Abstract
Context: Despite its wide clinical use, cyclophosphamide (CP), an alkylating chemotherapeutic agent, possesses many adverse effects, including hepatotoxicity. Because Origanum vulgare L. (Lamiaceae) has antioxidative properties, it might protect against above-mentioned damage.
Objective: This study evaluated the protective effects of O. vulgare extract on CP-induced liver toxicity.
Materials and methods: Mice were pretreated with aerial parts of O. vulgare ethanolic extract (intraperitoneally) at doses of 50, 100, 200, and 400 mg/kg for 7 consecutive days before the administration of a single 200 mg/kg intraperitoneal dose of CP 1 h after the last injection of O. vulgare. After 24 h, animals were anesthetized, blood samples and hepatic tissues were collected and used for biochemical and histological examination.
Results: Serum levels of hepatic markers were increased after CP treatment but restored in the O. vulgare-pretreated groups. The serum ALT, AST, and ALP of the CP group were 196.49 ± 3.82, 143.78 ± 4.79, and 203.18 ± 3.81 IU/l, respectively. However, pretreatment with 400 mg/kg O. vulgare significantly decreased the serum ALT, AST, and ALP to 52.49 ± 2.18, 44.78 ± 2.06, and 65.62 ± 1.73 IU/l, respectively (p < 0.001). Histological examinations also confirmed the protective effects of O. vulgare against CP-induced liver toxicity.
Discussion and conclusion: Our results reveal that O. vulgare with high amount of flavonoids and phenolic compounds induces potent hepatoprotective mechanisms against CP. Therefore, O. vulgare might help defend the body against the side effects, particularly hepatic damages induced by chemotherapeutic agents.
Introduction
Cyclophosphamide [N,N-bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide; CP], an oxazophosphorine derivative of the classical alkylating agent nitrogen mustard, is commonly used as a cancer chemotherapeutic. This drug also has significant immunosuppressive activity and is used clinically to treat autoimmune diseases and aid in renal and bone marrow transplantations (Dollery, Citation1999). However, despite its wide clinical use, CP also possesses many adverse effects, including hepatotoxicity in humans and experimental animals (Capel et al., Citation1979; Fraiser et al., Citation1991). The precise mechanism by which CP causes hepatic injury is poorly understood. However, CP requires metabolic activation by the hepatic microsomal cytochrome P450 oxidase system for both its therapeutic and toxic actions (Dollery, Citation1999). Metabolic conversion of CP leads to the formation of two cytotoxic metabolites, phosphoramide mustard and acrolein. Phosphoramide mustard is believed to have antineoplastic activity, but acrolein, a highly reactive metabolite with a short biological half-life, may be responsible for CP-induced liver damage (Honjo et al., Citation1988). Recent studies suggest that CP generates reactive oxygen species (ROS), such as the superoxide anion, hydroxyl radical and hydrogen peroxide (H2O2), during its oxidative metabolism and suppresses the liver’s antioxidant defense mechanisms (Bhattacharya et al., Citation2003; Stankiewicz et al., Citation2002).
Due to CP-induced cytotoxicity, a compound capable of protecting healthy cells and tissues against CP metabolites, such as acrolein and free radicals, is needed. Various studies show that antioxidant intake can help control reactions to chemotherapy and also minimize the adverse side-effects of antineoplastic drugs (Weijl et al., Citation1997). Biological compounds with antioxidant properties may contribute to the protection of cells and tissues against the deleterious effects of ROS and other free radicals induced by CP (Manda & Bhatia, Citation2003; Navarova et al., Citation1999). Compounds that reduce these side effects and stimulate immunity could be of great help in improving cancer treatment.
Recently, researchers have become interested in potential compounds of plant origin that are capable of minimizing the adverse effects of chemotherapy on normal cells without compromising its antineoplastic activity (Pratheeshkumar & Kuttan, Citation2010). Origanum vulgare L. (Lamiaceae) (OV), also known as oregano, is a flavoring herb widely used around the world. Origanum vulgare is an aromatic plant with a wide distribution throughout Asia, particularly in Iran. It is used to cure respiratory diseases, hypoglycemic disease, and leukemia (Sheibani et al., 2010). The major aqueous constituents of oregano are rosmarinic acid, eriocitrin, luteolin-7-oglucoside, apigenin-7-O-glucoside, origanol A and B, and ursolic acid (Sheibani et al., 2010). Rosmarinic acid and origanol A and B, which are the most abundant components of the aqueous extract of oregano, have antioxidative activities (Kulisic et al., Citation2007; Matsuura et al., Citation2003). Previous studies have reported that the essential oil of O. vulgare has antioxidant capacity, which has been linked to components such as thymol, carvacrol, δ-terpinene, and p-cymene (Halici et al., Citation2005; Odabasoglu et al., Citation2004; Russo et al., Citation2002). In addition, there are many reports showing that components of the aqueous oregano extract, such as ursolic acid and rosmarinic acid, exert potent antioxidant activities by scavenging free radicals (Di Sotto et al., Citation2010; Lambert et al., Citation2001).
We recently reported that O. vulgare pretreatment attenuates radiation-induced oxidative stress and subsequent DNA damage in human blood lymphocytes. The protective effect of O. vulgare on DNA could be explained by its ability to increase the activity of the antioxidant defense system, scavenge ROS that induce lipid peroxidation as well as peroxidative damage, and quench free radicals induced by internal radiation (Arami et al., Citation2013).
Because oregano contains high levels of phenolic compounds and has antioxidative properties, it is likely that O. vulgare could protect against CP-induced hepatotoxicity. Because O. vulgare has been used extensively as an additive agent and is thus regarded as safe, this study was undertaken to assess the effects of O. vulgare on the liver toxicity induced by CP. Histological examination of liver tissues was also used to confirm the possible mitigating effects of O. vulgare extract against CP-induced hepatotoxicity.
Materials and methods
Preparation of extracts
The dried powder of extract in our study was obtained by the maceration method. In maceration (for fluid extract), whole or coarsely powdered plant-drug is kept in contact with the solvent in a stoppered container for a defined period with frequent agitation until soluble matter is dissolved (Ncube et al., Citation2008). Origanum vulgare dried aerial parts of the plant was obtained from the Giah Essence Phyto-Pharmaceutical Co., Golestan, Iran. Aliquots of 300 g dried aerial parts of plant were macerated with 3000 ml of ethanol (75%) as an extraction solvent for 72 h at room temperature and the resulting extract was filtered through a filter paper. After evaporating the solvent under a reduced vacuum at temperatures below 50 °C, 35 g of dried powder of extract was obtained.
Animals
For this randomized, controlled animal study, male Naval Medical Research Institute (NMRI) mice weighing 22 ± 3 g were obtained from the Pasteur Institute of Iran (Amol). All animals were housed at room temperature, 22 ± 2 °C, with a 12/12 h light/dark cycle. The animals were acclimatized for 1 week before the study, and standard laboratory chow and water were given. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the University. The study protocol was approved by the Research Committee of Mazandaran University of Medical Sciences, Sari, Iran.
Experimental O. vulgare treatment
Animals were divided into six groups (Groups 1–6, n = 5 for each group). For the negative control (Group 1), mice received distilled water (10 ml/kg) via intraperitoneal (i.p.) injection for 7 d. The positive control mice (Group 2) received a single toxic CP dose (200 mg/kg, i.p.) (Shokrzadeh et al., Citation2014a) in distilled water (10 ml/kg) at seventh day of the experiment. In Groups 3–6, mice received different doses of O. vulgare (50, 100, 200, or 400 mg/kg, i.p.) in distilled water (10 ml/kg) each day for 7 consecutive days, which was followed by a single i.p. CP dose 1 h after the last O. vulgare administration. After 24 h CP administration, mice were anesthetized with petroleum ether. Blood (1 ml) was removed via cardiac puncture, plasma was obtained from the blood and rapidly frozen at −80 °C for later plasma analyses, including measuring the serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) activities. After blood sampling, all animals were euthanized with an ether overdose. The livers were then removed, washed three times with normal saline for complete blood removal, and kept in 10% neutral buffered formalin for histopathological examinations.
Estimation of serum levels of hepatic markers
The extent of hepatic damage was assessed by measuring the levels of ALP, ALT, and AST in the circulation (Bhattacharjee & Sil, Citation2007). Serum levels of hepatic markers, including ALT, AST, and ALP, were evaluated based on our previous report (Ahmadi et al., Citation2011). To estimate the ALT and AST serum activities, commercially available enzymatic kits (based on the reaction of 2,4-dinitrophenylhydrazine with pyruvate and/or oxaloacetate to yield a brown-colored complex in alkaline medium) were used. Serum ALP activity was measured using a spectrophotometric method. The results are expressed as IU/l.
Histopathological analysis
The livers were fixed in 10% neutral buffered formalin, sliced transversely, paraffin embedded, and prepared as 5 µm thick sections that were stained with hematoxylin and eosin (H&E) for light microscopic evaluation. Three factors, hepatocellular necrosis, level of inflammation in the portal area and lymphocytic inflammatory infiltrations, were evaluated using a semi-quantitative method. The level of damage was recorded based on a grading system (0–4): grade 0 = no damage, 1 = very low levels of damage, 2 = mild damage, 3 = moderate damage, and 4 = severe damage. Slides were viewed, and images were acquired using a microscope-mounted camera (Labomed, LX400) at 400× magnification. At least three random visual fields from each animal were scored in a blinded manner by two expert pathologists.
Statistical analysis
The data are represented as the mean ± SD of five mice. A one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test were used to assess statistical significance. A p value of less than 0.05 was considered significant.
Results
Serum levels of hepatic markers
The effects of O. vulgare extract pretreatment on the serum ALT, AST, and ALP activities after CP administration are shown in . Serum ALT, AST, and ALP activities were increased in all CP-treated mice relative to the untreated control animals (p < 0.001). All doses of O. vulgare pretreatment mitigated CP-induced toxicity and were associated with decreased serum ALT, AST, and ALP activities. The serum ALT, AST, and ALP activities of the CP groups were 196.49 ± 3.82, 143.78 ± 4.79, and 203.18 ± 3.81 IU/l, respectively, while the control groups showed significantly lower serum ALT, AST, and ALP activities of 51.26 ± 1.37, 44.56 ± 2.23, and 64.21 ± 4.08 IU/l, respectively (p < 0.001). The maximum reduction in serum ALT, AST, and ALP activities was observed in mice that were pretreated with 400 mg/kg O. vulgare, which significantly decreased the serum ALT, AST, and ALP activities to 52.49 ± 2.18, 44.78 ± 2.06, and 65.62 ± 1.73 IU/l, respectively, compared with the only CP-treated group (p < 0.001). These results clearly show that oregano has a suppressive effect on CP-induced hepatotoxicity in mice and indicate that in a dose-dependent manner, O. vulgare pretreatment in mice prevented the elevation of serum ALT, AST, and ALP activities following CP administration.
Table 1. Effects of different doses of O. vulgare pretreatment on the serum levels of hepatic markers after CP administration.
Hepatic histopathological examination
Representative light photomicrograph of hepatic histological section 24 h after CP administration (200 mg/kg) is compared with histological sections from untreated and O. vulgare-pretreated mice (400 mg/kg for 7 consecutive days) in . shows a control mouse liver section demonstrating the normal histological structure of the hepatocytes, sinusoidal space, and central vein. shows a liver section of a CP-treated mouse displaying necrotic hepatocytes with small crushed nuclei, portal space with severe inflammation and hepatocytes surrounded by lymphocytic infiltration. shows liver section of an O. vulgare-pretreated (400 mg/kg) mouse that also received CP with a normal central vein, normal sinusoidal space, nearly normal hepatocytes, and small portal space with mild lymphocytic infiltration.
Figure 1. Normal group; a liver section of a mouse showing the normal histological structure of a hepatocyte (A; yellow arrow), sinusoidal space (B; red arrow), and central vein (C; white arrow).
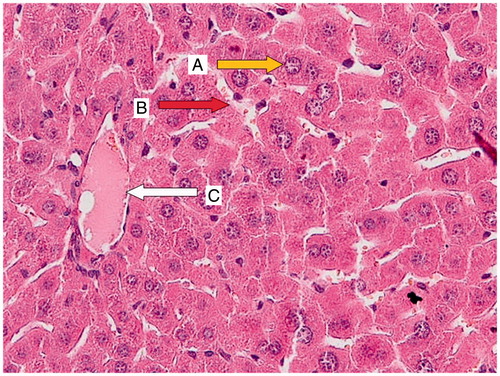
Figure 2. CP group (200 mg/kg); a liver section of a mouse showing necrotic hepatocytes with small crushed nuclei (A; yellow arrow), a portal space with severe inflammation (B; blue arrow), and hepatocytes surrounded by lymphocytic infiltration (C; green arrow).
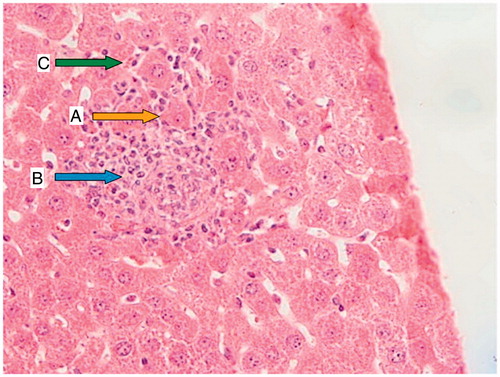
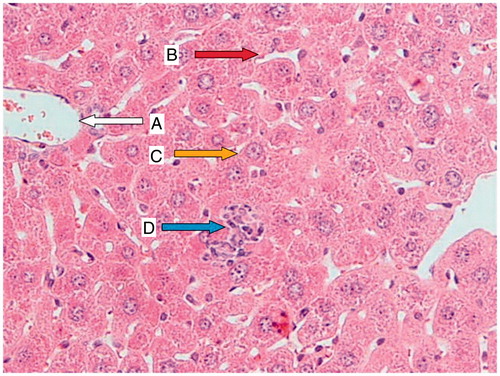
Figure 3. Origanum vulgare-pretreated animals (400 mg/kg) for 7 d before CP administration; a liver section of a mouse showing a normal central vein (A; white arrow), normal sinusoidal space (B; red arrow), nearly normal hepatocyte (C; yellow arrow), and small portal space with mild lymphocytic infiltration (D; blue arrow).
The semi-quantitative hepatic histopathological examination results are also summarized in . Briefly, the graded damage for all three factors was 0 and 4 for the non-treated control animals and single CP dose (200 mg/kg) animals, respectively, and these results indicate severe liver damage 24 h after CP administration. Origanum vulgare pretreatment for 7 consecutive days reduced the level of liver tissue damage in a dose dependent manner. These data support the serum toxicity marker enzyme results.
Table 2. The results of semi-quantitative hepatic histopathological examination.
Discussion
CP is effective against a wide spectrum of malignancies, such as leukemia and lymphoma and breast, lung, prostate, and ovarian cancers (Khan et al., Citation2004). The parent compound is inactive in vivo and in vitro and exerts its biological activities through metabolites, mainly phosphoramide mustard, in hepatic microsomal enzymes (McDonald et al., Citation2003). Normal tissue injury or damage is the major limitation of CP use, which gives rise to numerous side effects, and CP treatment also results in the production of ROS, which cause peroxidative damage to vital organs (Patel, Citation1987). In our recent study, CP administration induced testicular toxicity and oxidative stress in the testis of male mice (Chabra et al., Citation2014).
The hepatotoxicity of CP was observed as the therapeutic dose increased. Phosphoramide mustard and acrolein are two active CP metabolites produced by liver microsomal enzymes (De Jonge et al., Citation2005). CP's antineoplastic effects are associated with phosphoramide mustard, while acrolein is linked with its toxic side effects (Kern & Kehrer, Citation2002). To avoid these toxic side effects, a number of antioxidant agents could detoxify acrolein. Cellular mechanisms of toxicity are mediated by an increase in the free radicals through intracellular phosphoramide mustard and acrolein, the principle alkylating metabolites of CP (Tripathi & Jena, Citation2008). In the present study, CP administration damaged the liver, and this observation is consistent with the previous reports (Elkiran et al., Citation2007; Selvakumar et al., Citation2005). CP administration under different conditions and doses has been demonstrated to be an excellent model to produce the syndromes of both oxidative stress and hepatic damage (Mathew & Kuttan, Citation1997; Stankiewicz et al., Citation2002; Sulkowska et al., Citation1999). Hepatic dysfunction was the most common regimen-related toxicity reported in patients treated with CP and total body irradiation (McDonald & Frieze, Citation2008). Hepatic tissues were the primary sites for the microsomal activation of drugs. Hepatic activation of CP leading to the formation of toxic metabolites caused liver damage as measured by increased liver enzymes in serum.
Antioxidant compounds may contribute to the protection of cells and tissues against the deleterious effects of ROS and other free radicals induced by CP (Manda & Bhatia, Citation2003; Navarova et al., Citation1999). A large body of research is now focused on the antioxidant action of natural substances. Recently, the search of potential compounds of plant origin that are capable of minimizing chemotherapeutic toxicity to normal cells without affecting their antineoplastic activity has increased (Pratheeshkumar & Kuttan, Citation2010). In our recent study, O. vulgare had dose-dependent protective effects on lipid peroxidation induced by CP in lung tissues of mice. Administration of O. vulgare extract to mice for 7 consecutive days prior to injection of CP rescued the levels of several biomarkers associated with oxidative stress in mice lung tissue. Origanum vulgare extract also showed potent radical scavenging properties (Shokrzadeh et al., Citation2014b). In the present study, a single CP dose (200 mg/kg) resulted in a significant increase in the activities of serum ALT, AST, and ALP in mice. However, pretreatment with Oregano significantly lowered the serum levels of hepatic markers, and the levels were comparable with the control animal values. The restoration of the levels of these marker in animals pretreated with O. vulgare indicates the protective activity of O. vulgare in the liver. This protective effect might be from O. vulgare’s scavenging activity for the toxic metabolites produced during CP activation by liver microsomal enzymes. These data suggest a hepatoprotective role of O. vulgare.
In the current study, histopathological examination proved that CP causes liver damage as evidenced by the observation of necrotic hepatocytes with small crushed nuclei, a portal space with severe inflammation, and hepatocytes surrounded by lymphocytic infiltration. These observations might be caused by the membrane damaging potential of the CP's metabolites. These pathological changes correlated well with the altered enzyme activities, and these findings are supported by another previous study (Senthilkumar et al., Citation2006). Origanum vulgare pretreatment effectively alleviated the CP-induced hepatic histopathological changes; abnormal pathological findings, such as tissue injury and necrosis; and protected tissues from oxidative damage. These histopathological observations suggest that Oregano could protect tissues from damage and thus decrease the leakage of enzymes (AST, ALT, and ALP) into the circulation.
Conclusion
In our study, O. vulgare showed dose-dependent protective effects and reduced CP-induced hepatoxicity in mice. Administration of O. vulgare extract to mice for 7 consecutive days prior to CP injection attenuated the serum levels of hepatic markers. Histopathological examinations also confirmed the protective effects of Oregano against CP-induced liver damages. CP-induced tissue damage might be alleviated by antioxidant activity, free radical scavenging, increased activity of the antioxidant defense systems, and membrane stabilizing properties of O. vulgare. Because O. vulgare has been used extensively as an additive therapy and herbal medicine for several diseases, it could be a potential candidate for a safe supplemental agent against the side effects of chemotherapy.
Declaration of interest
The authors declare no conflict of interest. This study was supported by a grant from the Student Research Committee of the Mazandaran University of Medical Sciences, Sari, Iran.
References
- Ahmadi A, Ebrahimzadeh MA, Ahmad-Ashrafi S, et al. (2011). Hepatoprotective, antinociceptive and antioxidant activities of cimetidine, ranitidine and famotidine as histamine H2 receptor antagonists. Fundam Clin Pharmacol 25:72–9
- Arami S, Ahmadi A, Haeri SA. (2013). The radioprotective effects of Origanum vulgare extract against genotoxicity induced by (131)I in human blood lymphocyte. Cancer Biother Radiopharm 28:201–6
- Bhattacharjee R, Sil PC. (2007). Protein isolate from the herb Phyllanthus niruri modulates carbon tetrachloride-induced cytotoxicity in hepatocytes. Toxicol Mech Methods 17:41–7
- Bhattacharya A, Lawrence RA, Krishnan A, et al. (2003). Effect of dietary n-3 and n-6 oils with and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice. J Am Coll Nutr 22:388–99
- Capel ID, Jenner M, Dorrell HM, Williams DC. (1979). Hepatic function assessed (in rats) during chemotherapy with some anti-cancer drugs. Clin Chem 25:1381–3
- Chabra A, Shokrzadeh M, Naghshvar F, et al. (2014). Melatonin ameliorates oxidative stress and reproductive toxicity induced by cyclophosphamide in male mice. Hum Exp Toxicol 33:185–95
- De Jonge ME, Huitema AD, Holtkamp MJ, et al. (2005). Aprepitant inhibits cyclophosphamide bioactivation and thiotepa metabolism. Cancer Chemother Pharmacol 56:370–8
- Di Sotto A, Mazzanti G, Carbone F, et al. (2010). Inhibition by beta-caryophyllene of ethyl methanesulfonate-induced clastogenicity in cultured human lymphocytes. Mutat Res 699:23–8
- Dollery C. (1999). Therapeutic Drugs. Edinburgh: Churchill Livingstone
- Elkiran T, Harputluoglu H, Yasar U, et al. (2007). Differential alteration of drug-metabolizing enzyme activities after cyclophosphamide/adriamycin administration in breast cancer patients. Methods Find Exp Clin Pharmacol 29:27–32
- Fraiser LH, Kanekal S, Kehrer JP. (1991). Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs 42:781–95
- Halici M, Odabasoglu F, Suleyman H, et al. (2005). Effects of water extract of Usnea longissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine 12:656–62
- Honjo I, Suou T, Hirayama C. (1988). Hepatotoxicity of cyclophosphamide in man: Pharmacokinetic analysis. Res Commun Chem Pathol Pharmacol 61:149–65
- Kern JC, Kehrer JP. (2002). Acrolein-induced cell death: A caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact 139:79–95
- Khan TS, Sundin A, Juhlin C, et al. (2004). Vincristine, cisplatin, teniposide, and cyclophosphamide combination in the treatment of recurrent or metastatic adrenocortical cancer. Med Oncol 21:167–77
- Kulisic T, Krisko A, Dragovic-Uzelac V, et al. (2007). The effects of essential oils and aqueous tea infusions of Oregano (Origanum vulgare L. spp. hirtum), thyme (Thymus vulgaris L.) and wild thyme (Thymus serpyllum L.) on the copper-induced oxidation of human low-density lipoproteins. Int J Food Sci Nutr 58:87–93
- Lambert RJ, Skandamis PN, Coote PJ, Nychas GJ. (2001). A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91:453–62
- Manda K, Bhatia AL. (2003). Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol Toxicol 19:367–72
- Mathew S, Kuttan G. (1997). Antioxidant activity of Tinospora cordifolia and its usefulness in the amelioration of cyclophosphamide induced toxicity. J Exp Clin Cancer Res 16:407–11
- Matsuura H, Chiji H, Asakawa C, et al. (2003). DPPH radical scavengers from dried leaves of oregano (Origanum vulgare). Biosci Biotechnol Biochem 67:2311–16
- McDonald GB, Frieze D. (2008). A problem-oriented approach to liver disease in oncology patients. Gut 57:987–1003
- McDonald GB, Slattery JT, Bouvier ME, et al. (2003). Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood 101:2043–8
- Navarova J, Ujhazy E, Dubovicky M. (1999). Protective effect of the antioxidant stobadine against cyclophosphamide and irradiation induced oxidative stress. Gen Physiol Biophys 18 Spec No:112–19
- Ncube NS, Afolayan AJ, Okoh AI. (2008). Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. African J Biotech 7:1797–806
- Odabasoglu F, Aslan A, Cakir A, et al. (2004). Comparison of antioxidant activity and phenolic content of three lichen species. Phytother Res 18:938–41
- Patel JM. (1987). Stimulation of cyclophosphamide-induced pulmonary microsomal lipid peroxidation by oxygen. Toxicology 45:79–91
- Pratheeshkumar P, Kuttan G. (2010). Ameliorative action of Vernonia cinerea L. on cyclophosphamide-induced immunosuppression and oxidative stress in mice. Inflammopharmacology 18:197–207
- Russo A, Bonina F, Acquaviva R, et al. (2002). Red Orange extract: Effect on DNA cleavage. J Food Sci 67:2814–18
- Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P. (2005). Mitigation of oxidative stress in cyclophosphamide-challenged hepatic tissue by dl-α-lipoic acid. Mol Cell Biochem 272:179–85
- Senthilkumar S, Devaki T, Manohar BM, Babu MS. (2006). Effect of squalene on cyclophosphamide-induced toxicity. Clin Chim Acta 364:335–42
- Sheibani V, Hajializadeh Z, Afarinesh M. (2010). Evaluation of Origanum vulgare L. ssp. viridis leaves extract effect on discrimination learning and LTP induction in the CA1 region of the rat hippocampus. Iran J Basic Med Sci 14:161–9
- Shokrzadeh M, Chabra A, Ahmadi A, et al. (2014a). Hepatoprotective effects of Zataria multiflora ethanolic extract on liver toxicity induced by cyclophosphamide in mice. Drug Res [Epub ahead of print]. doi:10.1055/s-0034-1370932
- Shokrzadeh M, Ahmadi A, Chabra A, et al. (2014b). An ethanolic extract of Origanum vulgare attenuates cyclophosphamide-induced pulmonary injury and oxidative lung damage in mice. Pharm Biol [Epub ahead of print]. doi:10.3109/13880209.2013.879908
- Stankiewicz A, Skrzydlewska E, Makiela M. (2002). Effects of amifostine on liver oxidative stress caused by cyclophosphamide administration to rats. Drug Metabol Drug Interact 19:67–82
- Sulkowska M, Sulkowski S, Skrzydlewska E. (1999). The effect of pentoxifylline on ultrastructure and antioxidant potential during cyclophosphamide-induced liver injury. J Submicrosc Cytol Pathol 31:413–22
- Tripathi DN, Jena GB. (2008). Ebselen attenuates cyclophosphamide-induced oxidative stress and DNA damage in mice. Free Radic Res 42:966–77
- Weijl NI, Cleton FJ, Osanto S. (1997). Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev 23:209–40
