Abstract
Context: Clovers were chosen on the basis of traditional medicine recommendations, agricultural value, or available information on their promising chemical profiles.
Objective: This study evaluates and compares free radical scavenging and antioxidant properties of six clover species: Trifolium alexandrinum L. (Leguminosae), Trifolium fragiferum L., Trifolium hybridum L., Trifolium incarnatum L., Trifolium resupinatum var. majus Boiss., and Trifolium resupinatum var. resupinatum L.
Materials and methods: Free radical scavenging activity of the extracts (1.5–50 µg/ml) was estimated by reduction of 1,1-diphenyl-2-picrylhydrazyl (DPPH•) and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic) acid (ABTS•) radicals. The Trifolium extract effects on total antioxidant capacity of blood plasma were determined by the reduction of ABTS•+ and DPPH• radicals, as well as with the use of the ferric reducing ability of plasma (FRAP) assay.
Results: The UPLC analysis of chemical profiles of the examined extracts showed the presence of three or four groups of phenolic substances, including phenolic acids, clovamides, isoflavones, and other flavonoids. The measurements of free radical scavenging and ferric reducing ability of the examined clover extracts revealed the strongest effect for T. alexandrinum. Furthermore, antioxidant activity assays in human plasma have shown protective effects of all extracts against peroxynitrite-induced reduction of total antioxidant capacity.
Conclusions: Trifolium plants may be a rich source of bioactive substances with antioxidant properties. The examined extracts displayed free radical scavenging action and partly protected blood plasma against peroxynitrite-induced oxidative stress; however, the beneficial effects of T. alexandrinum and T. incarnatum seem to be slightly higher.
Introduction
Antioxidant properties are an important mechanism of beneficial activity of plant-derived compounds and extracts. For decades, plants have been considered to be a promising source of natural remedies. Ethnopharmacological surveys have shown that therapeutic use of even 80% of 122 plant-derived drugs may be related to their recommendations in traditional medicine (Fabricant & Farnsworth, Citation2001). The aim of this in vitro study was the evaluation of the biological properties of the plant extracts, prepared from the aerial parts of six clover species: Trifolium alexandrinum L. (Leguminosae), Trifolium fragiferum L. ssp. bonanni (Presl) Soj., Trifolium hybridum L., Trifolium incarnatum L., Trifolium resupinatum var. majus Boiss., and Trifolium resupinatum var. resupinatum L.
Trifolium genus includes over 240 species. Some of them are well-known forage plants that have been grown for centuries (i.e., Trifolium pratense L., Trifolium repens L., T. resupinatum L., T. incarnatum, T. hybridum, T. subterraneum L., and T. fragiferum); however, besides their significance in agriculture, clovers have also been used in traditional medicine of various cultures. The curative use of Trifolium species is mainly based on information derived from folk medicine, suggesting a wide range of therapeutic use of these plants. Seeds of T. alexandrinum have been used in Egypt as an antidiabetic remedy (Mohamed et al., Citation2000). Trifolium pratense and T. repens have been administered to cure sore throat, fever, pneumonia, meningitis, and feverish feeling in Pakistan (Khan et al., Citation2012), while in Turkey clovers such as T. repens, T. arvense L., and T. pratense have been used as expectorant, antiseptic, analgesic, sedative, and tonic mixtures (Sabudak et al., Citation2008).
The molecular mechanisms of physiological effects of the extracts from Trifolium species remain unknown. In contemporary medicine, the scientific interest in biological activity of clovers is mostly focused on therapeutic action of red clover (T. pratense) (Kolodziejczyk-Czepas, Citation2012). Trifolium pratense has been found to possess estrogenic activity, being a result of a high content of isoflavones, acting as natural ligands for estrogen receptors. Therefore, this clover species as a source of numerous herbal medicines, an alternative to the conventional estrogen replacement therapy, has been administered to alleviate menopausal complaints (Engelmann et al., Citation2009; Thompson Coon et al., Citation2007).
Biological activities and therapeutic properties of other clovers have not been well described; however, the number of scientific reports confirming the beneficial activity of Trifolium plant-derived extracts has been growing. Recently, Renda et al. (Citation2013) described results of an in vivo study on dermal wound healing potentials of aqueous methanol extracts from 13 Trifolium species (T. ambiguum M. Bieb., T. arvense, T. campestre Schreb., T. canescens Willd., T. hybridum var. anatolicum Boiss., T. hybridum var. hybridum L., T. pannonicum Jacq., T. pratense var. pratense L., T. purpureum var. purpureum Loisel., T. repens var. repens L., T. resupinatum var. microcephalum Boiss., T. spadiceum L., and T. trichocephalum M.Bieb.). It has been found that methanol extracts of T. canescens (the most efficient action) and T. pratense possessed better wound healing activity, compared with other examined Trifolium extracts.
Our previous experiments have shown the protective action of extracts from T. pallidum, T. pratense, and T. scabrum on blood platelets exposed to oxidative stress (Kolodziejczyk-Czepas et al., Citation2013a). In another study, we have found that the extracts of T. pallidum and T. scabrum might protect the components of blood plasma, including fibrinolytic proteins, against the peroxynitrite-induced oxidative damage (Kolodziejczyk-Czepas et al., Citation2013b). In the present work, we assess the free radical scavenging ability of six other clover species and their effect on the antioxidant capacity of human plasma under oxidative stress conditions in vitro. For this study, the examined clover species were selected on the basis of their use in folk medicine, agricultural value as well as knowledge of their promising chemical profile, including the results of a general phytochemical analysis of various Trifolium species, performed at the Institute of Soil Science and Plant Cultivation, State Research Institute, Pulawy (Oleszek et al., Citation2007).
Materials and methods
Plant material and reagents
Seeds of T. alexandrinum (TRIF 1237), T. fragiferum ssp. bonanni (Presl) Soj. (TRIF 208/79), T. hybridum ssp. bonanni (Presl) Soj. (TRIF 6/82), T. incarnatum (TRIF 1189), T. resupinatum var. majus (TRIF 61), and T. resupinatum var. resupinatum (TRIF 59) were obtained from Genebank, Leibniz Institute of Plant Genetics and Crop Plant Research (Gatersleben, Germany) and sown in experimental field at the Station of Vegetation Experiments of the Institute of Soil Science and Plant Cultivation – State Research Institute in Pulawy (Krolewska st17, Poland). The voucher samples (7/2011, 26/2011, 31/2011, 41/2011, 79/2011, and 77/2011, respectively) have been deposited at the Department of Biochemistry and Crop Quality of Institute (Pulawy). The aerial parts were harvested in year 2011 at the beginning of flowering (T. alexandrinum L. – 18 July, T. fragiferum L. ssp. bonanni (Presl) Soj. – 18 August, T. hybridum L. – 19 July, T. incarnatum L. – 18 July, T. resupinatum var. majus Boiss. – 29 July, T. resupinatum var. resupinatum – 19 July), freeze-dried and powdered. Stock solutions of the tested extracts were prepared in 30% dimethylsulfoxide (DMSO).
Peroxynitrite was synthesized according to the method of Pryor et al. (Citation1991). 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) for the ABTS assay was obtained from Sigma (Sigma-Aldrich, St. Louis, MO). 2,4,6-Tris(2-pyridyl)-S-triazine (TPTZ) was from Merck (Darmstadt, Germany). All other organic and non-organic reagents (of an analytical grade) were obtained from local commercial suppliers.
Preparation of Trifolium extracts
Phenolic fraction of each Trifolium species was isolated with the same method as previously described for Medicago sativa L. (Fabaceae) (Stochmal et al., Citation2001). Briefly, ground aerial parts of the plant material were extracted with 80% (v/v) methanol at room temperature for 24 h. After filtration, the extract was concentrated at 35 °C under reduced pressure. The crude extract was dissolved in distilled water and separated on a RP18 preparative column (60 × 100 mm, 40–60 µm, Merck). First, in order to remove carbohydrates, the column was washed with water and then phenolic fraction was eluted with a 40% (v/v) methanol.
Qualitative and quantitative analyses of the compounds in this fraction were done by using ultra performance liquid chromatography (UPLC) (solvent, 1% (v/v) acetic acid → 40% (v/v) acetonitrile over 10 min; column C18 50.0 × 2.1 mm, UPLC BEH; column temperature 50 °C; flow rate 0.35 ml/min). The content of the four groups of compounds (phenolic acids, flavonoids, isoflavones, and clovamides), determined in the examined phenolic fractions, is shown in (as mg per g of dry mass).
Table 1. Phytochemical composition of the investigated Trifolium extracts (phenolic fractions, prepared from aerial parts of six Trifolium species).
Isolation of blood plasma and preparation of samples
Blood samples from healthy volunteers were purchased from the Regional Centre of Blood Donation and Blood Treatment in Lodz, Poland. Plasma samples were preincubated for 10 min at 37 °C with extracts obtained from Trifolium species added to the final concentration range of 1.5–50 µg/ml, and then exposed to 200 µM peroxynitrite (ONOO−). Samples of plasma treated with peroxynitrite in the absence of Trifolium extracts were also prepared. Control samples were treated with neither the extracts nor peroxynitrite.
Determination of radical scavenging ability
Anti-free radical activities of six Trifolium extracts were assessed with two types of assays: reduction of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH•) and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic) acid radical (ABTS•) (Janaszewska & Bartosz, Citation2002). In experiments with DPPH• scavenging, a working reagent was 500 µM methanol solution of DPPH•. Stock solutions of the extracts were mixed with DPPH• solution, to obtain their final concentration range of 1.5–50 µg/ml. Similarly, for the estimation of ABTS• scavenging ability of the examined extracts, stock solutions of the extracts were added ABTS• solution, to obtain their final concentration range of 1.5–50 µg/ml in the reagent mixture. In order to visualize and compare the anti-free radical action of the individual extracts in full range of their concentrations, we started measurements at high absorbance values (over 1.0).
Determination of total antioxidant capacity (TAC) of plasma by the ABTS and DPPH assays
The ability to reduce ABTS• radical of the analyzed plasma samples was estimated according to the method of Re et al. (Citation1999). The 1,1-diphenyl-2-picrylhydrazyl radical (DPPH•) reduction assay, also applied for the evaluation of the total antioxidant capacity (non-enzymatic antioxidant capacity – NEAC) of blood plasma, was performed according to the protocol described by Janaszewska and Bartosz (Citation2002).
Ferric reducing ability of plasma
The determination of the antioxidant capacity of plasma with the use of the ferric reducing ability of plasma (FRAP) assay was carried out according to modified method of Benzie and Strain (Citation1996). Plasma samples were diluted 10 times, and then added to the reagent mixture in a volume ratio of 1:10:1:1, for plasma, acetate buffer (300 mM, pH 3.6), TPTZ (10 mM, in 0.04 M HCl), and FeCl3 (20 mM), respectively. This method was also used for the evaluation of ferric reducing ability of different concentrations of Trifolium extracts (1.5–50 µg/ml). In those assays, instead of blood plasma, solutions of the examined extracts were added to the reagent mixture in a volume ratio of 1:10:1:1. For the quantitative analysis of the obtained results (as equivalents of Fe2+), a standard curve ranging from 0 to 1.0 mM of FeSO4 was prepared.
Data analysis
In order to eliminate uncertain data, the Q-Dixon test was performed. The statistical significance of the observed differences was evaluated by the Kruskal–Wallis test (with the Conover–Inman correction). All the values were expressed as means ± SD.
Results
The UPLC analysis of the chemical profiles of the examined extracts (e.g., phenolic fractions) revealed the presence of three or four groups of phenolic substances (). The highest content of phenolics was found for T. alexandrinum (52.55 mg/g d.m.) and T. incarnatum (47.97 mg/g d.m.). In T. alexandrinum extract, the highest concentrations of isoflavones and clovamides were also detected (9.63 and 18.97 mg/g d.m., respectively), while T. incarnatum extract was particularly rich in flavonoids (41.54 mg/g d.m.). The phytochemical profile of other species (T. resupinatum var. resupinatum, T. resupinatum var. majus, T. hybridum, and T. fragiferum, ssp. bonanni) contained much lower concentrations of phenolic acids, flavonoids, and isoflavones. Besides T. alexandrinum extract, the presence of clovamides was found only in the phenolic fraction from T. hybridum ().
Measurements of free radical scavenging capacity of the extracts (DPPH• and ABTS• radical scavenging) provided some information on anti-free radical properties of six Trifolium extracts, and indicated on T. alexandrinum extract as the most effective free radical scavenger in these assays. The radical scavenging activities towards DPPH• and ABTS• radicals, recorded for the extracts of T. resupinatum var. resupinatum and T. resupinatum var. majus were also noticeably stronger, compared with the anti-free radical properties of other three extracts (T. incarnatum, T. hybridum, and T. fragiferum) (). Additionally, we evaluated the ferric reducing ability of all six clover extracts (), which confirmed our observations from determination of DPPH• and ABTS• radicals scavenging – extracts of T. alexandrinum, T. resupinatum var. resupinatum, and T. resupinatum var. majus displayed significantly stronger reducing effects.
Figure 1. Comparison of the reduction of DPPH• (panel A) and ABTS• (panel B) radicals by plant extracts, derived from six different Trifolium species. Free radical scavenging activity of the examined extracts was recorded as the absorbance (mOD) after 30 (for DPPH•) and 6 min (for ABTS•) of incubation. The figure is a graphical illustration of the observed effects and includes mean values of a measurement performed in triplicate.
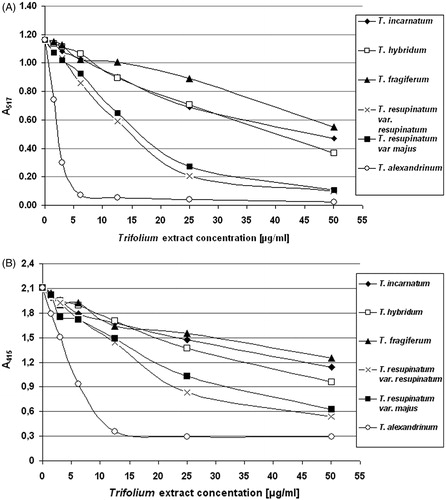
Table 2. Comparison of the ferric reducing ability of the examined Trifolium extracts. The ferric reducing ability of clover extracts was measured at the final concentration range of 1.5–50 µg/ml and expressed as mM of Fe2+. The table includes mean values ± SD of a measurement done in triplicate.
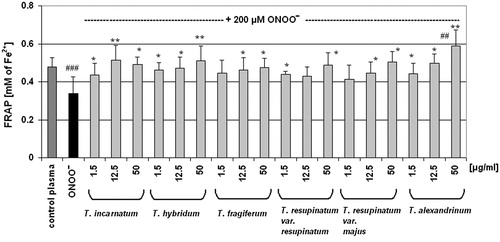
Experiments of the next stage of this work were designed to evaluate the antioxidant properties of Trifolium extracts in the protection of blood plasma under oxidative stress in vitro. The exposure of blood plasma to peroxynitrite resulted in a considerable decrease of its total antioxidant capacity, determined as reduction of ABTS•+ radical cation () or measured by DPPH• reduction (). The FRAP assay also was considerably diminished (). For the evaluation of the effects of Trifolium extracts on the total antioxidant capacity of blood plasma, we selected three concentrations (1.5, 12.5, and 50 µg/ml) from the concentration range of the examined extracts (1.5–50 µg/ml). In most cases, the pre-incubation of plasma samples with Trifolium extracts effectively diminished a harmful action of peroxynitrite. Analysis of the results from ABTS• and DPPH• reduction assays, as well as measurements of FRAP confirmed the protective action of Trifolium extracts on the antioxidant capacity of blood plasma under peroxynitrite-induced oxidative stress ().
Figure 2. Effect of Trifolium-derived extracts on total antioxidant capacity of blood plasma measured in the ABTS• assay. Antioxidant capacity of human plasma (control and exposed to 200 μM peroxynitrite) was determined spectrophotometrically by the ABTS• radical decolourization, and expressed as Trolox equivalents. Results are presented as means ± SD of five independent experiments: ###p < 0.001 for ONOO−-treated plasma (with or without extracts) versus control plasma, and *p < 0.05, **p < 0.01, ***p < 0.001 for plasma treated with ONOO− in the presence of extracts versus plasma treated with ONOO− in the absence of extracts.
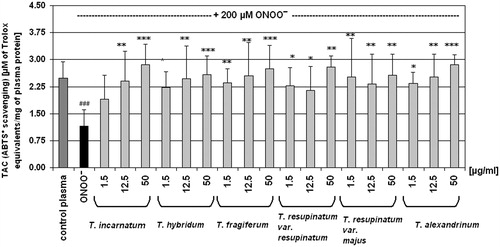
Figure 3. Effect of Trifolium-derived extracts on total antioxidant capacity of blood plasma determined in the DPPH• reduction assays. Antioxidant capacity of control samples and plasma exposed to 200 μM peroxynitrite was determined spectrophotometrically by the DPPH• radical reduction (decolourization), and expressed as Trolox equivalents. Results are presented as means ± SD of six independent experiments: ##p < 0.01 for plasma samples treated with ONOO− (in the presence or absence of the extracts) versus control plasma, and *p < 0.05, **p < 0.01 for plasma treated with ONOO− the presence of extracts versus plasma treated with ONOO− in the absence of the examined extracts.
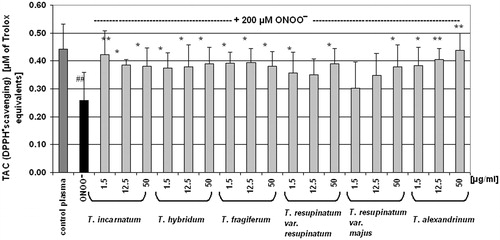
Figure 4. Effect of Trifolium-derived extracts on ferric reducing ability of plasma (the FRAP assay). The ability of blood plasma (control and exposed to 200 μM ONOO−) to reduce ferric ion to the ferrous ion was measured spectrophotometrically. Results (Fe2+ equivalents) are shown as means ± SD of six independent experiments: ##p < 0.01, ###p < 0.001 for ONOO−-treated plasma (with or without extracts) versus control plasma, and *p < 0.05, **p < 0.01 for plasma treated with ONOO− in the presence of extracts versus plasma treated with ONOO− in the absence of extracts.
Discussion
The phytochemical profile of Trifolium plants includes a wide range of polyphenolic compounds such as numerous flavonoids (including isoflavones), clovamides (caffeic acid esters), and other phenolic compounds (Oleszek & Stochmal, Citation2002; Oleszek et al., Citation2007; Sabudak & Guler, Citation2009), but individual species differ substantially in the content of these substances. In the present in vitro study, we assessed the free radical scavenging ability of the extracts prepared from the aerial parts of six clover species as well as their effects on the total antioxidant capacity of human plasma, under oxidative stress conditions. For the induction of oxidative stress, we used peroxynitrite (ONOO−), a highly reactive oxygen species, involved in the pathophysiology of various cardiovascular disorders, including atherosclerosis and cardiac reperfusion injury. Peroxynitrite, formed in a rapid reaction between superoxide anion () and nitric oxide (NO), is able to modify other molecules (proteins, lipids, and nucleic acids) by diverse mechanisms, including both direct reactions and damage induced by secondary radicals (Pacher et al., Citation2007). Because of the lack of specific enzymes responsible for deactivation of ONOO− in human tissues, scavenging of this molecule and reduction of its harmful effects depends mainly on endogenous and exogenous non-enzymatic antioxidants (Luo et al., 2010). It has been estimated that under in vitro conditions, the bolus addition of 0.25 mM ONOO− is equivalent to a steady-state level of 1 µM maintained for 7 min (Beckman et al., 1994). This range of ONOO− concentration is likely to occur at sites of inflammation, where production of rates of NO• and superoxide anion is considerably increased. Our previous experiments with various concentrations of peroxynitrite (1–1000 μM) demonstrated that it was able to modify the blood plasma components (proteins and lipids) at concentration much lower than 100 μM (Nowak & Wachowicz, Citation2001; Olas et al., Citation2004), but 200 μM ONOO− induces changes in plasma components at measurable level, allowing to estimate the antioxidant effects of the examined extracts.
Our measurements of free radical scavenging activity of the extracts (DPPH• and ABTS• radicals scavenging) indicated the T. alexandrinum extract was the most effective one in the assays. Experiments with the use of blood plasma confirmed antioxidant action of the examined extracts; however, the determination of influence of all the extracts on total antioxidant capacity and ferric reducing ability of blood plasma exposed to peroxynitrite did not reveal such evident differences between the examined extracts. This result may be an effect of a complexity of natural non-enzymatic antioxidant defense of blood plasma.
The observed antioxidant effect of Trifolium extracts may be attributed to scavenging of both peroxynitrite and radicals derived from its decomposition. The presence of hydroxyl groups in the structure of plant-derived phenolic and polyphenolic substances is critical for their antioxidant activity. For scavenging of peroxynitrite by flavonoids, a hydroxyl group at position 3 in the C-ring is of primary importance (Heijnen et al., Citation2001a); antioxidant activity of this group is enhanced by other –OH groups, present at positions 5 and 7, as well as by the double-bonded oxygen at position 4 and the ring oxygen at position 1 (Heijnen et al., Citation2001b). The antioxidant action of plant-derived phenolic acids, such as hydroxycinnamates, is also well known. Ferulic acid is able to deactivate hydroxyl radical and peroxynitrite, while chlorogenic and caffeic acid may scavenge peroxynitrite, organic free radicals, superoxide anion, hydroxyl, and peroxyl radicals (Shahidi & Chandrasekara, Citation2010).
Results of our experiments on anti-free radical properties do not reflect strictly the total content of phenolics in Trifolium extracts, but indicate a link between the chemical composition of the investigated species. Among the examined extracts, T. alexandrinum and T. incarnatum had the highest content of phenolic and polyphenolic substances, 52.55 and 47.97 mg/g of d.m., respectively. The DPPH• radical scavenging assay suggests the highest antiradical activity of T. alexandrinum extract, while the action of T. incarnatum was comparable with T. hybridum and T. fragiferum extracts. Results from the evaluation of the ferric reducing ability of the extracts were similar to those from the measurements of their anti-radical activity, including the strongest action of T. alexandrinum-derived extract. However, in our antioxidant assays, both T. alexandrinum and T. incarnatum seemed to be more effective than the other extracts. These effects of the examined clover extracts may be a consequence of diversity in phytochemical profile and differences in biochemical mechanisms of their protective action. Trifolium incarnatum extract was particularly rich in flavonoids (41.54 mg/g of d.m.), but T. alexandrinum contained isoflavones (18.97 mg/g of d.m.) and significant amounts (9.63 mg/g of d.m.) of clovamides. A considerable anti-free radical activity has been found for daidzein (Cai et al., Citation2012), genistein (Cai et al., Citation1997), and other isoflavones (Lengyel et al., Citation2013). The presence of clovamides (caffeic acid esters) in T. alexandrinum extract may additionally enhance its free radical scavenging action. According to Arlorio et al. (Citation2008), clovamide (N-caffeoyl-l-DOPA) possesses a high anti-radical activity (EC50: 9.238 ± 1.054 µM) measured as DPPH• radical scavenging. Ley and Bertram (Citation2003) reported that in the DPPH• and superoxide radical quencher assays, lipophilic clovamide derivatives might display radical scavenging activity equal to or higher than those of the standard antioxidants such as ascorbic acid and tocopherol. Furthermore, antioxidant properties of clovamide-rich T. pallidum extract was also demonstrated in our previous studies. This extract was able to significantly reduce oxidative stress-induced modifications of blood platelets and plasma in vitro, determined as the level of protein nitration and carbonylation, and well as peroxidation of blood platelets and plasma lipids (Kolodziejczyk et al., Citation2011).
Conclusions
The present study provides some new information on the biological activities of the extracts derived from six Trifolium species. Trifolium plants may be a rich source of bioactive substances with antioxidant properties, such as flavonoids (including isoflavones), phenolic acids, and clovamides. Our results indicate on a considerable anti-free radical and antioxidant effect of these plants that may be helpful in the protection of blood plasma components against oxidative stress-induced damage.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by Grants 506/1136 and 545/741 from University of Lodz (Lodz, Poland), as well as by statutory activities of Institute of Soil Science and Plant Cultivation – State Research Institute, (Pulawy, Poland).
Acknowledgements
Special thanks go to Michal B. Ponczek, Ph.D. and Michal Bijak, Ph.D. (Department of General Biochemistry, University of Lodz) for their assistance in statistical analysis.
References
- Arlorio M, Locatelli M, Travagilia F, et al. (2008). Roasting impact on the contents of clovamide (N-caffeoyl-l-DOPA) and the antioxidant activity of cocoa beans (Theobroma cacao L.). Food Chem 106:967–75
- Beckmann JS, Chen J, Ischiropoulos H, Crow JP. (1994). Oxidative chemistry of peroxynitrite. Methods Enzymol 233:229–40
- Benzie IFF, Strain JJ. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 239:70–6
- Cai Q, Rahn RO, Zhang R. (1997). Dietary flavonoids; quercetin; luteolin and genistein; reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Lett 119:99–107
- Cai Z, Zhang X, Lu DF, Gan JN. (2012). A novel chemiluminescence system for the determination of daidzein and its hydroxyl radical-scavenging capacity. Luminescence 27:256–61
- Engelmann NJ, Reppert A, Yousef G, et al. (2009). In vitro production of radiolabeled red clover (Trifolium pratense) isoflavones. Plant Cell Tiss Org Cult 98:147–56
- Fabricant DS, Farnsworth NR. (2001). The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109:69–75
- Heijnen CG, Haenen GR, van Acker FA, et al. (2001a). Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol in Vitro 15:3–6
- Heijnen CG, Haenen GR, Vekemans JA, Bast A. (2001b). Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ Toxicol Pharmacol 10:199–206
- Janaszewska A, Bartosz G. (2002). Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand J Clin Lab Invest 62:231–6
- Khan AV, Ahmed QU, Shukla I, Khan AA. (2012). Antibacterial activity of leaves extracts of Trifolium alexandrinum Linn. against pathogenic bacteria causing tropical diseases. Asian Pac J Trop Biomed 2:189–94
- Kolodziejczyk J, Olas B, Wachowicz B, et al. (2011). Clovamide-rich extract from Trifolium pallidum reduces oxidative stress-induced damage to blood platelets and plasma. J Physiol Biochem 67:391–9
- Kolodziejczyk-Czepas J. (2012). Trifolium species-derived substances and extracts – biological activity and prospects for medicinal applications. J Ethnopharmacol 143:14–23
- Kolodziejczyk-Czepas J, Wachowicz B, Moniuszko-Szajwaj B, et al. (2013a). Antioxidative effects of extracts from Trifolium species on blood platelets exposed to oxidative stress. J Physiol Biochem 69:879–87
- Kolodziejczyk-Czepas J, Olas B, Malinowska J, et al. (2013b). Trifolium pallidum and Trifolium scabrum extracts in the protection of human plasma components. J Thromb Thrombol 35:193–9
- Lengyel J, Rimarčík J, Vagánek A, Klein E. (2013). On the radical scavenging activity of isoflavones: Thermodynamics of O–H bond cleavage. Phys Chem Chem Phys 15:10895–903
- Ley JP, Bertram HJ. (2003). Synthesis of lipophilic clovamide derivatives and their antioxidative potential against lipid peroxidation. J Agric Food Chem 51:4596–602
- Luo Y, Pan J, Pan Y. (2010). Evaluation of the protective effects of Chinese herbs against biomolecule damage induced by peroxynitrite. Biosci Biotechnol Biochem 74:1350–4
- Mohamed KM, Hassanean HA, Ohtani K, et al. (2000). Chalcanol glucosides from seeds of Trifolium alexandrinum. Phytochemistry 53:401–4
- Nowak P, Wachowicz B. (2001). Studies on pig blood platelet responses to peroxynitrite action. Platelets 12:376–81
- Olas B, Nowak P, Kolodziejczyk J, Wachowicz B. (2004). The effects of antioxidants on peroxynitrite-induced changes in platelet proteins. Thromb Res 113:399–406
- Oleszek W, Stochmal A. (2002). Triterpene saponins and flavonoids in the seeds of Trifolium species. Phytochemistry 61:165–70
- Oleszek W, Stochmal A, Janda B. (2007). Concentration of isoflavones and other phenolics in the aerial parts of Trifolium species. J Agric Food Chem 55:8095–100
- Pacher P, Beckman JS, Liaudet L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424
- Pryor WA, Cueto R, Jin X, et al. (1991). A practical method for preparing peroxynitrite solutions of low ionic strength and free of hydrogen peroxide. Free Radic Biol Med 1:75–83
- Re R, Pellegrini N, Proteggente A, et al. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–7
- Renda G, Yalçın FN, Nemutlu E, et al. (2013). Comparative assessment of dermal wound healing potentials of various Trifolium L. extracts and determination of their isoflavone contents as potential active ingredients. J Ethnopharmacol 148:423–32
- Sabudak T, Dokmeci D, Ozyigit F, et al. (2008). Anti-inflammatory and antioxidant activities of Trifolium resupinatum var. microcephalum extracts. Asian J Chem 20:1491–6
- Sabudak T, Guler N. (2009). Trifolium L. – A review on its phytochemical and pharmacological profile. Phytother Res 23:439–46
- Shahidi F, Chandrasekara A. (2010). Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem Rev 9:147–70
- Stochmal A, Piacente S, Pizza C, et al. (2001). Alfalfa (Medicago sativa L.) flavonoids. Apigenin and luteolin glycosides from aerial parts. J Agric Food Chem 49:753–8
- Thompson Coon J, Pittler MH, Ernst E. (2007). Trifolium pratense isoflavones in the treatment of menopausal hot flushes: A systematic review and meta-analysis. Phytomedicine 14:153–9
