponins were analyzed by TLC chromatography on silica gel plates (silica gel 60, 0.02, Merck, Darmstadt, Germany) using the solvent system CH2Cl2:MeOH:H2O (64:32:5). Spots were detected after sulfuric acid revelation followed by heating at 110 °C.
Structure elucidation of saponins
The 1H and 13C NMR spectra were recorded on a Bruker Avance III 600 spectrometer (Billerica, MA) (1H-600.13 MHz) equipped with a 5-mm triple resonance inverse Cryoprobe TCI (1H–13C–15N) with a z gradient. Spectra were recorded in pyridine C5 D5N (99.99%) solvent (δ1H 8.74 ppm–δ13C 150.35 ppm) at 300 K. The 1H (600 MHz) and 13C NMR (150 MHz) data are reported in ppm downfield from tetramethylsilane. Hydrogen connectivity (C, CH, CH2, and CH3) information was obtained from edited HSQC and/or DEPTQ-135 experiments. Proton and carbon peak assignments were based on 1D (TOCSY) and 2D NMR analyses (COSY, HSQC-TOCSY, ROESY, HSQC, and HMBC). MS analyses were measured using a 3200 QStar Elite (Applied Biosystems SCIEX, Framingham, MA) mass spectrometer in a positive- and negative-ion mode. Flow infusion (10 µL/min) was employed with a methanol solution with ammonium acetate (3 mM), sodium iodide (1 mM), and potassium iodide (1 mM).
Cytotoxic activities
The cytotoxic activities of saponins were assessed on human normal fibroblasts and on six human tumor cell lines provided from ATCC-LGC Standards Sarl (Molsheim, France): breast adenocarcinoma cells (SK-BR-3), colon adenocarcinoma (HT-29), hepatocellular carcinoma (HepG2/3A), lung carcinoma (NCI-H1299), pancreatic carcinoma (BXPC-3), and prostate carcinoma (22RV1). They were compared with those of the conventional anticancer drug mitomycin C.
Cells were maintained in McCoy’s 5A (CHO-K1, SK-BR-3, HT-29), RPMI 1640 (NCI-H1299, BXPC-3, 22RV1, HepG2/3A), or DMEM (human fibroblasts) media supplemented with 10% bovine calf serum, 2 mM glutamine, and 100 (U/mL)/10 µg/mL penicillin/streptomycin mixtures. They were incubated at 37 °C in a humidified atmosphere containing 5% CO2. They were seeded in 96-well plates and incubated overnight. Various saponin concentrations were incorporated in triplicate cultures, and cells were incubated at 37 °C for 24 h. At the end of the incubation period, cells were subjected to three successive washes in phosphate buffer saline (PBS) and incubated in PBS containing 10% WST-1 for an additional 30 min. Cell viability was evaluated by the assessment of WST-1 absorbance at 450 nm in a microplate spectrophotometer. Results were expressed as percentages of cell viability in reference to the control (culture medium only), which corresponded to 100% cell viability. Dose–response curves were calculated by non-linear regression analysis with TableCurve V2 software (Marseille, France). The inhibitory concentration 50% (IC50) was defined as the concentration of saponin that induced a 50% decrease of viable cells.
Metabolic activation mixture (S9 mix)
A centrifuged supernatant (9000 × g) of a liver homogenate (S9) prepared from male Sprague–Dawley rats treated with a single injection of Aroclor 1254 (Peterson Environmental Services, Bethel, VT) (500 mg/kg body weight) was used for in vitro metabolic activation. The protein concentration in the S9 homogenate was 26 mg/mL. In the micronucleus assay, S9 mix contained 10% S9, 5 mM G6P, 4 mM NADP, 33 mM KCl, and 8 mM MgCl2.
Clastogenic/aneugenic activities
The clastogenic/aneugenic activities of saponins were evaluated by the standard cytokinesis-blocked micronucleus assay, according to the protocol described by Kirsch-Volder et al. (Citation2003). Experiments were performed on the Chinese Hamster Ovary cell line (CHO-K1). This cell line is characterized by a good genetic stability and by a short generation time, it has been well validated for genetic toxicology assays.
An amount of 50 000 cells was plated in chamber slides and incubated overnight at 37 °C. Various concentrations of saponins were added to the cell cultures with or without 10% S9 mix. Each set of experiments included duplicate cell cultures with four relevant tested doses. A solvent control (0.1% DMSO) was added to determine the spontaneous micronuclei levels in CHO cells. Two positive controls were also included to ensure the sensitivity of the assay and the S9 mix: 0.06 µg/mL mitomycin C without S9 mix and 5 µg/mL benzo[a]pyrene (BaP) with S9 mix. After a 3-h exposure period at 37 °C, cells were rinsed with PBS and incubated in a fresh medium containing cytochalasin B (3 µg/mL) to arrest cytokinesis. After an additional 24 h incubation period at 37 °C, cells were subjected to two successive washes with PBS and fixed with methanol (HPLC purity grade solvent). Air-dried slides were stained with 5% Giemsa stain in Milli-Q (Millipore Corporation, Billerica, MA) water for 15 min and examined at 1000 × magnification.
The proliferative index (PI) was considered as a measure of cytotoxicity (Kirsch-Volders et al., Citation2003). PI was determined by scoring the number of mononucleated (M1), binucleated (M2), and trinucleated (M3) cells among 500 Giemsa-stained cells with well-preserved cytoplasm: PI = (M1 + 2 × M2 + 3 × M3)/500. Micronucleated cells were scored for concentrations inducing less than a 50% decrease in the proliferative index: 2000 binucleated cells were examined and micronuclei were identified according to the morphological criteria previously defined by Kirsch-Volders et al. (Citation2003). Statistical differences between negative controls and treated samples were determined using the χ2 test.
The assay was considered positive when a dose–response relationship was established between the numbers of micronucleated cells and the concentrations of saponins, and when at least one concentration induced a significant increase of micronucleated cells as compared to the solvent-only control culture.
Anticlastogenic activity
The saponins were assessed for their protective effects against mitomycin C by the micronucleus assay (Kirsch-Volders et al., Citation2003). Three treatment protocols were used: pre-treatment, simultaneous treatment, and post-treatment protocols. In the pre-treatment protocol, various non-toxic concentrations of saponins were incorporated into duplicate cultures. After a 3-h exposure at 37 °C, cells were rinsed with PBS and placed in a fresh medium containing 0.06 µg/mL mitomycin C. Cells were incubated for 3 h at 37 °C, and the assay was continued according to the standard procedure (Kirsch-Volders et al., Citation2003). In the simultaneous treatment protocol, cells were placed in a fresh medium containing various non-toxic concentrations of saponins and 0.06 µg/mL mitomycin C. Cells were incubated at 37 °C, and the assay was continued according to the standard procedure. In the post-treatment protocol, 0.06 µg/mL mitomycin C was added in cell cultures. After a 3-h incubation period at 37 °C, cells were rinsed with PBS and placed in a fresh medium containing various non-toxic concentrations of saponins. Cultures were incubated at 37 °C for an additional 3 h period and the assay was continued according to the standard procedure.
Fe2+-chelating and DPPH scavenging activities
The chelating and antioxidant activities were assessed by the ferrous ion chelating method and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay. The ferrous ion chelating activity was determined according to Lim et al. (Citation2007). Equal volumes of 0.12 mM FeSO4, saponins at different dilutions, and 0.6 mM ferrozine were mixed. The solutions were allowed to stand for 10 min at room temperature, and the absorbance of Fe2+–ferrozine complex was measured at 562 nm. Ultra-pure water instead of sample solution was used as a negative control and ultra-pure water instead of ferrozine solution was used as a blank. EDTA–Na2 was used as the positive control. The chelating effect of EDTA increased linearly up to 80% with increasing concentrations, reaching progressively the saturation (data not shown). The ability of saponins to chelate ferrous ions was calculated by using the formula given by the procedure. All measurements were performed in duplicate.
The scavenging effects of the extracts for DPPH radical were determined by the method of Yan and Chen (Citation1995) with slight modifications. Serial dilutions of saponins were prepared in methanol. The basic procedure was to add an aliquot (1 mL) of test sample to 1 mL of DPPH 0.15 mM methanol solution. The mixture was vortexed for 1 min and then left to stand at room temperature for 30 min in the dark. The absorbance (A) was read at 517 nm, and the scavenging activity (%) (SA) was calculated as follows: SA (%): [1 − (Asample − Asample blank)/Acontrol] × 100. Sample solution (1 mL) plus methanol (1 mL) was used as a sample blank and DPPH solution (1 mL) plus methanol (1 mL) was used as a negative control. Catechin and ascorbic acid were used as the positive controls. Stock solutions of catechin (0.8 mg/mL) and ascorbic acid (0.8 mg/mL) were diluted with methanol to give concentrations ranging from 1.5 to 20 μg/mL. All measurements were performed in duplicate.
Results
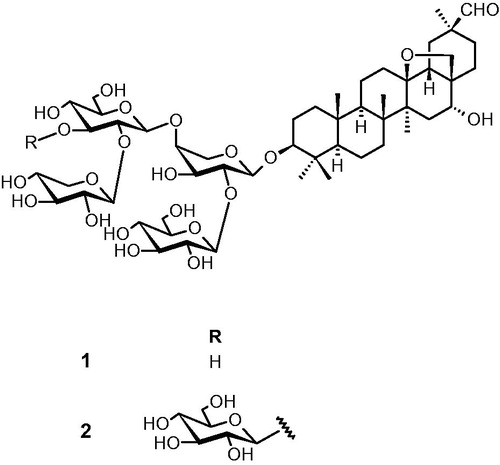
The 1H- and 13C-NMR spectra observed for saponins 1 and 2 revealed signals of six tertiary methyl, two pairs of oxygenated germinal protons, and an aldehyde group characteristics of a 13δ,28-epoxy oleanane skeleton moiety. Four and five anomeric protons were identified for saponins 1 and 2, respectively. Complete assignments of the resonance of each sugar unit and the sequence of the sugar chain were achieved by extensive 1D (1H, 13C, TOCSY) and 2D (HSQC, HMBC, ROESY, HSQC-TOCSY) NMR analysis. The chemical structures of saponins are shown in . Saponin 1 was established as saxifragilofin B (Park et al., Citation2010) whereas saponin 2 was identified as cyclamin (Reznicek et al., Citation1989) by comparing their spectroscopic data with those reported in the literature. Saxifragifolin B and cyclamin were isolated for the first time from C. libanoticum.
Results of the cytotoxicity assays are reported in . Both saxifragifolin B and cyclamin displayed interesting cytotoxic activities; their activities were generally higher than those of the established chemotherapy drug mitomycin C (IC50 between 0.45 and 21.73 µM).
Table 1. Cytotoxic activities of saxifragifolin B and cyclamin as compared with those of mitomycin C.
Saxifragifolin B was found to be the most active compound, with IC50 values ranging between 0.18 and 0.55 µM. It was 56-times more active than mitomycin C against breast adenocarcinoma cell line (SK-BR-3), and 37-fold against lung carcinoma cell line (NCI-H1299). Cyclamin demonstrated lower cytotoxic activities, with IC50 values ranging between 0.33 and 0.84 µM. It is interesting to note that, in contrast mitomycin C, the IC50 of saxifragifolin B and cyclamin weakly varied according to the tested cell lines. However, their cytotoxicity was not specific for the tumor cells, since they displayed low IC50 values (0.28 and 0.32 µM, respectively) with human fibroblasts.
Results of the standard micronucleus assay are reported in . For all the tested concentrations, ranging from 0.005 to 0.1 µM, saxifragifolin B did not induce micronuclei formation. Cyclamin was not directly active; however, it produced a significant increase of micronucleated cell levels after metabolic activation with S9 mix in a dose-dependent manner.
Table 2. Summary results of the standard micronucleus assay.
The results of the anticlastogenic effects of saxifragifolin B and cyclamin against mitomycin C are summarized in ; saxifragifolin B protected cells from the clastogenic effects of the antineoplastic drug mitomycin C in the pre-treatment and simultaneous treatment protocols. As expected, mitomycin C induced a significant increase of micronucleated cell levels (38–40 micronucleated cells per 1000) compared to the control culture. In the pre-treatment test, a dose-dependent decrease of micronucleated cell levels can be observed for saxifragifolin B concentrations between 0.001 and 0.01 µM. At this concentration of 0.01 µM, its protective capacity reached a maximum of 61%, and then decreased to 39% for the concentration of 0.05 µM.
Table 3. Summary results of the protective effects of saxifragifolin B against the clastogenic activity of mitomycin C.
In the simultaneous treatment test, a dose-dependent decrease of the micronucleated cell levels could also be observed for the concentrations of saxifragifolin B between 0.001 and 0.01 µM. Similarly, its protective capacity reached a maximum of 54% for the concentration of 0.01 µM, and then decreased to 12% for the concentration of 0.05 µM. It seemed that saxifragifolin had a better protective capacity when applied before mitomycin C treatment. On one hand, in the post-treatment test, no protective effect of saxifragifolin B was observed. On the other hand, cyclamin did not seem to have a protective anticlastogenic effect in all the applied treatments.
Ferrous ion chelating and DPPH scavenging activities of saxifragifolin B and cyclamin are displayed in . Saxifragifolin B and cyclamin exhibited practically the same ferrous ion chelating activity of 10.4% at the concentration of 67 µM. It was low as compared with those of EDTA–Na2 that exhibited this chelating activity at the concentration of 5 µM. Saxifragifolin B and cyclamin were also weak DPPH radical scavengers, with EC50 values of 0.45 and 0.96 mM, respectively, as compared with the commonly used reference antioxidant compounds catechin (EC50 = 8.6 µM) and ascorbic acid (EC50 = 14.2 µM).
Table 4. Antioxidant and ferrous chelating activities of saxifragifolin B and cyclamin versus DPPHa and Fe2+ ions.
Discussion
The genus Cyclamen has been poorly investigated for cytotoxic and anticlastogenic activities. In the present study, saxifragifolin B and cyclamin, two previously described saponins, were isolated for the first time from C. libanoticum. Also, the presence of saxifragifolin B as well as cyclamin in C. persicum was confirmed.
Saxifragifolin B is a triterpenoid tetrasaccharidic saponin. It has been previously isolated from different Chinese plants including Androsace saxifragifolia (Waltho et al., Citation1986) and Androsace umbellate (Song, Citation1998); these plants have been used in the treatment of sore throat, detumescence, and human cancers. Cyclamin is a triterpenoid pentasaccharidic saponin. It has been previously isolated from other Cyclamen species such as Cyclamen mirabile (Çaliş et al., Citation1997) and Cyclamen trocopteranthum (Mihci-Gaidi et al., Citation2010).
As a first step in evaluating the anticancer potential of saxifragifolin B and cyclamin, the cytototoxic activities of saponins were determined against various tumor cells. Both saxifragifolin B and cyclamin were shown to be cytotoxic at concentrations ranging from 0.18 to 0.84 µM. These results were consistent with previously published data. Saxifragifolin B, isolated from Androsace umbellate, has been shown to exhibit promising cytotoxic activities against human hepatoma cells (Hep3B and multidrug-resistant HepG2), human leukemia cells (HL-60), and human colon cancer cells (HT-29) (Zhang et al., Citation2007), whereas cyclamin has previously demonstrated cytotoxic activity against tumor colon cells (HT-29 and HCT 116), hepatoma cells (HepG2), and Caco-2 cells (Mihci-Gaidi et al., Citation2010). Their very close IC50 values indicated that the cytotoxic activity slightly depended on the chemical structure of the glycan part of the molecules. However, their strong cytotoxic activity against human fibroblasts showed a weak specificity for tumor cells. It partly explained the low variations observed for IC50 in the six tumor cell lines. It confirmed the results of Zhang et al. (Citation2007), which demonstrated that the cytotoxicity of saxifragifolin B was similar in MDR and non-MDR cells through a mechanism that involved activation of PARP and caspases, mitochondrial membrane potential collapse, cytochrome C release, and apoptosis. Comparison with mitomycin C indicated that saxifragifolin and cyclamin were 2–50-fold more active than the anticancer drug toward tumor cells. However, in contrast to saponins, mitomycin C was deprived from cytotoxicity against normal cells, and its activity greatly varied according to the tumor cell lines as previously demonstrated by Sasaki et al. (Citation2006). This could be due to the great variations in p-glycoprotein expression and/or MDR1 polymorphisms in tumor cells (Sasaki et al., Citation2006).
The standard micronucleus assay was applied to test the cytogenetic safety of saponins. Micronuclei are defined as chromosome fragments or whole chromosomes which lag during cell division due to the lack of a centromere or to a defect in cytokinesis (Kirsch-Volders et al., Citation2003). They may be produced by clastogenic or aneugenic compounds, according to a wide range of mechanisms which includes both genotoxic and epigenetic events leading to chromosome breaks (clastogenic effect) or default in mitotic spindle (aneugenic effect). The micronucleus assay allows the scoring of micronuclei in the cytoplasm of interphasic cells exposed in vitro or in vivo to clastogenic and/or aneugenic xenobiotics. It is considered as an efficient assay to evaluate the capacity of compounds to induce heritable chromosome mutations. It may also be used to identify protective agents that prevent cells from the clastogenic/aneugenic of genotoxic molecules such as mitomycin C. Results showed that both saxifragifolin B and cyclamin were devoid of direct intrinsic clastogenic/aneugenic activity. However, cyclamin, at the concentration of 0.05 µM, induced a significant increase of micronucleated cells after metabolism with S9 mix; indicating that different biological effects could be obtained depending on the nature of the glycan chain.
To the best of our knowledge, the present study determined for the first time the genotoxic safety of saxifragifolin B and the cytogenetic toxicity of cyclamin. Arslan and Orzgun (Citation2012) have shown that water extracts of Cyclamen trochopteranthum were able to modulate the expression of cytochromes P450 (CYP1A1, CYP1A2 CYP2E1, CYP2B6, CYP2C9, and CYP3A4) which are known to participate in the metabolism of drugs and carcinogens. The well-known carcinogenic agent mitomycin C was chosen as a DNA-damaging agent in the anticlastogenic assay testing saxifragifolin B. Mitomycin C is an anaziridine-containing molecule used as an antitumoral agent in cancer chemotherapy to treat gastrointestinal (Hofheinz et al., Citation2008), gynecological (Kahmann et al., Citation2010), breast, and bladder malignancies (Volpe et al., Citation2010).
This bifunctional alkylating compound is a potent DNA cross linker, specific for a guanine nucleoside. It induces DNA-damage leading to deletion mutations (Levin et al., Citation1984) and chromosome abnormalities (Krishna et al., Citation1989). It is commonly used as a positive control in the in vitro micronucleus assay (Kirsch-Volders et al., Citation2003). In order to fully explore the anticlastogenic effects of saxifragifolin B and cyclamin, three tests were performed including pre-treatment, simultaneous treatment, and post-treatment conditions. Results observed in the present study clearly established that saxifragifolin B displayed anticlastogenic activities under pre-treatment and simultaneous treatment conditions, whereas cyclamin did not exert any protective effect.
It is well known that excessive production of reactive oxygen species (ROS) is an important factor of chemo-induced and endogenous mutations (Ziech et al., Citation2011). The majority of environmental and industrial pollutants, including mitomycin C (Matsunaga et al., Citation2010), have been shown to be able to generate ROS. In the present study, and in order to elucidate their mechanism of action, the saponins were tested for their antioxidant activities. Both saxifragifolin B and cyclamin displayed weak antioxidant activities as was shown by DPPH radical scavenging and ferrous ion chelating assays. These results were consistent with previously published data (Hu et al., Citation2012) concerning the chelating properties of the compounds. They could be explained by the weak number of phenolic–OH groups in their structures (Santoso et al., Citation2004; Sedej et al., Citation2010). Therefore, it seems unlikely that the saxifragifolin B anticlastogenic activity could be due to its direct radical scavenging or metal chelating properties. In contrast, it could include other antioxidant mechanisms such as modulation of enzymes involved in cell defenses against xenobiotics and ROS.
In conclusion, this study demonstrated the interesting cytotoxic activities of both saxifragifolin B and cyclamin against SK-BR-3, HT-29, HepG2/3 A, NCI-H1299, BXPC-3, and 22RV1 cell lines. One of the most promising results of this work is the very potent cytotoxicity of saxifragifolin B against breast adenocarcinoma (SK-BR-3) and lung carcinoma (NCI-H1299) in comparison with mitomycin C. Also, this same compound protected the cells against the clastogenic effect of mitomycin C. Thus, saxifragifolin B could be considered as a possible cytotoxic drug with a preventive effect against environmental and chemotherapeutic exposures to genotoxic agents, it could be prescribed as a complementary medicine to improve the anticancer activities of conventional chemotherapeutic agents and to limit their adverse effects. However, additional experiments are needed to explore the combined cytotoxicity of saxifragifolin B with anticancer drugs and to study its potential protective effect against therapeutic or environmental carcinogens on in vivo models.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
We would like to thank Dr. L. Auezova, Dr. L. Boyer, and Mr. G. El Khoury for their valuable comments during the preparation of the manuscript. Also, we thank Mrs. M. Boudon and Mr. F. Mabrouki for their technical assistance.
References
- Arslan S, Ozgun O. (2012). Cyclamen trochopteranthum: Cytotoxic activity and possible adverse interactions including drugs and carcinogens. Chin J Integr Med 23. doi: 10.1007/s11655-012-1253-1
- Çaliş T, Satana ME, Yürüker A, et al. (1997). Triterpene saponins from Cyclamen mirabile and their biological activities. J Nat Prod 60:315–18
- Cragg GM, Newman DJ, Snader KM. (1997). Natural products in drug discovery and development. J Nat Prod 60:52–60
- Dall'Acqua S, Castagliuolo I, Brun P, et al. (2010). Triterpene glycosides with in vitro anti-inflammatory activity from Cyclamen repandum tubers. Carbohydr Res 345:709–14
- Hofheinz RD, Beyer U, Al-Batran SE, Hartmann JT. (2008). Mitomycin C in the treatment of gastrointestinal tumours: Recent data and perspectives. Onkologie 31:271–81
- Hu JL, Nie SP, Huang DF, et al. (2012). Extraction of saponin from Camellia oleifera cake and evaluation of its antioxidant activity. Int J Food Sci Technol 47:1676–87
- Huang WY, Cai YZ, Zhang Y. (2010). Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr Cancer 62:1–20
- Imanshahidi M, Hosseinzadeh H. (2008). Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res 22:999–1012
- Kahmann L, Beyer U, Mehlhorn G, et al. (2010). Mitomycin C in patients with gynecological malignancies. Onkologie 33:547–57
- Kirsch-Volders M, Sofuni T, Aardema M, et al. (2003). Report from the in vitro micronucleus assay working group. Mutat Res 540:153–63
- Krishna G, Kropko ML, Theiss JC. (1989). Use of the cytokinesis-block method for the analysis of micronuclei in V79 Chinese hamster lung cells: Results with mitomycin C and cyclophosphamide. Mutat Res 222:63–9
- Kupchan SM, Hemingway RJ, Knox JR, et al. (1967). Tumor inhibitors XXI. Active principles of Acer negundo and Cyclamen persicum. J Pharm Sci 56:603–8
- Levin DE, Marnett LJ, Ames BN. (1984). Genetics spontaneous and mutagen-induced deletions: Mechanistic studies in Salmonella tester strain TA102 (single-strand breaks/mutagenic gyrase inhibitors/DNA repair/multicopy plasmid). Proc Natl Acad Sci USA 81:4457–61
- Lim YY, Lim TT, Tee JJ. (2007). Antioxidant properties of several tropical fruits: A comparative study. Food Chem 103:1003–8
- Mahasneh AM, El-Oqlah AA. (1999). Antimicrobial activity of extracts of herbal plants used in the traditional medicine of Jordan. J Ethnopharmacol 64:271–6
- Matsunaga T, Tsuji Y, Kaai K, et al. (2010). Toxicity against gastric cancer cells by combined treatment with 5-fluorouracil and mitomycin C: Implication in oxidative stress. Cancer Chemother Pharmacol 66:517–26
- Mihci-Gaidi G, Ozbey S, Orhan I, et al. (2010). Triterpene saponins from Cyclamen trocopteranthum. Planta Med 76:818–21
- Park JH, Kwak JH, Khoo JH, et al. (2010). Cytotoxic effects of triterpenoid saponins from Androsace umbellata against multidrug resistance (MDR) and non-MDR cells. Arch Pharm Res 33:1175–80
- Primorac M, Sekulović D, Antonić S. (1985). In vitro determination of the spermicidal activity of plant saponins. Pharmazie 40:585–94
- Reznicek G, Jurenitsc, J, Robien W, Kubelka W. (1989). Saponins in Cyclamen species: Configuration of cyclamiretin C and structure of isocyclamin. Phytochemistry 28:825–8
- Santoso J, Yoshie-Stark Y, Suzuki T. (2004). Anti-oxidant activity of methanol extracts from Indonesian seaweeds in an oil emulsion model. Fisheries Sci 70:183–8
- Sasaki M, Okamura M, Ideo A, et al. (2006). Re-evaluation of tumor-specific cytotoxicity of mitomycin C, bleomycin and peplomycin. Anticancer Res 26:3373–80
- Schwartsmann G, Ratain MJ, Cragg GM, et al. (2002). Anticancer drug discovery and development throughout the world. J Clin Oncol 20:47–59
- Sedej IJ, Sakac MB, Misan AC, Mandic AI. (2010). Antioxidant activity of wheat and buckwheat flours. Proc Nat Sci 118:59–68
- Song L. (1998). Chinese Bencao. Vol. 6. Shanghai: Shanghai Science and Technology Press
- Speroni E, Cervellati R, Costa S, et al. (2007). Analgesic and antiinflammatory activity of Cyclamen repandum S. et S. Phytother Res 21:684–9
- Volpe A, Racioppi M, D’Agostino D, et al. (2010). Mitomycin C for the treatment of bladder cancer. Minerva Urol Nefrol 6:133–44
- Wall ME. (1992). Antimutagenic agents from natural products. J Nat Prod 55:1561–8
- Waltho J, Williams DH, Mahato SB, et al. (1986). Structure elucidation of two triterpenoid tetrasaccharides from Androsace saxifragifolia. J Chem Soc Perkin Trans 18:1527–31
- Yan GC, Chen HY. (1995). Antioxidant activity of various tea extracts in relation to their antimutagenecity. J Agric Food Chem 43:27–37
- Zhang DM, Wang Y, Tang MK, et al. (2007). Saxifragifolin B from Androsace umbellata induced apoptosis on human hepatoma cells. Biochem Biophys Res Commun 362:759–65
- Ziech D, Franco R, Pappa A, Panayiotidis MI. (2011). Reactive oxygen species (ROS)-induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res 711:167–73