Abstract
Context: Paeonia lactiflora Pall. (Ranunculaceae) has been used for more than 1000 years in traditional Chinese medicine for the treatment of gynecological problems, cramp, pain, giddiness, and congestion. Paeoniflorin, monoterpene glycosides isolated from P. lactiflora, possesses a variety of pharmacological activities. However, the pharmacological activity of the pharmacological activity of albiflorin, another main monoterpene glycoside, has not been well studied.
Objectives: The present study investigated the anti-inflammatory activities of paeoniflorin and albiflorin using models of lipopolysaccharides (LPS) induced RAW 264.7 cells.
Materials and methods: Production of nitric oxide (NO) was measured by the Griess colorimetric method. In addition, prostaglandin E2 (PGE2), interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) synthesis were analyzed using an enzyme-linked immunosorbent assay (ELISA). The protein expression of cyclooxygenase-2 (COX-2) was detected by a cell-based ELISA. The gene expression levels of inducible nitric oxide synthase (iNOS), COX-2, TNF-α, and IL-6 were detected by quantitative real-time reverse-transcription polymerase chain reaction (real-time RT-PCR).
Results: Compared with the LPS-induced group, the inhibition rates of NO, PGE2, TNF-α, and IL-6 production were 17.61, 27.56, 20.57, and 29.01% by paeoniflorin and 17.35, 12.94, 15.29, and 10.78% by albiflorin. The IC50 values of paeoniflorin and albiflorin on NO production were 2.2 × 10−4 mol/L and 1.3 × 10−2 mol/L, respectively. The protein expression of COX-2 was reduced by 50.98% with paeoniflorin and 17.21% with albiflorin. The inhibition rates of gene expression of iNOS, COX-2, IL-6, and TNF-α were 35.65, 38.08, 19.72, and 45.19% by paeoniflorin and 58.36, 47.64, 50.70, and 12.43% by albiflorin, respectively.
Conclusion: These results show that albiflorin has similar anti-inflammatory effects to paeoniflorin, which provides new evidence that albiflorin can serve as a new chemical marker for the quality control of Paeoniae Radix and the Chinese Pharmacopoeia can be updated.
Introduction
Herbaceous peony [Paeonia lactiflora Pall. (Ranunculaceae)], also named Chinese Paeony, is widely cultivated as an ornamental plant in gardens. Moreover, the herb Paeonia, known as “Shao Yao” in China, and dried root as a predominantly used form called as Paeoniae Radix, has been used for more than 1000 years in traditional Chinese medicine to treat gynecological problems, cramp, pain, giddiness, and congestion. The total glucosides of peony (TGP), extracted from Paeoniae Radix, has been approved by the State Food and Drug Administration of China (SFDA) as a novel treatment for rheumatoid arthritis since 1998. Total glucosides of Paeony Capsules, named “Pa-fu-lin” in China, used to reduce pain and inflammatory response of arthritis patients have been used in clinic about 20 years.
Paeoniflorin (), a monoterpene glycoside isolated from the aqueous extract of the dry root of Paeonia, has been identified as a main active ingredient responsible for the biological activities in the Chinese Pharmacopoeia. The modern pharmacological studies have shown that paeoniflorin has been widely studied as antioxidant, anticonvulsant, antithrombotic agent, cognitive enhancer or learning impairment attenuating agent, and neuroprotecting agent (Xiao et al., Citation2005; Ye et al., Citation2001; Zhong et al., Citation2009). However, little attention has been focused on the pharmacological studies of albiflorin (), another monoterpene glycoside isolated from the aqueous extract of Paeoniae Radix. In the present study, the effects of anti-inflammatory on paeoniflorin and albiflorin were investigated, which will provide new evidence to pay albiflorin more attention to a new chemical marker for the quality control of Paeoniae Radix and update the Chinese Pharmacopoeia.
Materials and methods
Reagents
The standard of paeoniflorin was purchased from the Chinese National Institute for Control of Pharmaceutical and Biological Products (Beijing, China). The standard of albiflorin was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Dulbecco’s modified Eagle’s medium-high glucose (DMEM), 2-(4,-5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), and lipopolysaccharides (LPS) from Escherichia coli 0111:B4 (LPS) were purchased from Sigma-Aldrich Co. (St. Louis, MO). Prostaglandin E2 Express EIA Monoclonal kit was obtained from Cayman Chemical (Ann Arbor, MI). IL-6 Mouse ELISA kit and TNF-α Mouse ELISA kit were obtained from Invitrogen (Carlsbad, CA). Mammalian Cell Lysis Kit and UNIQ-10 column Trizol total RNA extraction kit were bought from Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China). Improm-II Reverse Transcription System was purchased from Promega Corporation (Madison, WI). FastStart Universal SYBR Green Master (ROX) kit was purchased from Roche (Mannheim, Germany). Mouse anti-COX-2 monoclonal antibody was from BD Pharmingen (San Diego, CA) and Goat Anti-Mouse IgG Peroxidase Conjugate was from Merck-Calbiochem (Darmstadt, Germany).
Cells and cell culture
The RAW 264.7 murine macrophages cell line was obtained from Cell Culture Center of Chinese Academy of Medical Sciences (Beijing, China). RAW 264.7 cells were maintained in DMEM supplemented with 10% heat inactivated fetal bovine plasma (HI-FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified incubator containing 5% CO2. For the determination of cell viability, nitrite concentration as an index for NO synthesis, as well as cytokines, and chemokines in culture medium, the cells were plated at 5 × 105 cells/well in 96-well plates and treated with various concentrations of paeoniflorin and albiflorin in the presence of 0.2 μg/mL LPS for 18–24 h as indicated. Moreover, for the determination of protein levels of COX-2, cells were treated with various concentrations of paeoniflorin and albiflorin in the presence of 0.2 μg/mL LPS for 24 h. For real-time RT-PCR, the cells were pre-incubated with various concentrations of paeoniflorin and albiflorin for 2 h and were then treated with 0.2 μg/mL LPS for an additional 6 h. Paeoniflorin and albiflorin at various concentrations were dissolved in phosphate-buffered saline (PBS, pH 7.4) as the stock solution. Cells were treated with PBS as a vehicle control.
Cell viability assay (MTT assay)
RAW 264.7 cells were treated with various concentrations of paeoniflorin and albiflorin (10−8, 10−7, 10−6, and 10−5 mol/L) for 24 h. Then MTT (stock solution of 5 mg/mL) was added to a final concentration of 0.5 mg/mL, and the cells were incubated for an additional 4 h at 37 °C and 5% CO2. The medium was removed, the formazan precipitate was solubilized in 100 μL DMSO, and the absorbance was measured at 570 nm on a multifunctional microplate reader (FlexStation 3, Molecular Devices, Sunnyvale, CA).
Nitrite assay and detection of cytokines
RAW 264.7 cells were treated with various concentrations of paeoniflorin and albiflorin (10−8, 10−7, 10−6, and 10−5 mol/L) in the presence of 0.2 μg/mL LPS. About 18 h later, the medium was collected. The nitrite accumulated in culture medium was measured as an indicator of NO production based on a diazotization reaction using Griess reagent system (Promega, Madison, WI). Nitrite concentration was determined by a standard curve prepared with sodium nitrite dissolved in DMEM without phenol red supplemented with 5% HI-FBS. IL-6 and TNF-α secreted in the culture medium were quantified by ELISA kits (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Samples were assayed after a 10-fold dilution in the medium (DMEM without phenol red supplemented with 5% HI-FBS).
ELISA for PGE2 production and cell-based ELISA for COX-2 protein expression in RAW 264.7 cells
RAW 264.7 cells were seeded in 96-well plates treated with various concentrations of paeoniflorin and albiflorin (10−8, 10−7, 10−6, and 10−5 mol/L) in the presence of 0.2 μg/mL LPS. The cell supernatants were collected for the detection of PGE2 using a Prostaglandin E2 Express EIA Monoclonal kit (Cayman Chemical, Ann Arbor, MI) and the expression of COX-2 was determined by a slightly modified original protocol for the cell-based ELISA (Versteeg et al., Citation2000; Wang et al., Citation2011). After cultivation, cells were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 20 min at room temperature and washed three times with PBS containing 0.1% Triton X-100 (PBS/T). Endogenous peroxidase was quenched with 0.6% H2O2 in PBS/T for 20 min, and cells were washed three times in PBS/T. Following blocking with 10% FCS in PBS/T for 1 h, cells were incubated for 2 h at 37 °C with the primary antibody in PBS/T containing 1% BSA. After washing the cells four times with PBS/T for 5 min, the plate was incubated for 1 h at room temperature with secondary antibody in PBS/T containing 1% BSA. Subsequently, cells were washed and incubated with TMB substrate solution (Invitrogen, Carlsbad, CA) for 30 min at room temperature in the dark. The reaction was stopped with 50 μL of 2 N H2SO4, and the absorbance at 450 nm was determined on a multifunctional microplate reader. The data were corrected for differences in cell number by staining the cells with Janus Green B after the cell-based ELISA procedure (Raspotnig et al., Citation1999).
Real-time RT-PCR for detecting mRNA expression of TNF-α, IL-6, COX-2, and iNOS
Total RNA was isolated using Sangon UNIQ-10 column Trizol total RNA extraction kit according to the instructions of the manufacturer. RNA (1 μg) was reversely transcribed using ImProm-II Reverse Transcription System cDNA synthesis kit (Promega, Madison, WI). The real-time RT-PCR oligonucleotide primers used for mouse iNOS, COX-2, IL-6, TNF-α, and β-actin are shown in . The reactions were setup in duplicates in 25 μL total volumes with 1 μL of each primer (0.3 μM final concentrations), 12.5 μL of FastStart Universal SYBR Green Master (ROX) (Roche, San Francisco, CA), and 1 μL of template. The PCR cycle was as follows: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 60 °C for 1 min; and a melt curve analysis was performed at the end of each experiment to verify that a single product per primer pair was amplified. The amplification and analysis were performed using an ABI Prism 7500 Real-Time PCR System (Biosystems, Foster City, CA). Samples were compared using the relative CT method. The fold increase or decrease was determined relative to a blank control after normalized to a housekeeping gene using (Degois et al., Citation2005; Livak & Schmittgen, Citation2001).
Table 1. The real-time RT-PCR oligonucleotide primers.
Statistical analysis
All results were expressed as mean ± SD. Statistically significant differences between groups was determined by one-way analysis of variance (ANOVA) followed by Scheffe's multiple range tests. The criterion for statistical significance was p < 0.01 or p < 0.05.
Results
Effect of paeoniflorin and albiflorin on RAW 264.7 cells viability
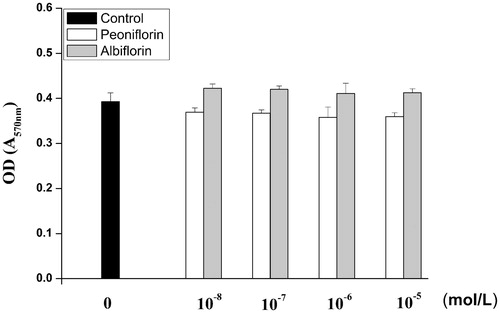
The cytotoxicity of paeoniflorin and albiflorin on RAW 264.7 cells was measured with the MTT assay. Cell viability was not significantly altered by paeoniflorin and albiflorin at up to 10−5 mol/L. These results suggest that concentrations of paeoniflorin and albiflorin below 10−5 mol/L were not toxic to RAW 264.7 cells. Therefore, for all experiments, cells were treated with paeoniflorin and albiflorin in the concentration range of 10−8–10−5 mol/L. The MTT assay results of paeoniflorin and albiflorin on RAW 264.7 cells are shown in .
Effect of paeoniflorin and albiflorin on LPS-induced NO production
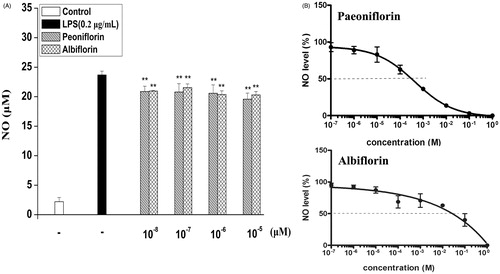
The effect of paeoniflorin and albiflorin on NO in LPS-induced RAW 264.7 cells was tested to investigate the anti-inflammatory effects. Concentrations of nitrite accumulated in the culture medium were estimated by the Griess reagent as an index for NO. RAW 264.7 cells were pretreated with different concentrations (10−8, 10−7, 10−6, and 10−5 mol/L) of paeoniflorin and albiflorin. It was found that paeoniflorin could inhibit LPS-induced NO production by 17.61% and albiflorin could inhibit NO production by 17.35% compared to the LPS-induced group (). The IC50 of paeoniflorin and albiflorin on NO production was 2.2 × 10−4 mol/L and 1.3 × 10−2 mol/L, respectively ().
Figure 3. Effects of paeoniflorin and albiflorin on LPS-induced NO in RAW 264.7 cells. RAW 264.7 cells were incubated with the indicated concentrations of paeoniflorin, albiflorin and 0.2 μg/mL LPS for 18 h. (A) The NO content of culture medium was analyzed by the Griess reagent method. (B) IC50 of paeoniflorin and albiflorin on LPS-induced NO in RAW 264.7 cells. Data represent means ± SD values from three independent experiments. **p < 0.01 (n = 6) compared with LPS treated cells alone.
Effects of paeoniflorin and albiflorin on LPS-induced cytokines TNF-α and IL-6
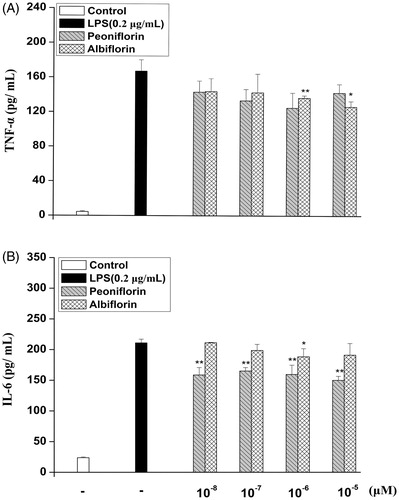
After treatment with paeoniflorin and albiflorin and activated with LPS (0.2 μg/mL), the secretion of IL-6 and TNF-α was detected by ELISA. As shown in , compared with the only LPS-induced group, albiflorin could reduce TNF-α production by 50.70%, paeoniflorin reduced TNF-α production by 20.57%. Paeoniflorin could reduce IL-6 production by 29.01%, and albiflorin reduced IL-6 production by 12.43%. Paeoniflorin could reduce IL-6 production stronger than albiflorin, however, albiflorin reduced TNF-α production stronger in the concentration of 10−6 and 10−5 mol/L compared with paeoniflorin.
Figure 4. Effects of paeoniflorin and albiflorin on LPS-induced cytokines TNF-α and IL-6 expression. RAW 264.7 cells were incubated with the indicated concentrations of paeoniflorin, albiflorin, and 0.2 μg/mL LPS for 18 h. TNF-α (A) and IL-6 (B) in the culture medium were analyzed by ELISA. Data represent means ± SD values from three independent experiments. *p < 0.05, **p < 0.01 (n = 6) compared with LPS treated cells alone.
PGE2 production and protein expression of COX-2 on paeoniflorin and albiflorin
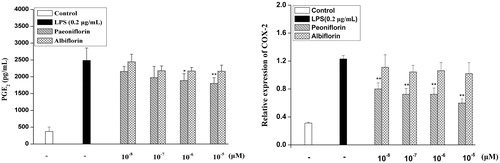
PGE2 production is modified by COX-2 catalyzing the metabolism of arachidonic acid. As shown in , paeoniflorin could decrease the overproduction of PGE2 by 27.56% induced by LPS. However, albiflorin could not inhibit the production of PGE2. The effects of paeoniflorin and albiflorin on COX-2 protein expression were studied by cell-based ELISA. Similarly, LPS treatment increased COX-2 protein expression levels, whereas COX-2 protein expression suppressed the induction of paeoniflorin by 50.98%. Albiflorin could not significantly suppress the COX-2 protein expression compared with the only LPS-induced group. The results showed that paeoniflorin has a strong inhibition on COX-2 protein expression compared with albiflorin.
Figure 5. Effects of paeoniflorin and albiflorin on LPS-induced PGE2 production and COX-2 protein expression. RAW 264.7 cells were incubated with the indicated concentrations of paeoniflorin, albiflorin, and 0.2 μg/mL LPS for 24 h. PGE2 production in the culture medium was analyzed by ELISA. The COX-2 protein expression was analyzed by cell-based ELISA. Data represent means ± SD values from three independent experiments. *p < 0.05, **p < 0.01 (n = 6) compared with LPS-treated cells alone.
Effects of paeoniflorin and albiflorin on LPS-induced mRNA expression of TNF-α, IL-6, iNOS, and COX-2
Since paeoniflorin and albiflorin were found to most potently inhibit the pro-inflammatory mediators, e.g., NO, TNF-α, IL-6, and PGE2 in supernatants and protein of COX-2, we investigated the effects of paeoniflorin and albiflorin on LPS-induced iNOS, TNF-α, IL-6, and COX-2 gene expression using real-time RT-PCR. The inhibitions of paeoniflorin and albiflorin on LPS-induced mRNA expression of TNF-α, IL-6, iNOS, and COX-2 are shown in . The results showed that paeoniflorin inhibited the mRNA expression of iNOS by 35.65% and albiflorin inhibited the mRNA expression of iNOS by 58.36% compared with the only LPS-induced group. The mRNA expression of iNOS was also decreased by paeoniflorin and albiflorin, confirming the suppressive effect on the NO production. The blocking effect of paeoniflorin and albiflorin on LPS-induced iNOS expression might have resulted from the transcriptional inhibition of iNOS gene.
Figure 6. Effect of paeoniflorin and albiflorin on LPS-stimulated mRNA expression of iNOS, COX-2, TNF-α, and IL-6. RAW 264.7 cells were pre-incubated with indicated concentrations of paeoniflorin and albiflorin for 2 h and were then treated with 0.2 μg/mL LPS for an additional 6 h. The mRNA expression of iNOS, COX-2, and TNF-α, IL-6 was analyzed by real-time RT-PCR. Data represent means ± SD values from three independent experiments. *p < 0.05, **p < 0.01 (n = 6) compared with LPS-treated cells alone.
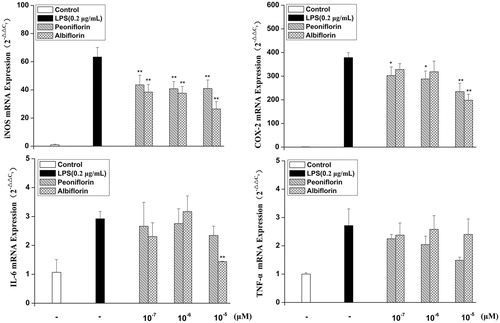
Albiflorin could inhibit the mRNA expression of IL-6 and COX-2 by 50.70 and 47.64% compared with the only LPS-induced group; however, it could not significantly inhibit the mRNA expression of TNF-α compared with the only LPS-induced group. Paeoniflorin could inhibit the mRNA expression of COX-2 by 38.08%, but not significantly inhibit the mRNA expression of TNF-α and IL-6 compared with the only LPS-induced group. The mRNA expression of IL-6 and iNOS was inhibited stronger by albiflorin compared with paeoniflorin; however, paeoniflorin inhibited the mRNA expression of COX-2 and TNF-α stronger compared to albiflorin.
Discussion
P. lactiflora is an Oriental medicinal herb containing monoterpene glycoside compounds such as paeoniflorin, albiflorin, and benzoyloxypaeoniflorin, and its dried root as a predominantly used form is called Paeoniae Radix (Goto et al., Citation1996). Paeoniflorin has been reported to reduce the inflammatory response in macrophages induced by LPS (Huang et al., Citation2008). Furthermore, paeoniflorin could protect RAW 264.7 macrophages from LPS-induced cytotoxicity and genotoxicity (Kim & Ha, Citation2009). Paeoniflorin could inhibit systemic inflammation in rat (Jiang et al., Citation2009) and suppress inflammatory mediator production in arthritic rats (Zheng et al., Citation2007). In the Chinese Pharmacopoeia, paeoniflorin has been authorized as the only detection indicator of quality control for Paeoniae Radix. However, little attention was paid to the pharmacological activity of another monoterpene glycoside, albiflorin. In the present study, a comparison of peoniflorin and albiflorin on anti-inflammatory was investigated.
NO is recognized as a mediator and regulator of inflammatory responses and is produced in high amounts by iNOS in activated inflammatory cells (Korhonen et al., Citation2005). The IC50 of paeoniflorin and albiflorin on NO production was 2.2 × 10−4 mol/L and 1.3 × 10−2 mol/L, respectively. It is reported that after oral administration of paeoniae to rats, paeoniflorin and albiflorin reached the maximum concentration of 2.6 × 10−6 mol/L at 30 min and 3.2 × 10−6 mol/L at 20 min, respectively (Gan et al., Citation2010). The blood concentration of paeoniflorin and albiflorin may be increased by a local drug delivery system, such as a transdermal delivery system. Paeoniflorin and albiflorin were found to inhibit LPS-induced NO production. The mRNA expression of iNOS was also decreased by paeoniflorin and albiflorin, confirming the suppressive effect on the NO production. The blocking effect of paeoniflorin and albiflorin on LPS-induced NO production might have resulted from the transcriptional inhibition of iNOS gene expression. The mechanism of various anti-inflammatory drug actions was at least shared by the inhibition of prostaglandin synthesis, which is mediated by cycloxygenase (COX) (Vane, Citation1971). Of the two isoforms of COX, COX-1 has been suggested to provide a physiologic level of PGs for normal platelet, stomach, and kidney function. Moreover, COX-2 has been found to be highly induced at inflammatory sites in animals as well as patients with inflammatory diseases (Masferrer et al., Citation1994; Seibert et al., Citation1994). The activity of COX-2 also could be affected directly by NO and iNOS (Moncada et al., Citation1991). The results showed that paeoniflorin and albiflorin inhibited the production of PGE2 and COX-2 mRNA and protein expression. The inhibitory effect of NO production and iNOS mRNA expression was almost equivalent by paeoniflorin and albiflorin. Furthermore, paeoniflorin could inhibit the COX-2 mRNA and protein expression, but albiflorin only inhibited COX-2 gene expression at the concentration of 10−5 mol/L and did not inhibit COX-2 protein expression. These interesting results showed that paeoniflorin and albiflorin had different effects on the NO–iNOS pathway and the PGE2–COX-2 pathway in inflammation and further studies should be performed in the future.
In inflammation progress, a series of cytokines and mediators contributed to evoking and regression of inflammation. TNF-α and IL-6 are the most critical cytokines involved in inflammation and their inhibition is regarded as a treatment strategy for inflammation-related diseases (Burger et al., Citation2006; Locksley et al., Citation2001; Rose-John et al., Citation2007), so we chose TNF-α and IL-6 as parameters to investigate the anti-inflammatory effect of paeoniflorin and albiflorin, respectively. The production of TNF-α was inhibited by albiflorin (10−6 and 10−5 mol/L) but not by paeoniflorin; the production of IL-6 was inhibited by paeoniflorin about 29.01% but inhibited by albiflorin about 10.78%. The mRNA expression of IL-6 was only down-regulated by albiflorin in the concentration of 10−5 mol/L and the mRNA expression of TNF-α was not down-regulated by paeoniflorin and albiflorin. The reason may be that the time point of TNF-α and IL-6 production was investigated in 18 h after LPS-induced RAW 264.7 cell, but the time point of the mRNA expression of TNF-α and IL-6 was 6 h after LPS-induced RAW 264.7 cell. The short affection time of paeoniflorin and albiflorin after LPS-induced RAW 264.7 cell may be the reason why the production of TNF-α and IL-6 was different from the mRNA expression of TNF-α and IL-6. Further research is needed to investigate paeoniflorin and albiflorin on mRNA expression and production of TNF-α and IL-6.
Conclusion
The current study compared the pharmacological effects of peoniflorin and albiflorin on anti-inflammatory responses. The results showed that not only peoniflorin but also albiflorin could inhibit iNOS and COX-2 gene expression induced by LPS as well as the subsequent production of NO and PGE2, protein expression of COX-2. Moreover, both could reduce the production of cytokines (TNF-α and IL-6) and gene expression of TNF-α and IL-6 induced by LPS in RAW 264.7 macrophages. These results showed that albiflorin has similar anti-inflammatory effects to peoniflorin, which provides new evidence regarding the importance of albiflorin as a new chemical marker for the quality control of Paeoniae Radix and suggest the Chinese Pharmacopoeia should be updated.
Declaration of interest
We have no conflict of interest in this research. This work was supported by the National Natural Science Foundation of China (81173469), Program for New Century Excellent Talents in University (NCET-09-0899), and the Key New Drug Creation and Manufacturing Program (No. 2012ZX09304007).
Acknowledgements
The authors are grateful to Yaozu xiang of Imperial College London, Hammersmith Hospital Campus for fruitful discussions.
References
- Burger D, Dayer JM, Palmer G, Gabay C. (2006). Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract Res Clin Rheumatol 20:879–96.
- Degois S, Schafer MK, Defamie N, et al. (2005). Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci 25:7121–33
- Gan PP, Zhong MZ, Huang X, et al. (2010). Pharmacokinetic comparisons of albiflorin and paeoniflorin after oral administration of Shaoyao–Gancao–Tang and Single Herb Paeony Decoction to rats. Planta Med 78:237–43
- Goto H, Shimada Y, Akechi Y, et al. (1996). Endothelium-dependent vasodilator effect of extract prepared from the roots of Paeonia lactiflora on isolated rat aorta. Planta Med 62:436–9
- Huang H, Chang EJ, Lee Y, et al. (2008). A genome-wide microarray analysis reveals anti-inflammatory target genes of paeonol in macrophages. Inflamm Res 57:189–98
- Jiang WL, Chen XG, Zhu HB, et al. (2009). Paeoniflorin inhibits systemic inflammation and improves survival in experimental sepsis. Basic Clin Pharmacol Toxicol 105:64–71
- Kim ID, Ha BJ. (2009). Paeoniflorin protects RAW 264.7 macrophages from LPS-induced cytotoxicity and genotoxicity. Toxicol In Vitro 23:1014–19
- Korhonen R, Lahti A, Kankaanranta H, Moilanen E. (2005). Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy 4:471–9
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–8
- Locksley RM, Killeen N, Lenardo MJ. (2001). The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 104:487–501
- Masferrer JL, Zweifel BS, Manning PT, et al. (1994). Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA 91:3228–32
- Moncada S, Palmer RM, Higgs EA. (1991). Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–42
- Raspotnig G, Fauler G, Jantscher A, et al. (1999). Colorimetric determination of cell numbers by Janus green staining. Anal Biochem 275:74–83
- Rose-john S, Waetzig GH, Scheller J, et al. (2007). The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets 11:613–24
- Seibert K, Zhang Y, Leahy K, et al. (1994). Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA 91:12013–17
- Vane JR. (1971). Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231:232–5
- Versteeg HH, Nijhuis E, Van DB, et al. (2000). A new phosphospecific cell-based ELISA for p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK, protein kinase B and cAMP-response-element-binding protein. Biochem J 350:717–22
- Wang QS, Cui YL, Wang YF, Chi W. (2011). Effects of compounds from bi-qi capsule on the expression of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Ethnopharmacol 136:480–7
- Xiao L, Wang YZ, Liu J, et al. (2005). Effects of paeoniflorin on the cerebral infarction, behavioral and cognitive impairments at the chronic stage of transient middle cerebral artery occlusion in rats. Life Sci 78:413–20
- Ye J, Duan H, Yang X, Zheng X. (2001). Anti-thrombosis effect of paeoniflorin: Evaluated in a photochemical reaction thrombosis model in vivo. Planta Med 67:766–7
- Zheng YQ, Wei W, Zhu L, Liu JX. (2007). Effects and mechanisms of paeoniflorin, a bioactive glucoside from paeony root, on adjuvant arthritis in rats. Inflamm Res 56:182–8
- Zhong SZ, Ge QH, Li Q, et al. (2009). Peoniflorin attenuates A-beta(1-42)-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci 280:71–8