Abstract
Context: Murraya paniculata (Linn) JACK (Rutaceae) is used in traditional medicine in the treatment of diabetes, inflammation, and microbial disorders.
Objective: This study determined the polyphenol composition and antimicrobial and acute toxicological activity of the hydroethanolic extract of M. paniculata leaves (EEMp).
Materials and methods: Chemical composition was evaluated by the Folin–Ciocalteu and AlCl3 assays and by HPLC-DAD. Antibacterial and modulatory activity was determined by the microdilution method. Toxicity was assessed with a single dose of EEMp administered orally at doses of 2000 and 5000 mg/kg body weight/day in male and female Swiss mice.
Results: Total phenolic content of the EEMp samples varied from 66.5 to 396.8 mg gallic acid equivalent/g of extract and flavonoid content varied from 0.3 to 31.1 mg quercetin equivalent/g of extract. The principal component identified by HPLC-DAD assay was ellagic acid. The results of oral acute toxicity showed no mortality, changes in hematological parameters, or CNS and ANS toxicities in rats. Biochemical analysis showed a significant increase in glucose and glutamic oxaloacetic transaminase activity and reduction in triglycerides and cholesterol for 5000 and 2000 mg/kg doses, respectively, when compared with the control group. Histopathological evaluation showed no significant microscopic changes. EEMp showed essentially no antimicrobial activity, but when aminoglycosides were combined with EEMp their MIC was reduced.
Conclusions: Significant effects were observed in the acute toxicity assay, but they had no clinical relevance. The results suggest that M. paniculata could be used as a source of natural products with antibacterial resistance-modifying activity, with lower toxicity.
Introduction
The widespread use of antibiotics in human and veterinary medicine increases antibiotic resistance. A patient infected with a drug-resistant strain of Escherichia coli or Staphylococcus aureus, for example, as opposed to a drug-sensitive organism, is more likely to require hospitalization and have a longer hospital stay and an increased risk of death. The evaluation of a new antimicrobial agent is of importance for new therapeutic strategies. Many constituents of natural origin, such as plant extracts, are still used in modern medicines to treat a wide variety of infectious diseases such as septicemia (Iwu et al., Citation1999; Saklani & Kutty, Citation2008). There is growing interest in the pharmacological evaluation of various plants used against bacterial resistance to antibiotics (Araruna et al., Citation2013; Oliveira et al., Citation2011).
Murraya paniculata (Linn) Jack and Murraya koenigi (L) Spreng (Rutaceae) are the most popular flavor plants in the genus Murraya and possess potential bioactivities. Murraya paniculata is native to and common throughout much of India, Burma, and Malacca and dry areas of Ceylon, and it is often grown in Thailand, Cambodia, South Vietnam, and East Africa (Parrotta, Citation2001). They are armed shrubs or small trees, and have 3- to 7-foliolate leaves and corymbose, fragrant inflorescences. Leaves of the genus Murraya have been commonly used as flavoring agents in Indian curry preparations since ancient times. On phytochemical investigation, researchers have reported that Murraya leaves contain flavonoids (Zhang et al., Citation2011, Citation2012), cinnamates, and coumarins (Aziz et al., Citation2010; Ito et al., Citation2005; Kinoshita & Shimada, Citation2002; Saied et al., Citation2008; Shabbir et al., Citation1997), alkaloids (Kong et al., Citation2007), and volatile oils (Chowdhury et al., Citation2008). These leaves have been found to be useful for hepatoprotective (Sathaye et al., Citation2011), antidiabetic (Arulselvan et al., Citation2006; Tembhurne & Sakarkar, Citation2009, Citation2010b), antimicrobial (Gautam et al., Citation2012b; Sundaram, Citation2011), anti-inflammatory (Gupta et al., Citation2010; Mathur et al., Citation2011), wound healing (Patidar et al., Citation2010), and antidiarrheal (Mandal et al., Citation2010) purposes.
The aim of this study was to evaluate the hydroalcoholic extract of plant leaves of M. paniculata for polyphenol and flavonoid content, oral acute toxicity, antibacterial, and antibacterial resistance-modifying activity against some human pathogens.
Materials and methods
Chemicals, instrumentation, and general procedures
All chemicals were of analytical grade. Methanol, acetic acid, gallic acid, caffeic acid, chlorogenic acid, and ellagic acid were purchased from Merck (Darmstadt, Germany). Quercetin, quercitrin, rutin, kaempferol, catechin, and epicatechin were acquired from Sigma Chemical Co. (St. Louis, MO). High performance liquid chromatography (HPLC-DAD) was performed with a Shimadzu Prominence Autosampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector, and LC solution 1.22 SP1 software (Shimadzu, Kyoto, Japan). Biochemicaal assays were performed using Labtest® biochemical kits (Shimadzu, Kyoto, Japan) for glucose, urea, creatinine, glutamic oxaloacetic transaminase (SGOT), glutamic pyruvic transaminase (SGPT), cholesterol, and triglycerides.
Bacterial strains
The bacterial strains used were Escherichia coli ATCC25922 (EC25922), Proteus vulgaris ATCC13315 (PV13315), Pseudomonas aeruginosa ATCC15442 (PA15442), Shiguella flexneri ATCC12022 (SF12022), Staphylococcus aureus ATCC12692 (SA12692), and resistant E. coli (EC27) and S. aureus (SA358), both clinical isolates with resistance profile identified and shown in and . All strains were maintained on slants of heart infusion agar (HIA, Difco Laboratories Ltd., Sparks, MD). Before the tests, the cells were grown overnight at 37 °C in brain heart infusion (BHI, Difco Laboratories Ltd, Sparks, MD).
Table 1. Antibiotic resistance profile of clinically isolated bacterial strains used.
Table 2. MIC values (μg/mL) of aminoglycosides in the absence and presence of 64 μg/mL EEMp.
Plant material
Leaves of M. paniculata were collected in Barbalha city in April 2011 in Ceara State, Brazil. The plant material was identified by Dr. Maria Arlene Pessoa da Silva and a voucher specimen (number 4301) was deposited in Dardano Andrade Lima Herbarium of the Regional University of Cariri, Crato, Brazil.
Preparation and standardization of plant extract
Leaves were submersed in ethanol–water (1:1 v/v) for 72 h; afterwards, the extract was filtered and concentrated using a rotary vacuum evaporator (model Q-344B – Quimis, Brazil) and hot water bath (model Q-214M2 – Quimis, Brazil) at 60 °C, with a yield of 7.6%. The crude extract was lyophilized and the dry power was used for experimental procedures.
Phytochemical analysis
Determination of total phenolic content
Total phenolic concentration in different extracts was determined by the spectrophotometric method using the Folin–Ciocalteau reagent. Briefly, 5 mL of distilled water, 0.5–1.0 mL of sample, and 1.0 mL of the Folin–Ciocalteau reagent were added to a 25 mL flask. Next 10 mL of 7% sodium carbonate solution was added followed by distilled water. The solution was allowed to stand at room temperature for 15 min, and the absorbance at 750 nm then recorded. Total phenolic content was standardized against gallic acid and expressed as mg gallic acid equivalents (GAE)/L. The linearity range for this assay was 0.5–5.0 mg GAE/L (R2 = 0.999), giving an absorbance range of 0.050–0.555 absorbance units.
Determination of total flavonoid content
Total flavonoid content was determined spectrophotometrically using aluminum chloride (2%). Absorbance was recorded at 415 nm after 10 min against a blank sample consisting of sample (5 mL) and methanol (5 mL) without aluminum chloride. The total flavonoid content was determined from a standard curve of quercetin at 0–50 µg/mL. The average of three different readings was used and then expressed in µg quercetin equivalent flavones per mg extract.
HPLC-DAD analysis
Reverse phase chromatographic analyses were carried out under gradient conditions using a C18 column (4.6 mm × 250 mm) packed with 5 μm diameter particles; the mobile phase consisted of water containing 2% acetic acid (A) and methanol (B), and the composition gradient was 5% (B) for 2 min, 25% (B) until 10 min, and 40, 50, 60, 70, and 80% (B) every 10 min, following the method described by Sabir et al. (Citation2012) with slight modifications. The extract of M. paniculata and mobile phase were filtered through a 0.45 μm membrane filter (Millipore, Billerica, CA) and then degassed in an ultrasonic bath prior to use. The extracts of M. paniculata were analyzed at a concentration of 15 mg/mL. The flow rate was 0.7 mL/min and the injection volume was 40 μL. The sample and mobile phase were filtered through a 0.45 μm membrane filter (Millipore) and then degassed in an ultrasonic bath prior to use. Stock solutions of standards references were prepared in the HPLC mobile phase at a concentration of 0.050–0.250 mg/mL catechin, epicatechin, quercetin, quercitrin, kaempferol, and rutin, and 0.020–0.200 mg/mL for gallic, chlorogenic, caffeic, and ellagic acids. Quantification was carried out by the integration of the peaks using the external standard method, at 257 nm for gallic acid; 280 nm for catechin and epicatechin; 325 nm for chlorogenic, ellagic, and caffeic acids; and 365 for quercetin, quercitrin, kaempferol, and rutin. The chromatographic peaks were confirmed by comparing its retention time with that of reference standards and by DAD spectra (200–600 nm). The calibration curves were as follows: gallic acid, Y = 12 540x + 1198.5 (r = 0.9997); chlorogenic acid: Y = 13 057x + 1356.1 (r = 0.9999); caffeic acid, Y = 12 965x + 1288.4 (r = 0.9991); ellagic acid, Y = 15 173x + 1185.5 (r = 0.9994); catechin, Y = 13 542x + 1267.3 (r = 0.9996); epicatechin, Y =14 258x + 1529.7 (r = 0.9991); rutin, Y = 14 756x +1198.7 (r = 0.9993); quercetin, Y = 15 071x + 1241.6 (r = 0.9985); quercitrin, Y = 11 691x + 1353.0 (r = 0.9989); and kaempferol, Y = 13 077x + 1241.9 (r = 0.9998). All chromatography operations were carried out at ambient temperature and in triplicate.
Limit of detection and limit of quantification
Limit of detection (LOD) and limit of quantification (LOQ) were calculated on the basis of the standard deviation of the responses and the slope using three independent analytical curves, as defined by Boligon et al. (Citation2012). LOD and LOQ were calculated as 3.3 and 10σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve.
Antimicrobial assay
Antimicrobial activity test
The antibacterial activity of the extracts was investigated by the microdilution method, as recommended by NCCLS (Citation2005), using the two strains E. coli (EC27) and S. aureus (SA358). Inocula of each bacterial strain were suspended in brain heart infusion broth (3.8% BHI) and used for bacterial growth (24 h, 35 ± 2 °C). Afterwards, the suspension was diluted to 1 × 106 CFU/mL in 10% BHI. A 100 μL aliquot of each dilution was distributed in 96-well plates plus extracts at different concentrations, resulting in 5 × 105 CFU/mL as the final bacterial concentration. The extracts were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 1024 μg/mL. Subsequently, serial dilutions were made to obtain a final concentration in the range of 512–518 μg/mL. All experiments were performed in triplicate, and the microdilution plates were incubated at 35 ± 2 °C for 24 h. For the evaluation of antibiotic resistance-modifying activity, the MICs of the antibiotics were determined at a sub-inhibitory concentration (MIC/8) in the presence and absence of the extract. The clinical bacterial isolates EC27 and SA358 were assayed with four different aminoglycosides at final concentrations of 2500–1 μg/mL. Antibacterial activity was detected using a colorimetric method by adding 25 μL of aqueous resazurin dye (0.01%) to each well at the end of the incubation period. The minimal inhibitory concentration (MIC) was defined as the lowest at which the extracts were able to inhibit bacterial growth, as indicated by resazurin color change.
Evaluation of acute toxicity
Experimental animals
Seven-week-old male and female Wistar rats, weighing 200–220 g were obtained from the Faculty of Medicine of Juazeiro do Norte. Animals were randomly assigned to control and treatment groups (5 rats/sex group). They were housed under standard environmental conditions of temperature at 24 ± 1 °C under a 12 h dark-light cycle, and were allowed free access to drinking water and standard pellet diet (PURINA, Brasil). Rats were kept in an experimental facility for 1 week to allow them to be acclimated prior to dosing. The Animal Ethics Committee of the Regional University of Cariri, Brazil, approved all experimental protocols (No. 0015/2011).
Administration
Animals were deprived of food but not water 16–18 h prior to dosing on day 0. According to the Organization of Economic Cooperation and Development guidelines for testing of chemicals 420 (OECD, 2001) with adaptations, doses of 5000 and 2000 mg/kg extract were given orally to test groups of rats, while the control group received water in the same volume by gavage using a ball-tipped stainless steel feeding needle.
Observation of toxicity signs
Body weight, signs of toxicity (general behavior, respiratory pattern, cardiovascular signs, motor activities, reflexes, and change in skin and fur), and mortality were observed after the administration at the first, second, fourth, and sixth hour and once daily for next 14 d. In all these experiments, animals were observed for 14 d for any signs of morbidity or mortality. On the 15th day, all rats were fasted overnight and then anesthetized with thiopental sodium (50 mg/kg). Rats were sacrificed for biochemical assays and necropsy. The internal organs were excised and weighed.
Hematological and biochemical analysis
Hematological analyses were determined immediately after collection. Parameters included the following: red blood cell (RBC) count, hemoglobin (Hb), hematocrit (Hct), platelet count, and differential leukocyte count (lymphocytes, monocytes, neutrophils, eosinophils, and basophils). For biochemical analysis, blood was centrifuged at 1480 × g for 10 min to obtain serum, which was stored at −20 °C. The following parameters were determined: glucose, urea, creatinine, SGOT, SGPT, cholesterol, and triglycerides. Assays were carried out using a BT-1007 (Biotecnica®/BR) semi-automation using Labtest® and Biotecnica® kits for biochemical analysis (Biotecnica, Varginha, Brazil).
Statistical analysis
Results were expressed as mean ± standard error of mean (S.E.M.). Statistical significance was determined by a two-way analysis of variance (ANOVA) followed by Bonferroni's post hoc multiple comparisons test, and p values less than 0.05 were considered significant.
Results
Phytochemical analysis
Total phenolic content of the EEMp samples (based on the Folin–Ciocalteu method) varied from 66.5 to 396.8 mg gallic acid equivalent/g of extract and flavonoid content (based on the colorimetric AlCl3 method) varied from 0.3 to 31.1 mg quercetin equivalent/g of extract. The HPLC profile of M. paniculata extract was also acquired, and HPLC analysis is shown in . The samples of M. paniculata contained other minor compounds in addition to gallic acid (retention time (RT) 13.56 min, peak 1), catechin (RT = 16.38 min, peak 2), chlorogenic acid (RT = 21.70 min, peak 3), caffeic acid (RT = 24.98 min, peak 4), ellagic acid (RT = 31.09 min, peak 5), epicatechin (RT = 37.24 min, peak 6), rutin (RT = 43.29 min, peak 7), quercitrin (RT = 46.17 min, peak 8), quercetin (RT = 50.13 min, peak 9), and kaempferol (RT = 56.11 min, peak 10) ().
Figure 1. High performance liquid chromatography showing phenolic and flavonoid profile of Murraya paniculata. Gallic acid (peak 1), catechin (peak 2), chlorogenic acid (peak 3), caffeic acid (peak 4), ellagic acid (peak 5), epicatechin (peak 6), rutin (peak 7), quercitrin (peak 8), quercetin (peak 9), and kaempferol (peak 10).
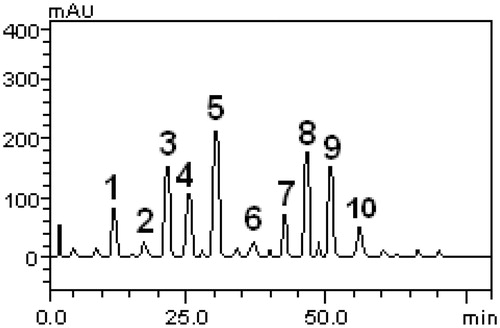
Table 3. Phenolic and flavonoid composition of Murraya paniculata.
Toxicological effect
In rats administered M. paniculata extract at 2000 and 5000 mg/kg by the oral route, there were no abnormal toxicity signs (such as piloerection, diarrhea, and alteration in locomotor activity) or deaths during the 14 d of observation. No significant change was observed in the hematological data ().
Results of biochemical parameters with the above doses of M. paniculata extract studied compared to control group are shown in . Male and female rats treated with 5000 and 2000 mg/kg showed a significant increase of 30.25% and 17.06% in the values of glucose, 33.8% and 40.3% in the glutamic oxaloacetic transaminase, and reductions in the levels of triglycerides by 27.08% and 43.26%, 24.3%, and 18.3% when compared with the control group.
Table 4. Effect of EEMp 5000 and 2000 mg/kg on hematological parameters in female/male Wistar rats treated orally.
Table 5. Effect on biochemical parameters after 14 d of oral administration of M. paniculata extract at 5000 and 2000 mg/kg.
Table 6. Effect of EEMp (5000 and 2000 mg/kg) by oral route on absolute (g) and relative organ weight (g/100 g of body weight) in male/female Wistar rats treated orally.
Necropsy was performed for macroscopic external evaluation of the heart, lung, liver, kidney, and spleen. These organs were carefully removed and individually weighed, and organ weight was expressed in absolute weight. The macroscopic analysis of target organs of the animals treated with EEMp did not reveal any abnormalities in their gross examinations as changes in color or texture when compared with the control group. The mean absolute weight of the tissues did not change, except for a decrease in relative weight of the liver (9.87 and 11.18%) at doses of 5000 and 2000 mg/kg, respectively, when compared with control groups (), but with no significant microscopic modifications.
Table 7. MIC values (μg/mL) of Murraya paniculata extract.
Microbiological effect
presents the results of antibacterial activity of the EEMp against the standard and multidrug-resistant strains of E. coli and S. aureus. These results demonstrated no antimicrobial activity of clinical significance, except against the SA358 bacterial strain with MIC of 512 μg/mL () (Matias et al., Citation2012).
shows the results of the modulatory effect on bacterial resistance to aminoglycosides against the E. coli and S. aureus strains. Interestingly, the addition of 64 μg/mL EEMp to the growth medium showed considerable reduction in the concentration of antibiotic needed to inhibit bacterial growth (reduction in MIC) for all the aminoglycosides tested, demonstrating a synergistic effect.
Discussion
Phytochemical analysis shows that M. paniculata extract contains carbohydrates, proteins, amino acids, and phenolic compounds (Gautam et al., Citation2012a), phytosterol and coumarins (Ito et al., Citation2005; Mesquita et al., Citation2008), alkaloids and flavonoids (Alitheen, Citation2012), and flavone derivatives (Lu et al., Citation2012). The flavone occurring, 5,7,3′,4′-tetramethoxyflavone, is one of the major polymethoxyflavones identified in M. paniculata (Lu et al., Citation2012). Similar concentrations of phenols and flavonoids, determined by the Folin and AlCl3 assays, have been reported by Gautam et al. (Citation2012a). These compounds are important for biological activity, as shown in the study of Pande et al. (Citation2009), who reported the use of the extract of Murraya koenigii for hepatoprotective activity.
Oral acute and chronic toxicity studies are important to determine the safety of drugs and plant products for human use. In our study, rats administered M. paniculata extract up to 5000 and 2000 mg/kg did not show any kind of abnormal behavior of clinical significance. Similar data of the acute toxicity assay were obtained for the extract of M. koenigi leaves (Gambhire et al., Citation2009) and M. paniculata, where no deaths were observed during the study period at the doses tested. In our study of toxicity, minor biochemical changes were observed in glucose, glutamic oxaloacetic transaminase, cholesterol, and triglycerides levels, and these observations have also been reported in other studies. A study on the glycemic and lipidemic effect of M. koenigii extract found a 37.1% reduction in triglyceride levels in treated animals (Kesari et al., Citation2007). Another study showed a significant increase in the blood glucose level and concomitant decrease in insulin in rats treated with M. koenigii leaf extract (Tembhurne & Sakarkar, Citation2010a), a finding in line with our data. Curry leaf (M. koenigii) extract was found to significantly decrease blood cholesterol level from 277.6 ± 16.6 (day 0) to 182.0 ± 15.3 mg/dL (day 10, p < 0.01 compared with the change in vehicle group) (Xie et al., 2006). El-Amin et al. (2013) showed that streptozotocin-induced diabetic rats administered aqueous extracts of M. koenigii (100 and 200 mg/kg p.o.) exhibited a significant decrease (p < 0.05) in cholesterol and triglyceride levels (55.6–64.6%) compared with metformin (62.7%); however, there was no significant effect on body weight or serum creatinine.
There are reports in the literature describing the antimicrobial effect of M. paniculata as well as other species of the genus Murraya (Aziz et al., Citation2010). Sundaram (Citation2011) showed that the ethanolic extract of leaves had not antimicrobial activity against E. coli. Gautam et al. (Citation2012b) correlated high content of phenolics and flavonoids in M. paniculata leaf extract with antibacterial properties against human pathogens. This activity could be explained by the phenols (e.g., gallic acid) (Oliveira et al., Citation2011), flavonoids and coumarins (e.g., 5,7,3′,4′-tetramethoxyflavone) (Wang et al., Citation1989) present in the extract. Studies on natural product extracts containing phenols and flavonoids have reported modulatory activity with regard to resistance to aminoglycosides, mainly affecting the cell membrane fluidity (Junior et al., Citation2011; Matias et al., Citation2012), as was observed in our study. However, this is first report of anti-bacterial resistance-modifying activity of M. paniculata with regard to aminoglycosides.
Conclusion
The present study investigated the chemical composition, acute oral toxicity, and antimicrobial activity of the extract of M. paniculata. Results showed a synergistic effect of the extract with aminoglycosides and small significant effect in the acute toxicity assay. Therefore, we suggest that extracts of M. paniculata could be used as a source of natural products with antibacterial resistance-modifying activity. Further, teratogenic, mutagenic, and carcinogenic studies are required for this product to be considered safe for human use.
Declaration of interest
The authors have declared that no competing interests exist. The authors are indebted to Brazilian Granting Agencies CAPES, CNPq and FUNCAP and whose support to our joint group made possible this research.
References
- Alitheen N. (2012). Bioactivity studies and chemical constituents of Murraya paniculata (Linn) Jack. Int Food Res Int 19:1307–12
- Araruna MK, Santos KK, da Costa JG, et al. (2013). Phenolic composition and in vitro activity of the Brazilian fruit tree Caryocar coriaceum Wittm. Eur J Integral Med 5:178–83
- Arulselvan P, Senthilkumar G, Sathish Kumar D, Subramanian S. (2006). Anti-diabetic effect of Murraya koenigii leaves on streptozotocin induced diabetic rats. Die Pharma 61:874–7
- Aziz S, Sukari M, Rahmani M, et al. (2010). Coumarins from Murraya paniculata (Rutaceae). Malaysian J Anal Sci 14:1–5
- Boligon AA, Sagrillo MR, Machado LF, et al. (2012). Protective effects of extracts and flavonoids isolated from Scutia buxifolia Reissek against chromosome damage in human lymphocytes exposed to hydrogen peroxide. Molecules 17:5757–69
- Chowdhury JU, Bhuiyan MNI, Yusuf M. (2008). Chemical composition of the leaf essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangladesh J Pharmacol 3:59–63
- El-Amin M, Virk P, Elobeid MA, et al. (2013). Anti-diabetic effect of Murraya koenigii (L) and Olea europaea (L) leaf extracts on streptozotocin induced diabetic rats. Pakistan J Pharm Sci 26:359–65
- Gambhire MN, Juvekar AR, Sakat SS. (2009). Evaluation of anti-inflammatory activity of methanol extract of Murraya koenigi leaves by in vivo and in vitro methods. Pharmacol Online 1:1072–94
- Gautam M, Gangwar M, Singh A, et al. (2012a). In vitro antioxidant properties of Murraya paniculata (L.) leaves extract. Inventi Rapid: Ethnopharmacol 2012:1–3
- Gautam MK, Gangwar M, Nath G, et al. (2012b). In vitro antibacterial activity on human pathogens and total phenolic, flavonoid contents of Murraya paniculata Linn. leaves. Asian Pac J Trop Biomed 2:S1660–3
- Gupta S, George M, Singhal M, et al. (2010). Leaves extract of Murraya koenigii linn for anti-inflammatory and analgesic activity in animal models. J Adv Pharm Technol Res 1:68–77
- Ito C, Itoigawa M, Onoda S, et al. (2005). Chemical constituents of Murraya siamensis: Three coumarins and their anti-tumor promoting effect. Phytochemistry 66:567–72
- Iwu M, Duncan AR, Okunji CO. (1999). New antimicrobials of plant origin. Perspectives on new crops and new uses. Alexandria (VA): ASHS Press, 457–62
- Junior FE, Matias EF, Oliveira DR, et al. (2011). Modulatory antibiotic activity and chemical composition of hydroalcoholic extract of Croton campestris. J Med Plants Res 5:4400–4
- Kesari AN, Kesari S, Singh SK, et al. (2007). Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J Ethnopharmacol 112:305–11
- Kinoshita T, Shimada M. (2002). Isolation and structure elucidation of a new prenylcoumarin from Murraya paniculata var. omphalocarpa (Rutaceae). Chem Pharm Bull 50:118–20
- Kong YC, Ng K, Wat K, et al. (2007). Yuehchukene, a novel anti-implantation indole alkaloid from Murraya paniculata. Planta Med 51:304–7
- Lu W-C, Sheen J-F, Hwang LS, Wei G-J. (2012). Identification of 5,7,3′,4′-tetramethoxyflavone metabolites in rat urine by the isotope-labeling method and ultrahigh-performance liquid chromatography–electrospray ionization–mass spectrometry. J Agric Food Chem 60:8123–8
- Mandal S, Nayak A, Kar M, et al. (2010). Antidiarrhoeal activity of carbazole alkaloids from Murraya koenigii Spreng (Rutaceae) seeds. Fitoterapia 81:72–4
- Mathur A, Verma SK, Singh SK, et al. (2011). Investigation of the antimicrobial, antioxidant and anti-inflammatory activity of compound isolated from Murraya koenigii. Int J Appl Biol Pharm Technol 2:470–7
- Matias EF, Santos FA, Silva JMF, et al. (2012). Screening the in vitro modulation of antibiotic activity of the extracts and fractions of Ocimum gratissimum L. Afr J Microbiol Res 6:1902–7
- Mesquita SG, Martinez MF, Romoff P, et al. (2008). Chemical constituents from leaves of Murraya paniculata (Rutaceae). Rev Bras Farmacogn 18:563–8
- NCCLS. (2005). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved standard, 6th ed. NCCLS document M7-A6. Wayne: NIH
- Oliveira DR, Brito-Junior FE, Bento EB, et al. (2011). Antibacterial and modulatory effect of Stryphnodendron rotundifolium. Pharm Biol 49:1265–70
- Organisation for Economic Co-operation and Development (OECD). (2001). Guideline for the Testing of Chemicals Section 4 (Part 425)
- Pande M, Gupta S, Pathak A. (2009). Hepatoprotective activity of Murraya koenigii Linn bark. J Herbal Med Toxicol 3:69–71
- Parrotta JA. (2001). Healing Plants of Peninsular India. 1st ed. Oxford & New York: CABI Publishing
- Patidar DK, Yadav N, Nakra V, et al. (2010). Wound healing activity of Murraya koenigii leaf extract. Int J Comp Pharm 4:1–2
- Sabir S, Ahmad S, Hamid A, et al. (2012). Antioxidant and hepatoprotective activity of ethanolic extract of leaves of Solidago microglossa containing polyphenolic compounds. Food Chem 131:741–7
- Saied S, Nizami SS, Anis I. (2008). Two new coumarins from Murraya paniculata. J Asian Nat Prod Res 10:515–19
- Saklani A, Kutty SK. (2008). Plant-derived compounds in clinical trials. Drug Discov Today 13:161–71
- Sathaye S, Bagul Y, Gupta S, et al. (2011). Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol-induced hepatotoxicity model. Exp Toxicol Pathol 63:587–91
- Shabbir M, Ziauddin Sultani S, Jabbar A, Iqbal Choudhary M. (1997). Cinnamates and coumarins from the leaves of Murraya paniculata. Phytochemistry 44:683–5
- Sundaram M. (2011). Studies on in vitro antibacterial, antifungal property and antioxidant potency of Murraya paniculata. Pak J Nutr 10:925–9
- Tembhurne S, Sakarkar D. (2009). Hypoglycemic effects of fruit juice of Murraya koenigii (L) in alloxan induced diabetic mice. Int J Pharm Tech Res 1:1589–93
- Tembhurne S, Sakarkar D. (2010a). Beneficial effects of ethanolic extract of Murraya koenigii (Linn.) leaves in cognitive deficit aged mice involving possible anticholinesterase and cholesterol lowering mechanism. Int J Pharm Tech Res 2:181–8
- Tembhurne S, Sakarkar D. (2010b). Protective effect of Murraya koenigii (L) leaves extract in streptozotocin induced diabetics rats involving possible antioxidant mechanism. J Med Plants Res 4:2418–23
- Wang Y, Hamburger M, Gueho J, Hostettmann K. (1989). Antimicrobial flavonoids from Psiadia trinervia and their methylated and acetylated derivatives. Phytochemistry 28:2323–7
- Xie J-T, Chang W-T, Wang C-Z, et al. (2006). Curry leaf (Murraya koenigii Spreng.) reduces blood cholesterol and glucose levels in ob/ob mice. Am J Chinese Med 34:279–84
- Zhang J-Y, Li N, Che Y-Y, et al. (2011). Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. J Pharm Biomed Anal 56:950–61
- Zhang J-Y, Li N, Zhou Y, et al. (2012). Simultaneous qualitative and quantitative determination of major polymethoxylated flavonoids in the leaves of Murraya paniculata by RRLC-DAD-ESI-MSn. Anal Meth 4:3399–406
