Abstract
Context: Schizandra chinensis Baill (Magnoliaceae) fruit extract (SCE) is considered a traditional herbal medicine for the treatment and alleviation of various diseases. Gastric cancer is the second most common cause of cancer-related death worldwide, and the first most common in Korea.
Objectives: This study investigates the mechanism of SCE-induced apoptosis in AGS human gastric cancer cells.
Materials and methods: SCE concentrations from 100 to 400 µg/ml were used. Cell viabilities were determined using MTT assay. Members of the Bcl-2 family and Bax were detected by Western blotting. RT-PCR was performed to measure the expression level of the Fas/FasL pro-apoptotic genes.
Results: SCE inhibited the proliferation AGS cells for 24 or 72 h (inhibition by 3.1% ± 5.2% at 100 µg/ml and 87.3% ± 7.6% at 400 µg/ml at 24 h and by 40.2% ± 5.3% 100 µg/ml and 95.3% ± 1.3% 400 µg/ml at 72 h) and increased the sub-G1 phase (25.3% ± 5.2% at 100 µg/ml and 370.2% ± 7.2% at 400 µg/ml) and the mitochondrial membrane depolarization (11.2% ± 2.1% at 100 µg/ml and 311.5% ± 6.1% at 400 µg/ml). The SCE-induced apoptotic cell death showed the down-regulation of Bcl-2, but up-regulation of Bax. Subsequently, SCE increased the expression level of Fas/FasL, activated caspase-9 and -3, and increased reactive oxygen species generation. Also, JNK II inhibitor or a p38 MAPK inhibitor inhibited SCE-induced cell death.
Discussion and conclusion: These results indicate that SCE might be an effective chemotherapeutic for the treatment of human gastric cancer.
Introduction
Gastric adenocarcinoma is one of the most common causes of cancer-related death. The death rate of gastric cancer has declined during the last two decades due to medical and pharmacologic advances, but the absence of more efficient drugs has become a major barrier to future progress. Therefore, many researchers are trying to discover new drugs against gastric cancer. Nevertheless, gastric cancer remains a major cause of death and morbidity, and when advanced, its prognosis is dismal (Dicken et al., Citation2005; Shin et al., Citation2011).
Schizandra chinensis Baill (Magnoliaceae) (SC) fruit is used in traditional herbal medicine. More than 40 lignans have been discovered in this plant, and these have been used to treat and to alleviate various diseases, including cancer (Gnabre et al., Citation2010). These lignans are abundant in the seeds and fruits of SC (Huang et al., Citation2007; Ikeya et al., Citation1979; Lu et al., Citation2009; Panossian et al., Citation2008; Slanina et al., Citation1997). Previous studies have shown that SC possesses neuroprotective effects on glutamate-induced apoptosis (Lee et al., Citation2012) and has the ability to protect the liver from injury induced by toxins substances, such as, those associated with hepatitis (Liu, Citation1989; Melhem et al., Citation2005). Schizandrin B and C are components of SC extract (Xu et al., Citation2011). Schizandrin B has been used to treat vascular injury (Chiu et al., Citation2004; Kim et al., Citation2011; Park et al., Citation2012) and schizandrin C has been reported to induce leukemia cell death (Park et al., Citation2009), and the deaths of human cancer cell lines (Min et al., Citation2008; Yasukawa et al., Citation1992). However, the underlying pharmacological mechanisms of SC extract are not clearly understood.
Apoptosis is known as programmed cell death and causes cell shrinkage, nuclear and DNA fragmentation, cell membrane blebbing, and eventually cellular dysfunction (Cobb et al., Citation1996; Hengartner, Citation2000; Ziegler & Groscurth, Citation2004). Apoptosis can be controlled by several genes, including caspases (a family of cystein-dependent aspartate-specific proteases) and by proteins of the Bcl-2 family (Allen et al., Citation1998; Burlacu et al., Citation2003). Losing apoptotic control can contribute to the survival rate of cancer cells and represents a possible approach to the treatment of cancer (Meiler & Schuler, Citation2006). The caspases and Bcl-2 family members are both known to play important roles in apoptosis. In particular, Bcl-2 proteins have long been believed to be involved in the regulation of mitochondrial outer membrane (MOM) permeability (an essential step of apoptotic cell death) and to interact with member of the pro-apoptotic and anti-apoptotic proteins. Members of the Bcl-2 family are well-known anti-apoptotic proteins and accelerate cell survival, whereas Bax is a pro-apoptotic protein (Chao et al., Citation1995).
Reactive oxygen species (ROS) are mostly generated in mitochondria and play an important role in apoptosis, such as in the regulation of cell growth, differentiation, and migration. Moreover, it has been reported that the depolarization of the mitochondrial membrane is induced by ROS, and that excessive ROS trigger numerous events in signal transduction pathways that mediate apoptosis, including c-Jun N-terminal Kinase (JNK) and mitogen-activated protein kinases (MAPKs), which lead to elevated expression levels of pro-apoptotic molecules in the cytosol (Aggeli et al., Citation2006; Lee et al., Citation2000).
In present study, we show that SCE induces ROS generation, which leads to apoptotic signaling via mitochondria-dependent and caspase-dependent pathways in human gastric cancer cells.
Materials and methods
Preparation of SCE
SC was purchased from Kwangmyungdang Medicinal Herbs (Ulsan, Korea), harvested in Kyungju, Kyungbook Province (Korea) in October, 2012, and authenticated by Professor Hyung Woo Kim (Division of Pharmacology, Pusan National University, School of Korean Medicine, Yangsan, Korea). Air-dried SC (100 g) was extracted by boiling with distilled water (1 L) for 2 h. The extract was filtered, evaporated, and lyophilized to yield 14.2 g of powder (a recovery yield of 14.2%). Stock solution was prepared by accurately weighing 10 mg of schizandrin (Sigma, St. Louis, MO) and dissolving it in 10 ml of methanol. Standard solution (STD) was a 10-fold dilution of stock solution, which was filtered through a 0.2 μm syringe filter. The final concentration of STD was 100 μg/ml.
Chromatographic conditions and standard preparation
The Smart LC system comprised a LC800 (GL Sciences, Tokyo, Japan) equipped with a built-in solvent delivery unit, autosampler, column oven, and UV–visible detector. Chromatographic separation was performed on an Inertsil ODS-4 column (2.1 × 50 mm, i.d. 2 μm; GL Sciences, Tokyo, Japan) at 37 °C. The mobile phase consisted of water (A) and acetonitrile (B). The gradient program used was follows: 10% (B) maintained for 1 min, 10–90% (B) over 2–9 min. The flow rate was set at 0.4 ml/min and the injection volume was 1 μl. The wavelength used to detect schizandrin was 250 nm. Acquired data were processed using a EZChrom Elite software (Ver. 3.3.2 SP1, Shiseido, Tokyo, Japan).
Cell culture and reagents
The human gastric adenocarcinoma cell lines (AGS) were used. The AGS cell line was established at the Cancer Research Center, College of Medicine, Seoul National University, Korea, and cells were propagated in a RPMI-1640 medium (Gibco-BRL, St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum and 20 μg/ml penicillin and streptomycin in a 5% CO2 atmosphere at 37 °C. All cell culture reagents used were obtained from Invitrogen (Grand Island, NY). C-Jun N-terminal Kinase (JNK) II inhibitor, SB203580, and N-acetyl-l-cysteine (NAC) were purchased from Tocris (Bristol, UK). All other reagents were supplied by Sigma (St. Louis, MO).
Cell viability assay
Cell viability was assessed using a MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. AGS cells were seeded into the well of 12-well plates and then cultured in RPMI-1640 supplemented with other reagents for 72 h. After incubation, 100 μl of MTT solution (5 mg/ml in PBS) was added to each well, and the plates were incubated for 4 h at 37 °C. After removing the supernatant and shaking with 200 μl of dimethyl sulfoxide (Jersey Lab Supply, Livingston, NJ) for 30 min, the absorbances were measured at 570 nm. All experiments were repeated at least three times.
Measurement of cell cycle
In order to determine disruption of the AGS cell cycle, we used propidium iodine (PI) stain (Nicoletti et al., Citation1991; Wang et al., Citation2005). About 1 × 106 cells were placed in an e-tube, and 700 μl of ice-cold fixation buffer (ethyl alcohol) was slowly added with vortexing. Tubes were sealed with parafilm and incubated at 4 °C overnight. Samples were spun for 3 min at 106 g at 4 °C, and supernatants was aspirated and discarded. Cell pellets were resuspended in 200 μl of PI staining solution (PI [5 mg/ml] 2 μl and RNase 2 μl in PBS 196 μl) and then, spun at 20 817 g for 5 s. After 30 min in the dark at room temperature, samples were analyzed using a fluorescence-activated cell sorter (FACScan; Becton-Dickinson, Mountain View, CA) at λ = 488 nm using a Cell-Quest software (Becton-Dickinson, Franklin Lakes, NJ). The DNA content distribution of normally growing cells is characterized by two peaks, the G1/G0 and G2/M phases. On one hand, the G1/G0 phase represents normal functioning and the resting state of the cell cycle and has most diploid DNA content, whereas the DNA content in the G2/M phase is more than diploid. On the other hand, cells in the sub-G1 phase have least DNA content, are termed hypodiploid, and represent DNA fragmentation (Wang et al., Citation2005).
Assessment of mitochondrial membrane depolarization
Mitochondrial membrane depolarization was evaluated using the JC-1 fluorescence probe, according to the manufacturer's instructions (Molecular Probes, Eugene, OR). AGS cells were labeled with 2 μM JC-1 for 30 min at 37 °C and then analyzed by flow cytometry using a 488 nm excitation wavelength and 530/30 or 585/42 nm bypass emission filters. Cells without red fluorescence were regarded to manifest mitochondrial membrane depolarization.
Western blot analysis
Total cell extracts (5 × 106 cells) were prepared using a RIPA buffer (Cell Signaling Technology Inc., Danvers, MA) containing 1 mM phenylmethylsulfonyl fluoride (PMSF). Amounts of proteins were measured using the Bradford method (Bio-Rad Laboratories, Hercules, CA). Equal amounts of proteins were fractionated by SDS-PAGE and transferred to PVDF membrane (Bio-Rad Laboratories, Hercules, CA). Blots were blocked for at least 1 h with 5% non-fat dry milk prior to being incubated with antibodies against bcl2, bax, and β-actin at 4 °C for overnight, and then incubated with secondary antibodies conjugated to HRP at room temperature for 1 h, the bands of interest were visualized by chemiluminescence (SuperSignalWest Femto; Thermo Fisher Scientific, Rockford, IL).
Reverse transcription PCR analysis
Total RNA from each cell was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA), and converted to cDNA using AccuPower RT-PreMix (Bioneer Co., Daejeon, Korea). Specific DNA sequences were amplified with AccuPower PCR-PreMix (Bioneer Co.). The PCR primers used in this study were as follows: forward primer for Fas 5′-ATGCTGGGCATCTGGACCCTCCTA-3′ and reverse primer 5′-TCTGCACTTGGTATTCTGGGTCCG-3′; forward primer for FasL 5′-ACTTCCGGGGTCAATCTTGC-3′ and reverse primer 5′-TAGAACATCTCGGTGCCTGTA-3′; forward primer for β-actin 5′-CAAGAGATGGCCACGGCTGCT-3′ and reverse primer 5′-TCCTTCTGCATCCTGTCGGCA-3′. Amplified products were analyzed in 1.0% agarose gels under UV light, and images were captured using the GelDoc-It TS Imaging System (UVP, Upland, CA).
Caspase assay
Caspase-3 and -9 assay kits (Cellular Activity Assay Kit Plus) were purchased from BioMol (Plymouth, PA). After experimental treatments, cells were centrifuged (1000 g, 4 °C, 10 min), washed with PBS, resuspended in ice-cold cell lysis buffer, and incubated on ice for 10 min. Samples were centrifuged at 10 000 g (4 °C, 10 min), and the supernatant was removed. Supernatant samples (10 μl) were then incubated with 50 μl of substrate (400-μM Ac-DEVD-pNA) in 40 μl of assay buffer at 37 °C. The absorbance at 405 nm was read at several time-points. pNA concentrations in samples were determined using a standard plot of absorbances at different pNA concentrations. The pan-caspase inhibitor zVAD-fmk (Calbiochem, Millipore Corporation, Billerica, MA) was used to validate the assay method.
Measurement of ROS production
Intracellular ROS generation in AGS cells was determined using carboxy-H2DCFDA [5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate; Molecular Probes, Eugene, OR]. Briefly, after various pharmacological treatments, cells were treated with 100 μM carboxy-H2DCFDA in culture medium, incubated at 37 °C for 30 min, washed with PBS, and then fluorescence was measured using a FACScan (Becton-Dickinson, Mountain View, CA) at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Statistical analysis
Data are expressed as means ± SEMs. Differences were evaluated using Student's t-test. Statistical significance was accepted for p values <0.05.
Results
Identification of schizandrin in SC extract
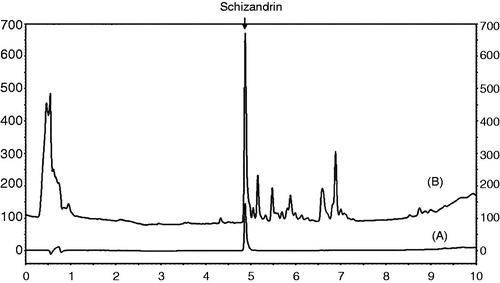
The schizandrin peak was detected at a retention time of 4.870 min ().
Induction of apoptosis by SCE in AGS cells
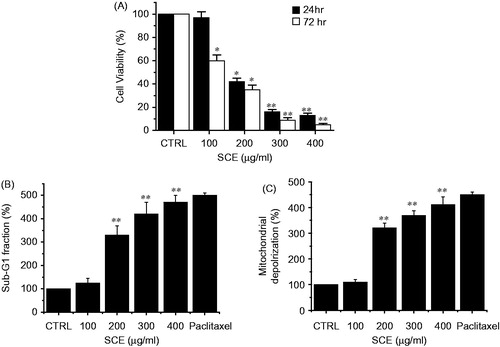
To investigate whether SCE suppresses AGS cell growth, MTT assays were performed after 24 or 72 h of culture with various concentrations of SCE. We observed that cell viability was remarkably reduced by SCE treatment. The addition of 100, 200, 300, or 400 µg/ml SCE in culture medium inhibited AGS survival by 3.1 ± 5.2%, 58.3 ± 6.1%, 84.1 ± 6.5%, or 87.3 ± 7.6% by the MTT assay at 24 h and by 40.2 ± 5.3%, 65.1 ± 6.2%, 91.0 ± 8.1%, and 95.3 ± 1.3% at 72 h (n = 5, respectively; ). To determine whether SCE induces apoptosis, cell cycle and mitochondrial membrane depolarization analysis were carried out by flow cytometry. Cells were harvested after treatment with SCE for 72 h (at concentration from 100 to 400 µg/ml; ). Cell-cycle analysis indicated that percentages of sub-G1 cells were significantly and dose-dependently increased by SCE. The sub-G1 phase was markedly increased by 25.3 ± 5.2% at 100 µg/ml, 230.1 ± 6.3% at 200 µg/ml, 321.3 ± 5.4% at 300 µg/ml, and 370.2 ± 7.2% at 400 µg/ml by flow cytometry (n = 6, respectively; ). In addition, mitochondrial membrane depolarization (an early event of an intrinsic apoptosis signaling) was elevated by SCE. The mitochondrial membrane depolarization by SCE was markedly increased by 11.2 ± 2.1% at 100 µg/ml, 220.4 ± 3.3% at 200 µg/ml, 268.4 ± 4.3% at 300 µg/ml, and 311.5 ± 6.1% at 400 µg/ml by flow cytometry (n = 5, respectively; ). To confirm apoptosis, we used a positive control, paclitaxel (10 nM), which is an anticancer drug. Paclitaxel induced increases in the sub-G1 level and mitochondrial membrane depolarization activity. These results suggested that SCE has an anti-cancer effect and that this is closely associated with the induction of apoptosis in AGS cells.
Figure 2. Induction of apoptosis in AGS cells by SCE in vitro. (A) AGS cells were cultured in the presence of SCE at the indicated concentrations for 24 or 72 h. Cell viabilities were determined by MTT assay. Viabilities of cells significantly decreased in a dose- and time-dependent manner. The number of viable cells after SCE treatment is expressed as a percentage of untreated cells. (B) Sub-G1 fractions are expressed as percentages of untreated cells. Cells were treated with the indicated concentrations of SCE for 72 h. (C) Mitochondria membrane depolarization is expressed as percentages of untreated cells. Cells were treated with the indicated concentrations of SCE for 72 h. Paclitaxel was used as a positive control. Values are means ± SEMs. *p < 0.05, **p < 0.01 compared with CTRL. CTRL, untreated AGS cells.
SCE induced apoptosis via a mitochondria- and caspase-dependent pathway in AGS cells
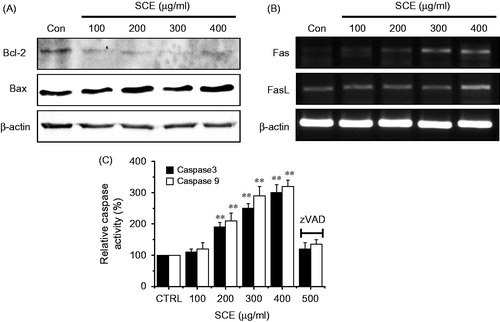
We investigated whether SCE-induced apoptotic cell death in AGS cells was regulated by B-cell lymphoma protein-2 (Bcl-2), which directly interacts with mitochondria. The expression levels of Bcl-2 (anti-apoptotic) and of Bax (pro-apoptotic) was measured by western blotting after being exposed to various concentrations (from 100 to 400 µg/ml) of SCE for 24 h. The results obtained revealed that Bcl-2 expression was markedly inhibited by SCE, whereas the Bax expression was up-regulated (). Since the Fas/FasL system is a key player in the transduction pathway for death receptor-mediated apoptosis, we examined the involvement of the Fas/FasL system in AGS cells treated with SCE by RT-PCR. Fas and FasL expression levels were both up-regulated by SCE (). Because it is known that caspase activation is required for apoptotic cell death, we performed caspase activity assays to observe the activities of caspase-9 and -3 in AGS cells. We found that caspase activities were dose dependently elevated in the presence of SCE (from 100 to 400 µg/ml) for 72 h, and that these activities were repressed by zVAD-fmk, a pan-caspase inhibitor (). Therefore, these results suggest that SCE-induced apoptosis is mediated by a mitochondria- and caspase-dependent pathway in AGS cells.
Figure 3. Down-regulation of the expression level of Bcl-2, and the up-regulation of Bax, Fas/FasL, pro-apoptotic genes, and caspase-9 and -3 activities in AGS cells. (A) Western blot analysis was performed using AGS cells treated with different SCE concentrations for 24 h. Bcl-2 expression was markedly down-regulated by SCE, whereas Bax expression was up-regulated. (B) RT-PCR was performed using AGS cells treated with different SCE concentration for 24 h. Fas and FasL were up-regulated by SCE. (C) Cells were cultured with SCE at the indicated concentrations for 72 h prior to caspase assays. The caspase activity of untreated cells was taken to be 100%. Pan-caspase inhibitor, zVAD-fmk, (zVAD) at 20 µM was treated to validate the analytical method. Values are means ± SEMs. **p < 0.01. ß-Actin was used as a loading control. CTRL, untreated AGS cells.
SCE-induced apoptosis through c-Jun N-terminal kinase (JNK) and p38 MAPK pathway in AGS cells
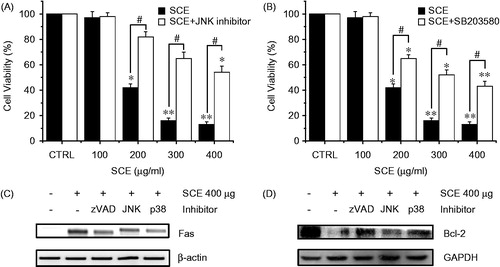
Since we found elevated Fas/FasL (pro-apoptotic) expression levels in AGS cells treated with SCE, we speculated that JNK and p38 MAPK were involved in SCE-induced apoptosis. To identify the involvement of the JNK/p38 MAPK signaling pathway in SCE-induced cell death, the cell viability was measured after treatment with various concentrations (from 100 to 400 µg/ml) of SCE for 72 h with or without JNK II inhibitor or SB203580 (a p38 MAPK inhibitor) using the MTT assay. Our results showed that treatment with JNK II inhibitor or SB203580 notably inhibited SCE-induced cell death, particularly after treatment with 200 µg/ml of SCE. The addition of 100, 200, 300, or 400 µg/ml SCE with JNK II inhibitor in culture medium inhibited the survival of AGS cells by 2.2 ± 1.1%, 18.2 ± 4.2%, 35.3 ± 3.5%, and 46.2 ± 5.2%. Also, the addition of 100, 200, 300, or 400 µg/ml of SCE with SB203580 in culture medium inhibited the survival of AGS cells by 2.1 ± 1.1%, 35.1 ± 3.4%, 48.2 ± 4.6%, and 57.1 ± 4.3%, respectively, by the MTT assay (n = 5; ). To confirm the involvement of the JNK/p38 MAPK signaling pathway in SCE (400 µg/ml)-induced cell death, we experimented the change of expression level of the Fas by RT-PCR and the Bcl-2 by western blotting. Fas expression levels were decreased by zVAD, JNK, or p38 inhibitors () and Bcl-2 expression was markedly increased by zVAD, JNK, or p38 inhibitors (). These results suggest that both JNK and p38 MAPK are involved in SCE-induced apoptosis in AGS cells.
Figure 4. The effect of SCE on the MAPK-signaling pathway in AGS cells. MTT assays were used to determine cell viabilities in the presence of (A) JNK II inhibitor or (B) SB203580 (a p38 MAPK inhibitor). Cells were treated with the indicated concentrations of SCE with JNK II inhibitor or SB203580 (both at 10 µM) for 72 h. (C) RT-PCR was performed using AGS cells treated with zVAD, JNK, or p38 inhibitor. Fas were decreased by zVAD, JNK, or p38 inhibitor. (D) Western blot analysis was performed using AGS cells treated with zVAD, JNK, or p38 inhibitor. Bcl-2 expression was markedly increased by zVAD, JNK, or p38 inhibitor. Values are means ± SEMs. *p < 0.01, **p < 0.01, #p < 0.01 compared with the CRTL. #p < 0.05 compared with each. CTRL: untreated AGS cells.
SCE-induced apoptosis was mediated by intracellular ROS generation in AGS cells
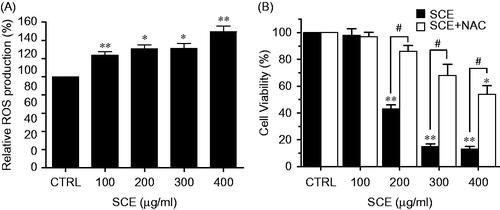
Since intracellular ROS play important roles in apoptosis, we examined whether SCE is capable of generating ROS in AGS cells. Cells were treated with various concentrations (from 100 to 400 µg/ml) of SCE for 24 h and levels of ROS generation were measured by flow cytometry. As shown in , ROS generation significantly increased in a dose-dependent manner up to almost 1.6-fold versus the untreated control. In addition, we measured cell viabilities after treating cells with SCE (from 100 to 400 µg/ml) for 24 h plus N-acetyl-l-cysteine (NAC), a ROS scavenger. As shown in , NAC remarkably abolished SCE-induced cell death. Thus, these results suggest that SCE enhances the generation of ROS.
Figure 5. SCE enhanced ROS accumulation in AGS cells. (A) Intracellular ROS was detected in AGS cells after treatment with the indicated concentration of SCE for 24 h. ROS production is expressed as percentages of untreated cells. (B) Cells were treated with SCE and NAC for 24 h and cell viabilities were determined by MTT assay. Values are means ± SEMs. *p < 0.05, **p < 0.01 versus CRTL. #p < 0.05 compared with each. CTRL, untreated AGS cells.
Discussion
SC has long been used as a traditional herbal medicine to treat coughs, dysentery, night sweats, spontaneous sweating, amnesia, and insomnia and to stimulate the immune system (Huang et al., Citation2007; Ikeya et al., Citation1979; Lee et al., Citation1990; Lu et al., Citation2009; Panossian et al., Citation2008; Slanina et al., Citation1997). It has been reported SC can also be used to induce the cell death of human cancer cells, and it has been suggested that SCE be considered a potential chemotherapeutic agent (Park et al., Citation2009).
Apoptosis is a process of gene-mediated cell death that is essential for removing unwanted cells in biological and physiological system (Thompson, Citation1995). It has been proposed that two major pathways can lead to apoptosis (Adams et al., Citation2007), namely, the extrinsic and intrinsic pathways. The extrinsic pathway is initiated by extracellular signals that result in the binding of ligands, such as Fas ligand (FasL) (Wajant, Citation2002). The Fas/FasL genes are pro-apoptotic genes that are activated by nuclear transcription factor activating protein-1 (AP-1). The intrinsic mitochondrial pathway is regulated by Bcl-2 family proteins (anti-apoptotic proteins). The Bcl-2 family has been shown to contain 17 or more members in mammalian cells (Cory et al., Citation2002), and to importantly regulate cell death processes. Bax (pro-apoptotic) shares regions of homology with Bcl-2 (Moldoveanu et al., Citation2006; Suzuki et al., Citation2000). Importantly, Bcl-2/Bax ratios are involved in the loss of mitochondrial membrane potential (Desagher et al., Citation2000; Tsujimoto et al., Citation1998). The down-regulation of Bcl-2 family proteins and the up-regulation of Bax proteins could cause permeabilization of the MOM. Mitochondria play crucial roles by the apoptotic proteins, such as cytochrome c (Green et al., Citation1998; Jeong et al., Citation2008; Spierings et al., Citation2005). Previous studies have shown that cytochrome c has two main functions, that is, it controls cellular metabolism and apoptosis and activates the caspase cascade (Budihardjo et al., Citation1999; Cai et al., Citation1998; Czerski & Nuñez, Citation2004; Simonian et al., Citation1997). The caspases are a family of proteins that belong to a group of enzymes called cysteine proteases and caspases, and are major executors of apoptotic processes (Earnshaw et al., Citation1999).
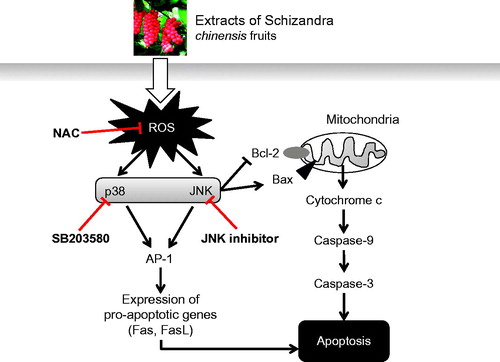
Taken together, we found that SCE down-regulated Bcl-2 protein levels but up-regulated Bax protein levels, promoted the release of cytochrome c to cytosol, and thus, activated caspase-9 and -3 and led to AGS apoptosis. Therefore, we propose a signaling pathway for SCE-induced apoptosis in AGS cells. As shown in , our findings suggest that SCE-induced cell death acts via a ROS-mediated JNK/p38 MAPK signaling pathway that provokes the up-regulations of pro-apoptotic genes.
Figure 6. Hypothetical schematic signaling pathway of SCE-induced apoptosis in AGS cells. SCE promotes pro-apoptotic factor Bax protein expression and decreases anti-apoptotic factor Bcl-2 protein expression and these are followed by cytochrome c release into the cytosol. Cytochrome c can activate the caspase-9 cascade followed by the caspase-3 cascade, which leads to cell death via the intrinsic apoptotic pathway. Another possible pathway is the extrinsic apoptotic pathway, which acts through ROS-mediated JNK/p38 MAPK. The Fas/FasL system (a pro-apoptotic system) is provoked by AP-1.
Conclusion
We provide evidence that SCE causes cell death via the extrinsic and intrinsic pathway in human gastric cancer cells. Furthermore, we suggest that SCE be considered a useful potential anticancer agent.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This study was supported by the Traditional Korean Medicine R&D Project, Korean Ministry of Health & Welfare (HI12C1886 and B120008) and the Research Fund Program of Research Institute for Basic Sciences, Pusan National University, Korea, 2013, Project no. RIBS-PNU-2013-114.
References
- Adams JM, Cory S. (2007). The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–37
- Aggeli IK, Gaitanaki C, Beis I. (2006). Involvement of JNKs and p38-MAPK/MSK1 pathways in H2O2-induced upregulation of heme oxygenase-1 mRNA in H9c2 cells. Cell Signal 18:1801–12
- Allen RT, Cluck MW, Agrawal DK. (1998). Mechanisms controlling cellular suicide: Role of Bcl-2 and caspases. Cell Mol Life Sci 54:427–45
- Budihardjo I, Oliver H, Lutter M, et al. (1999). Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15:269–90
- Burlacu A. (2003). Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med 7:249–57
- Cai J, Yang J, Jones DP. (1998). Mitochondrial control of apoptosis: The role of cytochrome c. Biochim Biophys Acta 10:139–49
- Chao DT, Linette GP, Boise LH, et al. (1995). Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med 182:821–8
- Chiu PY, Ko KM. (2004). Schisandrin B protects myocardial ischemia-reperfusion injury partly by inducing Hsp25 and Hsp70 expression in rats. Mol Cell Biochem 266:139–44
- Cobb JP, Hotchkiss RS, Karl IE, Buchman TG. (1996). Mechanisms of cell injury and death. Br J Anaesth 77:3–10
- Cory S, Adams JM. (2002). The Bcl2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–56
- Czerski L, Nuñez G. (2004). Apoptosome formation and caspase activation: Is it different in the heart? J Mol Cell Cardiol 37:643–52
- Desagher S, Martinou JC. (2000). Mitochondria as the central control point of apoptosis. Trends Cell Biol 10:369–77
- Dicken BJ, Bigam DL, Cass CJ, et al. (2005). Gastric adenocarcinoma: Review and considerations for future directions. Ann Surg 241:27–39
- Earnshaw WC, Martins LM, Kaufmann SH. (1999). Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424
- Gnabre J, Unlu I, Chang TC, et al. (2010). Isolation of lignans from Schisandra chinensis with anti-proliferative activity in human colorectal carcinoma: Structure-activity relationships. J Chromatogr B Analyt Technol Biomed Life Sci 878:2693–700
- Green SR, Reed JC. (1998). Mitochondria and apoptosis. Science 281:1309–12
- Hengartner MO. (2000). The biochemistry of apoptosis. Nature 407:770–6
- Huang X, Song F, Liu Z, Liu S. (2007). Studies on lignan constituents from Schisandra chinensis (Turcz.) Baill. fruits using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. J Mass Spectrom 42:1148–61
- Ikeya Y, Taguchi H, Yosioka I, Kobayashi H. (1979). The constituents of Schizandra chinensis Baill. I. Isolation and structure determination of five new lignans, gomisin A, B, C, F and G, and the absolute structure of schizandrin. Chem Pharm Bull (Tokyo) 27:1383–94
- Jeong SY, Seol DW. (2008). The role of mitochondria in apoptosis. BMB Rep 41:11–22
- Kim EY, Baek IH, Rhyu MR. (2011). Cardioprotective effects of aqueous Schizandra chinensis fruit extract on ovariectomized and balloon-induced carotid artery injury rat models: Effects on serum lipid profiles and blood pressure. J Ethnopharmacol 134:668–75
- Lee MS, Chao J, Yen JC, et al. (2012). Schizandrin protects primary rat cortical cell cultures from glutamate-induced apoptosis by inhibiting activation of the MAPK family and the mitochondria dependent pathway. Molecules 18:354–72
- Lee PJ, Camhi SL, Chin BY, et al. (2000). AP-1 and STAT mediate hyperoxia-induced gene transcription of heme oxygenase-1. Lung Cell Mol Physiol 279:L175–82
- Lee YW, Voyksner RD, Pack TW, et al. (1990). Application of countercurrent chromatography/thermospray mass spectrometry for the identification of bioactive lignans from plant natural products. Anal Chem 62:244–8
- Liu GT. (1989). Pharmacological actions and clinical use of fructus schizandrae. Chin Med J 102:740–9
- Lu Y, Chen DF. (2009). Analysis of Schisandra chinensis and Schisandra sphenanthera. J Chromatogr A 1216:1980–90
- Meiler J, Schuler M. (2006). Therapeutic targeting of apoptotic pathways in cancer. Curr Drug Targets 7:1361–9
- Melhem A, Stern M, Shibolet O, et al. (2005). Treatment of chronic hepatitis C virus infection via antioxidants: Results of a phase I clinical trial. J Clin Gastroenterol 39:737–42
- Min HY, Park EJ, Hong JY, et al. (2008). Antiproliferative effects of dibenzocyclooctadiene lignans isolated from Schisandra chinensis in human cancer cells. Bioorg Med Chem Lett 8:523–6
- Moldoveanu T, Liu Q, Tocilj A, et al. (2006). The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell 24:677–88
- Nicoletti I, Migliorati G, Pagliacci MC, et al. (1991). A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139:271–9
- Panossian A, Wikman G. (2008). Pharmacology of Schisandra chinensis Bail: An overview of Russian research and uses in medicine. J Ethnopharmacol 118:183–212
- Park C, Choi YW, Hyun SK, et al. (2009). Induction of G1 arrest and apoptosis by schisandrin C isolated from Schizandra chinensis Baill in human leukemia U937 cells. Int J Mol Med 24:495–502
- Park EJ, Chun JN, Kim SH, et al. (2012). Schisandrin B suppresses TGFβ1 signaling by inhibiting Smad2/3 and MAPK pathways. Biochem Pharmacol 83:378–84
- Shin AS, Kim JS, Park SH. (2011). Gastric cancer epidemiology in Korea. J Gastric Cancer 11:135–40
- Simonian PL, Grillot DA, Nuñez G. (1997). Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood 90:1208–16
- Slanina J, Táborská E, Lojková L. (1997). Lignans in the seeds and fruits of Schisandra chinensis cultured in Europe. Planta Med 63:277–80
- Spierings D, McStay G, Saleh M, et al. (2005). Connected to death: The (unexpurgated) mitochondrial pathway of apoptosis. Science 310:66–7
- Suzuki M, Youle RJ, Tjandra N. (2000). Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell 103:645–54
- Thompson CB. (1995). Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–62
- Tsujimoto Y. (1998). Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 3:697–707
- Wajant H. (2002). The Fas signaling pathway: More than a paradigm. Science 296:1635–6
- Wang BJ, Won SJ, Yu ZR, Su CL. (2005). Free radical scavenging and apoptotic effects of cordycepin sinensis ractionated by supercritical carbon dioxide. Food Chem Toxicol 43:543–52
- Xu Y, Liu Z, Sun J, et al. (2011). Schisandrin B prevents doxorubicin-induced chronic cardiotoxicity and enhances its anticancer activity in vivo. PLoS One 6:e28335
- Yasukawa K, Ikeya Y, Mitsuhashi H, et al. (1992). Gomisin A inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology 49:68–71
- Ziegler U, Groscurth P. (2004). Morphological features of cell death. News Physiol Sci 19:124–8