Abstract
Context: Delonix elata (L.) Gamble (Fabaceae) has been used in the Indian traditional medicine system to treat rheumatism and inflammation.
Aim: To assess the anti-inflammatory effect of Delonix elata flowers and to isolate the active principle.
Materials and methods: The prompt anti-inflammatory constituent was isolated from Delonix elata flower extracts using bioassay guided fractionation in liposaccharide (LPS) stimulated RAW 264.7 macrophage cell line. The anti-inflammatory activity of extracts/fractions/sub-fractions/compounds (10, 25, and 50 µg/ml) was evaluated by estimating the levels of nitric oxide (NO), TNF-α, and IL-1β after 24 h of LPS induction (1 μg/ml). The isolated active compound was subjected to NMR, IR, and UV analyses for structure determination.
Results: In an attempt to search for anti-inflammatory constituents, the active pure principle was isolated and crystallized as a white compound from Delonix elata flowers methanol extract. This active compound (50 µg/ml) decreased the release of inflammatory mediators levels such as NO (0.263 ± 0.03 µM), TNFα (160.20 ± 17.57 pg/ml), and IL-1β (285.79 ± 15.16 pg/ml) significantly (p < 0.05); when compared to the levels of NO (0.774 ± 0.08 µM), TNFα (501.71 ± 25.14 pg/ml), and IL-1β (712.68 ± 52.25 pg/ml) from LPS-stimulated macrophage cells. The active compound was confirmed as hesperidin with NMR, IR, and UV spectroscopy data. This is the first report of this compound from Delonix elata flowers.
Conclusion: The findings of the study support the traditional use of Delonix elata flowers to treat inflammation.
Introduction
The immune system plays a defense role in our body through adaptive immune response (highly specific) and innate immune response (non-specific) against foreign agents. But the undesired immune response causes inflammation/tissue injury. Inflammation is an essential aspect of host response to infection and injury. But excessive or aberrant inflammation proceeds to a chronic state, associated with undecorated human diseases (Serhan & Savill, Citation2005).
Macrophages and neutrophils are the primary phagocytic cells of the innate immune system among several types of immune cells. Macrophages are potent secretory cells that release a variety of inflammatory mediators, including pro-inflammatory and cytotoxic cytokines, chemokines, proteolytic enzymes, and reactive oxygen/nitrogen species, all of which have been implicated in the pathogenesis of tissue injury (Jung et al., Citation2010). Mainly, it secretes nitric oxide (NO), TNF-α, IL-1β, and IL-6, which in turn participate in the mediation of acute phase responses to injury (Jung et al., Citation2010). TNF-α itself can stimulate several functional cells to produce other pro-inflammatory mediators including NO, IL-1, and IL-6 (Chandel et al., Citation2000). IL-1β is able to activate macrophages to induce the production of cytokines, reactive oxygen intermediates, and prostaglandin. NO plays a pivotal role on cell survival and death, and shows various pro-inflammatory effects on many cell types (Garcia & Stein, Citation2006). Clinically, non-steroidal anti-inflammatory drugs are available to control inflammatory diseases, but these drugs show side effects that led us to screen for a lead compound/molecule from natural products without any side effects.
Delonix elata (L.) Gamble (Fabaceae) is a medium-sized tree found in East Africa, Southern Arabia, and to western India. This tree is a traditional medicinal plant that is rich in polyphenols (Hegazi, Citation2011); leaves and flowers of this tree are bipinnate and yellowish-white in color, respectively. This plant has been recommended by the traditional healers of South India (Mutheeswaran et al., Citation2011) and also in other regions for the treatment of rheumatic pain and inflammation (Ahluwalia, Citation1968; Deshmukh et al., Citation1999; Hegazi, Citation2011; Kapoor & Kapoor, Citation1980; Rao et al., Citation1997; Samvatsar & Diwanji, Citation1999). Mainly the flowers and leaves (Sethuraman & Sulochana, Citation1986) of this plant are used for the treatment of rheumatic pain and inflammation. The whole plant extract (excluding root) showed antibacterial, antifungal, antiviral, anticancer (Atal et al., Citation1978), and insecticidal activity (Bhakuni et al., Citation1969). Preliminary studies have shown the anti-inflammatory effect of this plant (Pradeepa et al., Citation2012; Sethuraman & Sulochana, Citation1986). Despite these reports, no studies have been made to identify the active constituents present in this plant.
The present study was designed to evaluate the traditional claim of Delonix elata flowers and aimed at bioassay-guided isolation of an anti-inflammatory compound from this plant.
Materials and methods
Collection of plant materials
The flowers of Delonix elata were collected during the months of January–March 2012 from Arakkonam, Vellore District, Tamil Nadu, India, and authenticated by Dr. P. Pandikumar, taxonomist, Entomology Research Institute, Loyola College, Chennai. The voucher specimens (ERI-ICMR-ART-01) were deposited at the herbarium of Entomology Research Institute, Loyola College, Chennai, for future use.
Extraction
The Delonix elata flowers, shade-dried, coarsely powdered, was used for extraction. Dry powder of flowers (2 kg) were taken in an aspirator bottle separately and soaked with methanol (6 l) for extraction. This mixture was shaken occasionally for 48 h at 30 °C. Then the extract was filtered before drying using Whatmann filter paper No. 2 on a Buchner funnel and the solvent was removed by vacuum distillation in a rotary evaporator at 40 °C. This procedure was repeated three-times and all the obtained extracts were combined and placed in glass bottles and refrigerated until use.
Fractionation of flower methanol extract
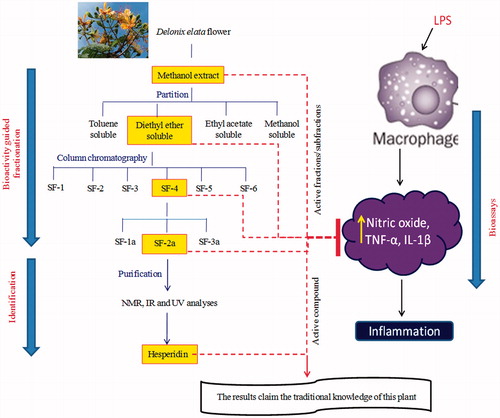
The flower methanol extract (50 g) was mixed in water and partitioned successively in toluene, diethylether, and ethyl acetate (150 ml × 3 times). The yield of the fractions was toluene soluble (5 g), diethylether (4 g), ethyl acetate (7.5 g), and methanol (15 g). The collected fractions were bioassayed in liposaccharide (LPS) stimulated RAW 264.7 macrophage cell line by estimating the levels of NO, TNF-α, and IL-1β. The active diethylether soluble fractions were then taken for further fractionations. The diethylether soluble part (3 g) was packed in a silica gel column (Qualigens, 60–120 mesh, 100 g) with hexane and eluted with 100% hexane, 100% benzene, 100% chloroform, and then with the mixture of chloroform and methanol in the ratios of 95:5, 9:1, 85:15, 4:1, 7:3, 2:3, 1:1, 1:3, and 0:1 (2 l each). All the fractions were then bioassayed and the active fractions which were eluted between 95:5 and 9:1 (chloroform in methanol; 1 g) were combined for further purifications (SF-4). This combined fraction, that contained three spots in TLC, was further re-chromatographed in a silica gel column and sub-fractionated into three fractions with 7.5, 10, and 12.5% (chloroform in methanol) and also bioassayed. Further purification was carried on the active sub-fraction (10% chloroform in methanol; 500 mg) (SF-2a) that showed single spot in TLC (chloroform [7.5]:methanol [2]) and again the activity was confirmed. The compound was subjected to NMR, IR, and UV analyses. The fractionation of flowers of Delonix elata is shown in .
Cell culture
RAW 264.7 macrophage cells were incubated in tissue culture flasks containing Dulbecco’s minimal eagle’s medium (DMEM), fetal bovine serum (FBS), streptomycin, and penicillin (100 U/l) in a humidified atmosphere containing 5% CO2 at 37 °C for confluence. After discarding the media, 1 ml of trypsin (0.25%) was added and kept in the incubator for 5 min. The flask was then gently tapped to detach the cells and then the flask was completely rinsed by adding 3 ml of DMEM-FBS media and the contents were centrifuged at 1000 rpm for 10 min. After discarding the supernatant, the cell pellet was dissolved in DMEM-FBS media. The viability of the trypsinised (trypsin–EDTA) RAW 264.7 cells was checked after staining with 0.4% trypan blue staining solution (1:4) diluted in phosphate buffered saline (PBS) and the cells were counted using Neubauer chamber and then diluted based on requirement.
Bioassay setup
RAW 264.7 cells were seeded in 96-well plates and were pre-incubated with the extract/fractions/compound of Delonix elata flower samples at different concentrations (10, 25, and 50 µg/ml; dissolved in 5% DMSO in serum-free DMEM medium), 30 min before stimulation with 1 μg/ml of LPS. The setup was incubated for 24 h at 37 °C in a humidified atmosphere containing 5% CO2. The LPS (1 μg/ml) stimulated cells were served as inflammation control; unstimulated cells were served as a negative control.
MTT assay
RAW 264.7 cells were plated at 2×103 cells/200 µl of medium and incubated in 96-well plates for the cell proliferation assay. The cells were treated with extract/fractions/compound in various concentrations (10–1000 µg/ml) to assess the toxicity of the compound in the absence of LPS. After 24 h of treatment, MTT solution (5 mg/ml PBS; 20 µl/well) (Sigma Aldrich, New Delhi, India) was added. After 4 h of incubation at 37 °C in a humidified 5% CO2 atmosphere, the absorbance of 570 nm was measured using an ELISA microplate reader (Yang et al., Citation2008).
NO production
The macrophage cells were plated 4 × 105 cells/ml of medium and incubated in 24-well plates for NO production. The cells were treated with extract/fractions/compound in various concentrations (10, 25, and 50 µg/ml) with or without LPS stimulation. After incubation (24 h), the level of NO production was monitored by measuring the nitrite concentration in the supernatant of cultured medium using the Griess reagent (Ignacio et al., Citation2001).
Estimation of cytokines
The macrophage cells were plated 106 cells/ml and cultured for cytokine quantification. The supernatants were collected by centrifugation at 2500 rpm for 20 min at 18 °C after 24 h, and assayed for IL-1β and TNF-α using ELISA kits (eBiosciences, San Diego CA) according to the manufacturer’s instructions. LPS-treated cells were considered as a positive control and the cells without LPS treatment were considered as a negative control.
Instrumentation for the identification of the active compound
IR spectra were recorded on a Perkin–Elmer FT-IR grating spectrometer (PerkinElmer, Inc, Akron, OH) in KBr disc. 1H and 13C NMR were recorded in D2O at 400 and 100 MHz in a Bruker instrument, respectively. Tetramethylsilane (TMS) was used as the internal standard. Electron ionization mass spectra (EI-MS) was analyzed on a Schimadzu instrument (Shimadzu Scientific Instruments, Columbia, MD) at 70 eV by the direct inlet method. Chemical shifts are given in δ-scale.
Statistics
Data obtained within the groups were expressed as mean ± SEM for triplicate independent experiments. The statistical significance of the data was analyzed with a one-way ANOVA followed by Tukey’s HSD using SPSS program (Version 11.5, SPSS Inc., Chicago, IL) and the significance level was set at p ≤ 0.05.
Results
Effect of extract/fractions/active compound isolated from Delonix elata flowers on cell viability
In bioassay-guided fractionation of Delonix elata flowers, the active extract/fractions/sub-fractions/active compound were assessed for the toxicity using MTT assay on RAW 264.7 cell line in the absence of LPS. The treatment with extract/fractions/sub-fractions/compound in various concentrations (10–1000 µg/ml) did not affect the viability of the cells. Thus the dosage of active fractions/sub-fractions/compound was fixed between 10 and 50 µg/ml concentrations for further studies.
Bioassay guided fractionation of Delonix elata flowers in LPS-induced cells
Effect of Delonix elata flower methanol extract
The levels of NO (0.756 ± 0.01 µM), TNFα (419.95 ± 21.46 pg/ml), and IL-1β (775.62 ± 36.81 pg/ml) were elevated highly on LPS-stimulated cells, when compared with unstimulated cells. The treatment with Delonix elata flower methanol extract significantly (p ≤ 0.05) reduced the levels of these markers in a dose-dependent manner. The maximum inhibition of NO (0.319 ± 0.02 µM), TNFα (179.45 ± 18.55 pg/ml), and IL-6 (376.06 ± 10.07 pg/ml) levels were observed on 50 µg/ml of Delonix elata methanol extract ().
Figure 2. Effect of Delonix elata flower methanol extract on LPS-induced RAW 264.7 cell line. (a) NO; (b) TNF-α and IL-1β; levels of LPS-induced RAW 264.7 cell lines. Values are mean ± SEM for triplicate independent experiments; values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05).
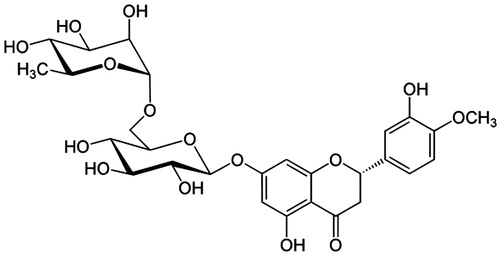
Effect of diethylether (ET) soluble fractions of Delonix elata flower methanol
In LPS-stimulated cells, the levels of NO (0.774 ± 0.03 µM), TNFα (430.84 ± 20.21 pg/ml), and IL-1β (756.68 ± 38.75 pg/ml) were highly elevated when compared with unstimulated cells. The treatment with ET soluble portion of Delonix elata flower methanol extract significantly (p ≤ 0.05) reduced the levels of these markers in a dose-dependent manner. There was a decrease in the levels of NO (0.303 ± 0.01 µM), TNFα (144.31 ± 10.45 pg/ml), and IL-1β (292.96 ± 17.57 pg/ml) at 50 µg/ml concentration of ET soluble portion ().
Table 1. Effect of toluene (TO), diethylether (ET), ethyl acetate (EA), and methanol (MeOH) soluble fractions of Delonix elata flower methanol extract on LPS-induced RAW 264.7 cell line.
Effect of sub-fractions from the ET soluble fractions of Delonix elata flower methanol extract
The release of NO (0.776 ± 0.03 µM), TNFα (464.45 ± 23.90 pg/ml), and IL-1β (752.30 ± 39.15 pg/ml) levels were elevated highly on LPS-stimulated cells, when compared with unstimulated cells. The treatment with 95:5 and 9:1% of chloroform in methanol (SF-4) of ET soluble portion of Delonix elata flower methanol extract significantly (p ≤ 0.05) reduced the levels of these markers in a dose-dependent manner. The maximum inhibition of NO (0.323 ± 0.01 µM), TNFα (126.42 ± 11.43 pg/ml), and IL-6 (318.08 ± 18.56 pg/ml) levels were observed on 50 µg/ml of SF-4 ().
Table 2. Effect of sub-fractions from the diethylether soluble fractions of Delonix elata flower methanol extract on LPS-induced RAW 264.7 cell line.
Effect of sub-fractions from SF-4 of the ET soluble fractions of Delonix elata flower methanol extract
depicts the results of treatment with SF-2a of SF-4 of the ET soluble portion of Delonix elata flower methanol extract. It significantly (p ≤ 0.05) reduced the levels of pro-inflammatory markers in a dose-dependent manner. In LPS-stimulated cells, the levels of NO (0.766 ± 0.03 µM), TNFα (474.50 ± 20.11 pg/ml), and IL-1β (715.49 ± 51.98 pg/ml) were highly elevated when compared with unstimulated cells. There was a decrease in the levels of NO (0.313 ± 0.02 µM), TNFα (120.15 ± 14.88 pg/ml), and IL-1β (334.00 ± 24.99 pg/ml) at 50 µg/ml concentration of SF-2a ().
Table 3. Effect of sub-fractions from SF-4 of the diethylether soluble fractions of Delonix elata flower methanol extract on LPS-induced RAW 264.7 cell line.
Effect of active compound isolated from Delonix elata flowers in LPS-induced cells
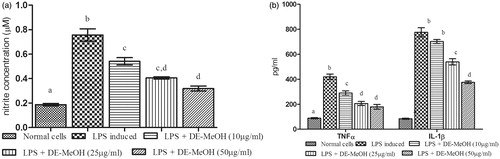
The active compound was isolated from the SF-2a portion. depicts the results of treatment with the active compound from Delonix elata flower. It significantly (p ≤ 0.05) reduced the pro-inflammatory markers in a dose-dependent manner. In LPS-stimulated cells, the levels of NO (0.774 ± 0.08 µM), TNFα (501.71 ± 25.14 pg/ml), and IL-1β (712.68 ± 52.25 pg/ml) were highly elevated when compared with unstimulated cells. The results showed to be decreased the levels of NO (0.263 ± 0.03 µM), TNFα (160.20 ± 17.57 pg/ml), and IL-1β (285.79 ± 15.16 pg/ml) at 50 µg/ml concentration of active compound ().
Figure 3. Effect of active compound (hesperidin) isolated from Delonix elata flowers on LPS-induced RAW 264.7 cell line. (a) NO; (b) TNF-α and IL-1β; levels of LPS-induced RAW 264.7 cell lines. Values are mean ± SEM for triplicate individual experiments; values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05).
Structure determination of active compound - hesperidin
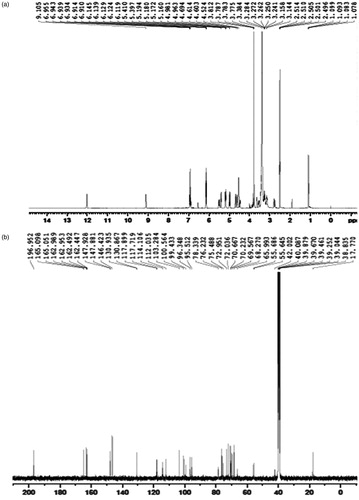
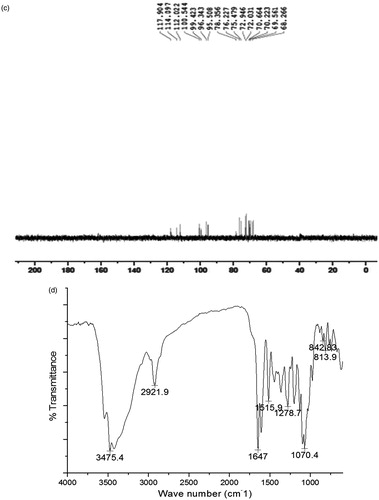
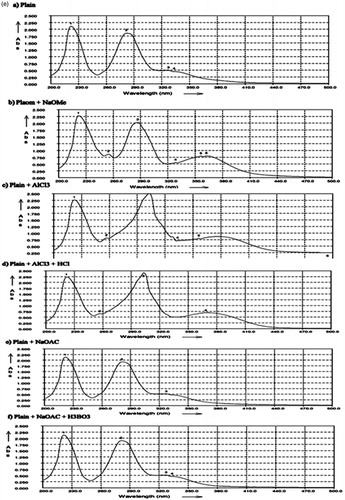
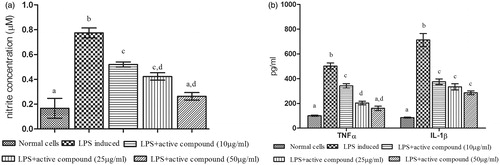
The active compound was found as white crystals from methanol containing melting point 272 °C along with molecular weight as 610 (C28H34O15). IR νmax (KBr) cm−1 showed peaks at 3475 (hydroxyl), 2922, 2842, 1647 (carbonyl), 1610, 1515, 843, 814, 760 (aromatic), 1070 (C–O–H bending), 1040, 1278. UV λmax (MeOH) nm showed the peaks at 282 and 327. In AlCl3 + HCl analysis, the peaks were observed at 303 and 369 and also in NaOMe analysis, the peaks were observed at 287 and 357. 1H NMR (DMSO-d6, 400 MHz) showed 6.94 (1 h, d, J = 2.0 Hz, H-2); 6.92 (2H, M, H-5, and H-6), 6.12 (1H, d, J = 2.4 Hz, H-6), 6.14 (1H, d, J = 2.4 Hz, H-8), 3.78 (3H, S, 4′-OMe), 5.50 (1H, d, J = 8.0 and 3.2 Hz, H-2), 2.78 (1H, dd, J = 17.6 and 3.2 Hz, H-3ax), H-3eq (merged with the solvent peak), 4.97 (1H, d, J = 7.2 Hz, H-1″), 4.60 (1H, d, J = 3.2 Hz, H-1″′), 1.08 (3H, d, J = 6.8 Hz, H-6″′). 13C NMR (δ, DMSO-d6, 100 MHz) showed 78.36 (C-2), 42.00 (C-3), 196.95 (C-4), 162.99 (C-5), 96.35 (C-6), 165.18 (C-7), 95.51 (C-8), 162.45 (C-9), 103.23 (C-10), 130.87 (C-1′), 114.10 (C-2′), 146.42 (C-3′), 147.93 (C-4′), 112.04 (C-5′), 117.90 (C-6′), 99.42 (C-1″), 72.23 (C-2″), 75.48 (C-3″), 72.03 (C-4″), 76.23 (C-5″), 65.99 (C-6″), 100.54 (C-1″′), 69.56 (C-2″′), 70.66 (C-3″′), 72.95 (C-4″′), 68.27 (C-5″′), 17.77 (C-6″′). The NMR, IR, and UV () analysis confirmed the compound to be hesperidin () (Lin et al., Citation2007). The spectroscopy data were comparable with those of hesperidin reported in the literature with 2S configuration (Inove et al., Citation2010; Maltese et al., Citation2009). This is the first report of this compound from this plant.
Figure 4. The spectroscopic data of hesperidin of the flowers methanol extract of Delonix elata. (a) 1H NMR spectrum, (b) 13C NMR spectrum, (c) DEPT NMR spectrum, (d) IR spectrum, and (e) UV spectrum.
Discussion
Macrophages are the first line of host defense against inflammation and play an important role in innate and adaptive immune responses (Verstovsek et al., Citation1992). Overproduction of the inflammatory mediators by activated macrophages has been implicated in the pathophysiology of many inflammatory diseases (Saravanan et al., Citation2012). The regulation of undesired immune response is necessary for the prevention of many severe diseases development from acute to chronic stage. Non-steroidal anti-inflammatory drugs are clinically common as an inhibitor of NF-kB-dependent inflammatory genes of TNF-α and IL-1β (Lee & Burckart, Citation1998); but these drugs carry the risk of gastrointestinal toxicity thus, their use is limited (Huh et al., Citation2011). Nowadays, the discovery of many pharmacological new drugs contains a natural products or natural product analogs. Many medicinal plants provide relief comparable with that of conventional therapies in the treatment of diseases (Verpoorte, Citation1999) and also they are excellent sources of lead compounds, in the search of new drugs.
In the present study, the bioassay-guided fractionation of Delonix elata flowers methanol extract was carried out for anti-inflammatory activity using the RAW 264.7 cell line. Delonix elata flowers were strategically fractionated and evaluated for their impact on NO, TNF-α, and IL-1β levels in RAW 264.7 cell lines. Lipopolysaccharide (LPS), a constituent of the outer membrane of Gram-negative bacteria, is an endotoxin (Guha & Mackman, Citation2001) and it stimulates innate immunity by regulating the production of inflammatory mediators like NO, TNF-α, IL-6, prostanoids, and leukotrienes (Opal, Citation2007). LPS was used to activate macrophages for the screening of anti-inflammatory agents from Delonix elata in this study.
NO plays a role in the regulation on blood vessel tone, inflammation, mitochondrial functions, and apoptosis (Beltran et al., Citation2002). In LPS-stimulated cells, the levels of NO was significantly increased; but the active extract/fractions/sub-fractions from Delonix elata flowers suppressed the release of NO levels due its anti-inflammatory property. TNF-α is an autocrine stimulator as well as a potent paracrine inducer of other inflammatory cytokines (Butler et al., Citation1995); but the levels of TNF-α was ameliorated by the treatment of active extracts/fractions/sub-fractions. Controlled levels of IL-1β were observed by the treatment of Delonix elata active extract/fractions/sub-fractions; that also revealed that the activation of macrophages was significantly ameliorated by the treatment. The active fractions/sub-fractions which showed maximum ameliorative effects on pro-inflammatory markers was selected for further bioassays. Finally, in an attempt to search for anti-inflammatory constituents, the active subfraction was purified and a pure compound was obtained.
The pure compound was crystallized as a white compound from methanol (m.p. 272 °C) and it was confirmed as hesperidin based on 1H and 13C NMR. It was analyzed as C28H34O15. It gave brown color with alcoholic ferric chloride and reddish pink color with Mg/HCl. It gave a single spot on TLC over silica gel (Rf: 0.41) with chloroform:methanol (7.5:2) as the developing system. UV spectrum showed peaks at 282 with shoulder at 327. The peaks were shifted to 303 and 369 with AlCl3 + HCl showed chelated hydroxyl at C-5. The peaks were shifted to 287 and 357, thereby showed the absence of 4′OH. The IR spectrum showed peaks at 3475 (hydroxyl), 1647 (carbonyl), 1610, 1515, 843, 814, and 760 (aromatic). The large peak at 1070 was due to C–O–H bending. 1H NMR also confirmed the structure to be hesperidin. The two meta-coupled protons H-6 and H-8 in ring A appeared at δ 6.12 and 6.14 each as a doublet (J = 2.4 Hz). H-2 appeared as the double doublet at δ 5.50 (J = 8 and 3.2 Hz) showing 2 S configuration. Accordingly H-3ax appeared as one-proton double doublet at δ 2.78 (J = 17.6 and 3.2 Hz). H-3eq was merged with the solvent peak. H-2′ in ring B appeared as a meta-coupled proton (J = 2 Hz) at δ 6.94. H-5′ and H-6′ were found as a two-proton multiplet at δ 6.92. H-1″ of glucose appeared a doublet (J = 7.2 Hz) at δ 4.97. H-1″′ of rhamnose appeared as a one-proton doublet (J = 3.2 Hz) at δ 4.60. The rhamnose methyl H-6″′ appeared as a three-proton doublet (J = 6.8 Hz) at δ 1.08. The 13C NMR also confirmed the compound to be hesperidin. The above physical and spectroscopy data were comparable with those of hesperidin reported in the literature with 2S configuration (Inove et al., Citation2010; Maltese et al., Citation2009).
Hesperidin, a bioflavonoid, is an abundant and inexpensive by-product of citrus species; highly present in sweet orange and lemon (Garg et al., Citation2001). It has been reported recently for significant anti-inflammatory (Coelho et al., Citation2013), anti-arthritic (Li et al., Citation2008; Umar et al., Citation2013), analgesic (Emim et al., Citation1994), immunomodulatory (Kawaguchi et al., Citation1999), wound healing (Hasanoglu et al., Citation2001), antiallergic, and anti-anaphylactic activities (Kubo & Matsuda, Citation1992). In this study, bioactivity-guided fractionation of Delonix elata flowers led to hesperidin as a major anti-inflammatory constituent. The presence of herperidin in Delonix elata flowers might play a significant role in the modulation of inflammatory markers such as NO, TNF-α, and IL-1β on LPS-induced RAW 264.7 cell line. Thus, this study has confirmed the traditional claim of Delonix elata flowers to treat inflammation with the presence of hesperidin.
Conclusion
In conclusion, the traditional knowledge on the use of Delonix elata was scientifically validated for its anti-inflammatory properties. The isolated active fractions/sub-fractions/compound from Delonix elata flowers significantly attenuated the release of NO, TNFα, and IL-1β levels in LPS-induced RAW 264.7 macrophage cell line. The NMR, IR, and UV spectroscopy data confirmed the compound to be hesperidin with a 2S configuration. This is the first report of this compound from Delonix elata flowers. These findings support the traditional use of Delonix elata flowers for future biomedical studies.
Acknowledgements
The authors are thankful to Mr. S. Mutheeswaran, Entomology Research Institute, Loyola College, Chennai for his help in plant collection.
Declaration of interest
The authors report no conflict of interest. The authors are thankful to Indian Council of Medical Research (Ministry of Health and Family Welfare), New Delhi, for financial support (Sanction number: 59/33/2009/BMS/TRM).
References
- Ahluwalia KS. (1968). Medicinal plants of Kerala-IV. Nagarjun 11:300–3
- Atal CK, Srinivastava JB, Wali BK, et al. (1978). Screening of Indian plants for biological activity: Part. VIII. Indian J Exp Biol 16:330–49
- Beltran B, Quintero M, Gracia-Zaragoza E, et al. (2002). Inhibition of mitochondrial respiration by endogenous nitric oxide: A critical step in Fas signaling. PNAS 13:8892–7
- Bhakuni DS, Dhar ML, Dhar MM, et al. (1969). Screening of Indian plants for biological activity: Part II. Indian J Exp Biol 7:250–62
- Butler DM, Maini RN, Feldmann M, Brennan FM. (1995). Modulation of pro-inflammatory cytokine release in rheumatoid synovial membrane cell cultures: Comparison of monoclonal anti TNF-a antibody with interleukin- 1 receptor antagonist. Eur Cytokine Netw 6:225–30
- Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. (2000). Role of oxidants in NF–κB activation and TNF-α genetranscription induced by hypoxia and endotoxin. J Immunol 165:1013–21
- Coelho RC, Hermsdorff HH, Bressan J. (2013). Anti-inflammatory properties of orange juice: Possible favorable molecular and metabolic effects. Plant Food Hum Nutr 68:1–10
- Deshmukh VR, Muratkar GD, Rothe SP. (1999). Preliminary observation on the medicinal and economically important leguminous plant species from Amravati tehsil. J Econ Taxon Bot 23:283–9
- Emim JADS, Oliveira AB, Lapa AJ. (1994). Pharmacological evaluation of the anti-inflammatory activity of a Citrus bioflavonoid, hesperidin and the isoflavanoids duartin and claussequione, in rats and mice. J Pharm Pharmacol 46:118–22
- Garcia X, Stein F. (2006). Nitric oxide. Semin Pediatr Infect Dis 17:55–7
- Garg A, Garg S, Zaneveld LJD, Singla AK. (2001). Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res 15:655–69
- Guha M, Mackman N. (2001). LPS induction of gene expression in human monocytes. Cell Signal 13:85–94
- Hasanoglu A, Ara C, Ozen S, et al. (2001). Efficacy of micronized flavonoid fraction in healing of clean and infected wounds. Int J Angiol 10:41–4
- Hegazi GAEM. (2011). In vitro studies on Delonix elata L., an endangered medicinal plant. World Appl Sci J 14:679–86
- Huh JE, Hong JM, Baek YH, et al. (2011). Anti-inflammatory and anti-nociceptive effect of Betula platyphylla var. japonica in human interleukin-1β-stimulated fibroblast-like synoviocytes and in experimental animal models. J Ethnopharmacol 135:126–34
- Ignacio SRN, Ferreira JLP, Almeida MB, Kubelka CF. (2001). Nitric oxide production by murine peritoneal macrophages in vitro and in vivo treated with Phyllanthus tenellus extracts. J Ethnopharmacol 74:181–7
- Inove T, Tsubakai S, Ogawa K, et al. (2010). Isolation of hesperidin from peels of thinned citrus unshiu fruits by microwave-assisted extraction. Food Chem 123:542–7
- Jung HW, Mahesh R, Park JH, et al. (2010). Bisabolangelone isolated from Ostericum koreanum inhibits the production of inflammatory mediators by down-regulation of NF-kappaB and ERK MAPkinase activity in LPS stimulated RAW 264.7 cells. Int Immunopharmacol 10:155–62
- Kapoor SL, Kapoor LD. (1980). Medicinal plant wealth of the Karimnagar district of Andhra Pradesh. Bull Med Ethnobot Res 1:120–44
- Kawaguchi K, Kikuchi S, Takayanagi K, et al. (1999). Colony stimulating factor inducing activity of hesperidin. Planta Med 65:365–6
- Kubo M, Matsuda H. (1992). Anti-allergic agents containing hesperidin. Patent Japan Kokai Tokkyo Koha, 64295, 428
- Lee JI, Burckart GJ. (1998). Nuclear factor kappa B: Important transcription factor and therapeutic target. J Clin Pharmacol 38:981–93
- Li R, Li J, Cai L, et al. (2008). Suppression of adjuvant arthritis by hesperidin in rats and its mechanisms. J Pharm Pharmacol 60:221–8
- Lin A-S, Shibano M, Nakagawa-Goto K, et al. (2007). Cancer preventive agents. 7. Antitumor-promoting effects of seven active flavonolignans from Milk Thistle (Silybum marianum) on Epstein–Barr virus activation. Pharm Biol 45:735–8
- Maltese F, Erkelens C, Kooy FVD, et al. (2009). Identification of natural epimeric flavone glycosides by NMR spectroscopy. Food Chem 116:575–9
- Mutheeswaran S, Pandikumar P, Chellappandian M, Ignacimuthu S. (2011). Documentation and quantitative analysis of the local knowledge on medicinal plants among traditional Siddha healers in Virudhunagar district of Tamil Nadu, India. J Ethnopharmacol 137:523–33
- Opal SM. (2007). The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int J Med Microbiol 297:365–77
- Pradeepa K, Krishna V, Girish KK, et al. (2012). Antinociceptive activity of Delonix elata leaf extract. Asian Pac J Trop Biomed 2:S229–31
- Rao RVK, Rao GP, Rao BG. (1997). Anti-inflammatory activity of the leaves and bark of Delonix elata. Anc Sci Life 17:141–3
- Samvatsar S, Diwanji VR. (1999). Plants used for rheumatism by the tribals of Western M.P. J Econ Taxon Bot 23:305–14
- Saravanan S, Prakash Babu N, Pandikumar P, et al. (2012). Immunomodulatory potential of Enicostema axillare (Lam.) A. Raynal, a traditional medicinal plant. J Ethnopharmacol 140:239–46
- Serhan CN, Savill J. (2005). Resolution of inflammation: The beginning programs the end. Nat Immunol 6:1191–7
- Sethuraman MG, Sulochana N. (1986). The antiinflamatory activity of Delonix elata Gamble. Curr Sci 55:343–4
- Umar S, Kumar A, Sajad M, et al. (2013). Hesperidin inhibits collagen-induced arthritis possibly through suppression of free radical load and reduction in neutrophil activation and infiltration. Rheumatol Int 33:657–63
- Verpoorte R. (1999). Exploration of nature’s chemodiversity: The role of secondary metabolites as leads in drug development. Drug Disc Today 3:232–8
- Verstovsek S, Maccubbin D, Mihich E. (1992). Tumoricidal activation of murine resident peritoneal macrophage by interleukin-2 and tumor necrosis factor. Cancer Res 52:3880–5
- Yang XY, Liu CH, Liang X, Sun J. (2008). Effects of mineralization liquid on rat’s osteoblast proliferation and differentiation. Hua Xi Kou Qiang Yi Xue Za Zhi 26:656–9