Abstract
Context: Trichophyton rubrum is the most common fungus causing chronic dermatophytosis in humans. Antifungal activity of promising agents is of great interest. Geraniol and citronellol are monoterpenes with antimicrobial properties.
Objective: This study aimed to investigate the inhibitory effects and possible mechanism of antifungal activity of geraniol and citronellol against strains of T. rubrum.
Materials and methods: The minimum inhibitory concentration (MIC) of each drug against 14 strains was determined by broth microdilution. The effects of the drugs on dry mycelial weight, conidial germination, infectivity on human nail fragments, and morphogenesis of T. rubrum were analyzed. The effects on the cell wall (test with sorbitol) and cell membrane (release of intracellular material and ergosterol biosynthesis) were investigated.
Results: MIC values of geraniol ranged between 16 and 256 µg/mL while citronellol showed MIC values from 8 to 1024 µg/mL. The drugs (MIC and 2 × MIC) inhibited the mycelial growth, conidia germination, and fungal growth on nail fragments. The drugs (half of MIC) induced the formation of wide, short, and crooked hyphae in T. rubrum morphology. With sorbitol, geraniol MIC was increased by 64-fold and citronellol by 32-fold. The drugs caused leakage of intracellular material and inhibited ergosterol biosynthesis.
Discussion: The results suggest that the drugs damage cell wall and cell membrane of T. rubrum through a mechanism that seems to involve the inhibition of the ergosterol biosynthesis.
Conclusion: This study confirms that geraniol and citronellol can be regarded as potential drugs for controlling T. rubrum growth, with great potential against agents of dermatophytosis.
Introduction
Dermatophytosis is a fungal infection in keratinized tissues caused by dermatophytes. This mycosis exhibits a cosmopolitan profile and ranks among the most common infectious diseases that affect humans (Seebacher et al., Citation2008; Silveira-Gomes et al., Citation2013). Dermatophytes are specialized fungi in keratin degradation of infecting the stratum corneum, hair, claws, or nails. Trichophyton rubrum is the most frequent in clinical cases of dermatophytosis, which has been isolated from various superficial sites of infection. Trichophyton rubrum is also responsible for deep and extensive infections with possible systemic dissemination in immunocompromised patients (Da Silva et al., Citation2014; Wu et al., Citation2013).
The treatment of dermatophytoses is often long, involving the use of drugs of the allylamine and azole class mainly. A successful treatment with antifungal agents may be often inappropriate because T. rubrum infections are associated with recidivism and drug resistance (Peres et al., Citation2010). The low diversity of chemical classes and the emergence of resistant strains propel research for new drugs with therapeutic potential (Miceli et al., Citation2011). In this context, the geraniol and citronellol are presented as some promising biologically active drugs. These compounds are monoterpenes (C10H16), produced from combination of two isoprene units (C5H8), prevalent in essential oils of aromatic medicinal plants such as Cymbopogon winterianus Jowitt ex Bor (Poaceae) (Oliveira et al., Citation2011) and other aromatic plants (Freire et al., Citation2012; Kim & Park, Citation2012).
Geraniol is a monoterpene with an alcohol group and two double bonds. Researchers have shown that geraniol is an agent with prominent repellent (Semmler et al., Citation2014), insecticide (Jeon et al., Citation2009), and antitumor activities (Wiseman et al., Citation2007). Citronellol only differs from geraniol by having a double bond in its structure. Citronellol demonstrated repellent (Semmler et al., Citation2014), larvicide (Hierro et al., Citation2004), antinociceptive, and anti-inflammatory action (Brito et al., Citation2012). Additionally, geraniol and citronellol have also shown antifungal activity (Aoudou et al., Citation2010; Van Zyl et al., Citation2006).
Terpenes are a group of antimicrobial compounds that are active against a broad spectrum of microorganisms, including yeasts and molds. Several studies have been performed emphasizing their effect on plasma membrane integrity; however, less is known about their action on T. rubrum (Hyldgaard et al., Citation2012). In the present study, experiments were performed to investigate the antifungal effect of geraniol and citronellol against T. rubrum strains and possible mechanisms involved in their antifungal activity, contributing to the search of natural drugs for dermatophytosis treatment.
Materials and methods
Test drugs
Geraniol (purity: 98%), citronellol (purity: 95%), and ketoconazole (purity: > 98%) were purchased from Sigma-Aldrich® (Sao Paulo, Brazil). The drugs were dissolved in DMSO (dimethylsulfoxide) and sterile distilled water was used to obtain solution at 1024 µg/mL. DMSO concentration did not exceed 0.5% in the assays.
Fungal strains
Dermatophytes strains were obtained from the Laboratory of Mycology (LM). They included one strains from the American Type Culture Collection (ATCC 1683) and clinical isolates obtained from nails (LM 222, LM 309, and LM 333), skin (LM 98, LM 130, LM 422, LM 582, LM 600, LM 713, LM 720, and LM 722), and scalp (LM 640 and LM 710) of patients with dermatophytosis. Stock inocula were prepared from a 10-d old culture in potato dextrose agar (Difco Lab., Detroit, MI) at 28 °C. Fungal colonies were covered with 5 mL of sterile saline solution (0.9%), the surface gently scraped with a sterile loop, and transferred to a sterile tube. These suspensions were shaken for 2 min, left in rest for 5 min, and the supernatant containing the conidia was collected. Inocula were standardized at 0.5 tube of McFarland scale (106 CFU/mL). Quantification was confirmed by plating 0.01 mL of a 1:100 dilution of each suspension in Sabouraud dextrose agar (SDA) (Difco Lab., Detroit, MI) (Barros et al., Citation2006).
Determination of minimum inhibitory concentration (MIC)
MIC values of geraniol and citronellol were determined by the broth microdilution method (Santos & Hamdan, Citation2005). For the tests, 100 µL of Sabouraud dextrose broth (SDB) (Difco Lab., Detroit, MI) was added to all wells of 96-well plates. Next, serial dilutions 2-fold were performed to obtain concentrations varying between 1024 and 1 µg/mL. Finally, 10 μL of inocula were added to wells, and the plates were incubated at 28 °C for 5 d. Negative control (without drugs) was performed to confirm the conidia viability. Sensitivity control to DMSO (0.5%) was also performed. MIC was defined as the lowest concentration capable of visually inhibiting 100% the fungal growth. The results were expressed as the geometric mean of three experiments.
Effects on dry mycelial weight
The interference of geraniol and citronellol on mycelial growth was performed by a dry mycelial weight test and it was used the strains T. rubrum ATCC 1683 and T. rubrum LM 422. This assay was performed in triplicate (Sharma & Tripathi, Citation2008). In a sterile test tube, 4.5 mL of SDB with the drugs (MIC and 2 × MIC) were added 0.5 mL of the inocula. Control experiment was performed contained sterile distilled water in place of the drugs. The tubes were incubated at 28 °C for 12 d. Cultures were filtered through a sterile filter paper (retention of particles: 11 µm). The mycelia were dried at 60 °C for 10 min. The filter paper containing dry mycelia was weighed and percent of mycelia produced was calculated, considering that control produced 100% of dry mycelia weight.
Effects on conidia germination
Geraniol and citronellol were tested to evaluate the effectiveness on the germination of T. rubrum conidia (ATCC 1683 and LM 422). Negative control was performed. Doubly concentrated SDB (500 µL) containing the drugs at the MIC and 2 × MIC was added to sterile tubes, mixed with 500 µL of inocula, and then immediately incubated at 28 °C. Samples were taken at 24 h to determine the number of germinated and ungerminated conidia was in a hemocytometer. The percentage of germinated conidia was calculated. The test was performed in triplicate (Liu et al., Citation2007).
Effects on morphogenesis
Morphogenesis alterations induced by geraniol and citronellol in T. rubrum ATCC 1683 and T. rubrum LM 422 were analyzed by the slide culture technique, in duplicate. A block of SDA containing the drugs (half of MIC) was transferred to the center of a glass slide, in a moist chamber. After that, a sample of mycelia was inoculated onto the agar medium block. The moist chamber plates were incubated at 28 °C for 5 d. After incubation time, two slides of each test were fixed in lactophenol–cotton blue stain and 10 microscopy fields on each slide were examined under light microscopy at 400×. A control assay without drugs was performed. Predominant structural changes observed in the tests were recorded as strong (+ + +), intermediate (+ +), low (+), or absent (−) (Gunji et al., 1983).
Infectivity in vitro assay
The interference of geraniol and citronellol on infection in vitro of T. rubrum strains (ATCC 1683 and LM422) was performed as described by Takasuka (Citation2000), with some modifications. Fingernail clippings were collected from healthy volunteers (five males and five females) with no obvious infection on their nails. Before the tests, this study was submitted and approved by the Ethics Committee in Research, Centro de Ciências da Saúde, Universidade Federal da Paraíba (Brazil) (protocol no. 0157/11). Human nail fragments of approximately 1 × 1 mm were treated with ethanol for 15 min and dried at room temperature. Each nail fragment in 96-well plates was soaked in 5 µL of the conidial suspension (T. rubrum ATCC 1683 and LM422) for 1 h. Next, 200 µL of drugs at MIC/2, MIC, and 2 × MIC were added. The plates were incubated at 28 °C for 6 d, and the fungal growth was observed by light microscopy. Nail fragments soaked in 200 mL distilled water were used as a control. The test was performed in triplicate. There was no obvious difference of growth rate among nails from different individuals.
Release of intracellular material
Measurement of the release of 260 nm absorbing material from T. rubrum ATCC 1683 was conducted using 2 mL aliquots of the fungal inocula after the addition of the geraniol and citronellol (MIC and 2 × MIC). After 4 and 8 h at 28 °C, the fungal cells were centrifuged at 3000 rpm for 5 min, and the supernatant was examined for leakage of intracellular material by measuring the absorbance at 260 nm, primarily nucleotides, with a UV–visible spectrophotometer (Shimadzu UV-1650 PC, Shimadzu, Columbia, MD) and quartz cuvette. Potassium hydroxide solution (25% diluted with ethanol at 70%) was used as a lysing agent. The blank tubes contained each drug at the same concentration. Control flasks without drugs were tested similarly. Rate of release of intracellular material was calculated by comparing the test values with the lysing agent (rate: 100%). The experiment was performed in triplicate (Lunde & Kubo, Citation2000).
Sorbitol effect assay
MIC values of geraniol and citronellol with T. rubrum ATCC 1683 were determined by microdilution, in the absence and presence of 0.8 M of sorbitol (VETEC Química Fina Ltd, Rio de Janeiro, Brazil). MIC was determined after 5 d. This assay was made in triplicate and the geometric mean values were calculated (Escalante et al., Citation2008).
Sterol quantitation assay
Aliquots of T. rubrum ATCC 1683 inocula (1 mL) were added to sterile tubes with 9 mL of SDB containing geraniol or citronellol (MIC and 2 × MIC) and incubated for 5 d at 28 °C. The fungal cells were harvested by centrifugation at 3000 rpm for 5 min and washed once with sterile distilled water. The wet weight of the cell pellet was determined. Lysing agent (3 mL) was added to each pellet and vortex mixed for 1 min. Cell suspensions were incubated in an 85 °C water bath for 1 h. Sterols were then extracted by the addition of a mixture of 1 ml of sterile distilled water and 3 ml of n-heptane followed by vigorous vortex mixing for 3 min. The n-heptane layer was transferred to tubes and stored under refrigeration for 24 h. An aliquot of sterol extract was examined by measuring the absorbance at 281.5 nm and 230 nm with a UV–visible spectrophotometer (Shimadzu UV-1650 PC, Shimadzu, Columbia, MD) and quartz cuvette. This assay was performed in triplicate. Ketoconazole was used as a control drug. Ergosterol content was calculated as the percentage of the wet weight of the cell as reported by Arthington-Skaggs et al. (Citation1999).
Statistical analysis
The results were expressed in mean ± SE. Differences between the means were statistically compared using unpaired t-test. The values were considered significantly different when p < 0.05.
Results
MIC values of geraniol and citronellol against T. rubrum strains are shown in . The MIC of geraniol ranged between 16 and 256 µg/mL. Geraniol inhibited the growth of 50% of T. rubrum strains at 32 µg/mL. Citronellol also showed antifungal activity with a MIC value varying from 8 to 1024 µg/mL with 50% of the strains tested inhibited at 64 μg/mL. No fungal growth inhibition by DMSO was detected and all strains were found to grow in the absence of drugs. To study the interference of geraniol and citronellol on mycelial growth, conidial germination, morphogenesis and infectivity in vitro, the strains T. rubrum ATCC 1683 and LM 422 were chosen. Both isolates had MIC values of 32 µg/mL (geraniol) and 128 µg/mL (citronellol).
Table 1. MIC values (µg/mL) of geraniol and citronellol against Trichophyton rubrum strains by microdilution method.
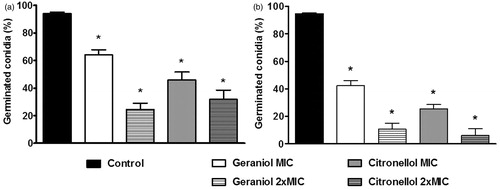
The effect of MIC and 2 × MIC of the drugs on the mycelial growth was determined by measuring the dry mycelia mass (). shows that all tested concentrations of geraniol and citronellol inhibited significantly the mycelial growth of T. rubrum ATCC 1683 (p < 0.05) compared with the control (100% mycelia yield). Regarding the LM 422 strain, the effects were similar once they inhibiting the mycelial growth effectively (). The percentage of germinated conidia of T. rubrum LM 422 and ATCC 1683 are recorded in . At their MICs, the drugs significantly inhibited conidial germination (p < 0.05), with the effects most evident at 2 × MIC.
Figure 1. Percentage of dry mycelial weight produced by Trichophyton rubrum in the absence (control) and presence of geraniol (MIC: 32 g/mL; 2 × MIC: 64 g/mL) and citronellol (MIC: 128 g/mL; 2 × MIC: 256 g/mL). (a) T. rubrum ATCC 1683; (b) T. rubrum LM 422. Control produced 100% of dry mycelial weight. *p < 0.05 compared with control.
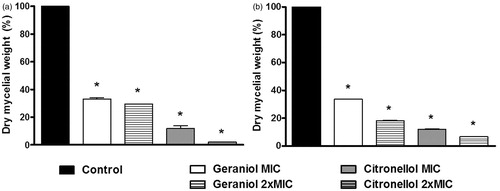
Figure 2. Percentage of germinated conidia of Trichophyton rubrum ATCC 1683 (a) and LM 422 (b) in the absence (control) and presence of geraniol (MIC: 32 g/mL; 2 × MIC: 64 g/mL) and citronellol (MIC: 128 g/mL; 2 × MIC: 256 g/mL). *p < 0.05 compared with control.
The strains T. rubrum ATCC 1683 and LM 422 were grown on SDA in the absence and presence of the drugs (half of MIC) for observation of morphological changes (). In control test, it was found the production of long hyaline hyphae, narrow, and septate, with drop-shaped conidia, arranged laterally in conidiophores and it was not observed macroconidia. Geraniol and citronellol induced similar morphologic changes in both strains. A few conidia were observed, although they were normally distributed along the conidiophores. Macroconidia were not observed in any of the test groups. The normal formation of hyphae of T. rubrum was affected in the presence of all drugs, with the formation of wide short and crooked hyphae. These abnormalities are not characteristic of the species, as can be viewed in control description. Furthermore, chlamydoconidia production by T. rubrum was induced by geraniol ().
Figure 3. Rate of release of intracellular material from Trichophyton rubrum ATCC 1683 absorbing at 260 nm in the absence (control) and presence of geraniol (MIC: 32 µg/mL; 2 × MIC: 64 µg/mL) and citronellol (MIC: 128 µg/mL; 2 × MIC: 256 µg/mL). Lysing agent induced 100% of release of intracellular material. *p < 0.05 compared with control.
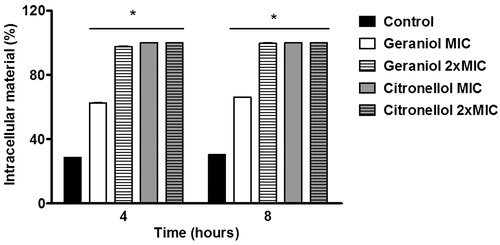
Table 2. Observations detected in microculture of Trichophyton rubrum in the absence (control) and presence of geraniol and citronellol (MIC/2).
The effect of geraniol and citronellol (MIC/2, MIC, and 2 × MIC) on the infectivity of T. rubrum strains (ATCC 1683 and LM 422) was assessed by the human nail model in vitro. Growth of T. rubrum was significantly inhibited by each drug at MIC and 2 × MIC. However, sub-inhibitory concentrations were not able to impair the capacity of T. rubrum strains grow on human nails fragments.
Further experiments were conducted only on T. rubrum ATCC 1683 because both fungal strains showed similar results on previous tests. With sorbitol, the geraniol MIC was increased by 64-fold and citronellol by 32-fold compared with tests without sorbitol (). Fungal growth was observed in control. Geraniol and citronellol caused significantly leakage of intracellular material compared with control (no drugs), after 4 h of drug incubation (p < 0.05), as can be seen in .
Table 3. MIC values (µg/mL) of geraniol and citronellol in the absence and presence of sorbitol (0.8 M) against Trichophyton rubrum ATCC 1683.
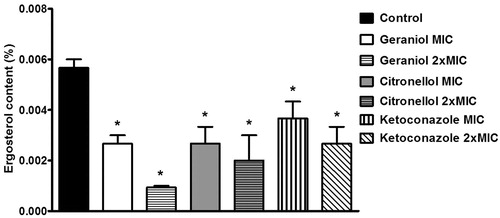
Geraniol, citronellol, and ketoconazole inhibited the sterol production by fungal cells (p < 0.05) (). Ketoconazole was used as a control in this experiment because it inhibits the biosynthetic pathway of ergosterol. Geraniol (MIC) showed results similar those obtained with ketoconazole (MIC) (p < 0.05), exerting more evident effects when the drugs were compared at their 2 × MIC (p < 0.0001). Citronellol showed results similar to ketoconazole when both were tested at 2 × MIC. Geraniol (MIC) and citronellol (MIC and 2 × MIC) showed results similar those obtained with ketoconazole at the same concentrations (p < 0.05). However, geraniol was more potent than ketoconazole when the drugs were compared at their 2 × MIC (p < 0.05).
Figure 4. Ergosterol content as a percentage of the wet weight of the Trichophyton rubrum ATCC 1683 cell in the absence (control) and presence of geraniol (MIC: 32 µg/mL; 2 × MIC: 64 µg/mL), citronellol (MIC: 128 µg/mL; 2 × MIC: 256 µg/mL) and ketoconazole (MIC: 16 µg/mL; 2 × MIC: 32 µg/mL). *p < 0.05 compared with control.
Discussion
In our study, geraniol and citronellol showed pronounced antifungal efficacy against all tested T. rubrum strains. According to the criteria proposed by Sartoratto et al. (Citation2004), geraniol and citronellol showed strong antifungal activity against T. rubrum because showed MIC values were lower than 500 µg/mL. In the literature, geraniol proved to be active against strains of Aspergillus spp, Fusarium oxysporum, and Penicillium digitatum (Aoudou et al., Citation2010; Kim & Park, Citation2012), Candida spp. (Van Zyl et al., Citation2006). The antifungal activity of citronellol is also reported against Aspergillus spp, Fusarium spp, Penicillium spp (Aoudou et al., Citation2010). Antifungal activity of geraniol and citronellol against Trichophyton spp. was demonstrated by Shin and Lim (Citation2004) and Khan and Ahmed (Citation2011) with high MIC values, in contrast to our results.
In this study, the drugs impaired the fungal growth by decreasing the dry mycelia production of T. rubrum. In the dermatophytosis pathogenesis, dermatophytes produce filamentous hyphae during the infection process. It is important because they can exacerbate the damage and penetrate into the deeper layers keratinized tissues (Brand, Citation2012). Therefore, the dry mycelial mass does not reflect the total live cells, but equally important, it reflects the production of fungal cell material. Moreover, dermatophytosis can be caused by the adherence of T. rubrum asexual conidia upon contact with the stratum corneum of the host. The conidia then germinate and the mycelium forms (Liu et al., Citation2007). For this reason, this study became prominent because it is the first report about the inhibition of T. rubrum conidia germination by geraniol and citronellol. The inhibitory effect of the terpenes and essential oils on conidia germination and dry mycelia production of Aspergillus and Trichophyton species has been previously reported (Khan & Ahmad, Citation2011).
Fungal morphogenesis is a major factor in the pathogenesis of dermatophytosis; its interference may reflect the impairment of the fungal growth and infectivity (Brand, Citation2012). It became evident the chlamydoconidia production by T. rubrum in the presence of geraniol, a fact not observed with citronellol. The formation of chlamydoconidia is considered as an indicator of stress conditions because they are produced by many fungi because they might allow survival in harsh environmental conditions as a resistant fungal form (Khan & Ahmad, Citation2011; Lin & Heitman, Citation2005). This can also be related to the ability of T. rubrum to produce chlamydoconidia as an important defense mechanism against toxic agents, as observed in our results (Ghahfarokhi et al., Citation2004).
The nail model in vitro of infectivity is cited as a simple way to simulate the condition of the infection site. It is a useful method for evaluating the therapeutic efficacy of antifungal agents against strains of T. rubrum (Schaller et al., Citation2009). Takasuka (Citation2000) showed that T. rubrum conidia can germinate and grow in vitro using nails as the only source of nutrition. Considering that fungal culture on nails might be a useful system for evaluating anti-dermatophyte compounds to predict clinical efficacy, our results are relevant because we found that all drugs tested could inhibit the growth of T. rubrum on nail fragments. It is worth noting that the drugs showed similar antifungal activity in two culture conditions: SDB and nails. Finally, our studies support the potential use of the drugs as antifungal agents.
The sorbitol assay was used to investigate the possible interference of drugs on the T. rubrum cell wall since it has extreme importance for growth and viability of fungi. The fungal cell wall serves to protect the fungus from environmental stresses, particularly osmotic changes Thus, drugs that act on the cell wall cause lysis of fungal cells in the absence of an osmotic stabilizer (sorbitol), but fungi can grow in the presence of sorbitol (Escalante et al., Citation2008; Levitz, Citation2010). Therefore, our results suggest that geraniol and citronellol interfere with the cell wall. Similarly, Souza et al. (Citation2010) evaluated the effect of Origanum essential oils on the cell wall of Aspergillus flavus and confirmed the antifungal action by sorbitol test.
In the literature, the mechanism of action of monoterpenes is not fully understood, but it is reported that they induce membrane disruption of microorganisms which increases fluidity and permeability, inducing disturbances in membrane function (Di Pasqua et al., Citation2007). With this approach, we investigated whether the drugs damage fungal cell membranes. In this way, any damage to the cell membrane could be evidenced by the detection of intracellular components, primarily nucleotides, which show a strong absorption at 260 nm (Lunde & Kubo, Citation2000). Exposure of T. rubrum cells to geraniol and citronellol caused fast loss of 260 nm absorbing material. These findings assume that the drugs induced damage and loss of cytoplasm membrane integrity in T. rubrum cells. Given the evidence presented above, it became important to clarify how these effects are caused.
Ergosterol is a sterol found in the cell membranes of yeasts and filamentous fungi and functions as an important regulator of membrane fluidity. Changes in the ergosterol biosynthetic pathway also inhibit fungal growth (Van Minnebruggen et al., Citation2010). In this perspective, the content of sterols produced by T. rubrum ATCC 1683 in the presence of geraniol, citronellol, and ketoconazole (positive control) at MIC and 2 × MIC was quantified. Ergosterol and an intermediate – 24(28)-dehydroergosterol (DHE) – absorb energy at 281.5 nm, while only DHE shows intense absorption at 230 nm. Changes in absorption patterns are indicative of interference with the ergosterol synthesis route (Arthington-Skaggs et al., Citation1999). Notably, the drugs inhibited ergosterol biosynthesis as ketoconazole. The scientific literature reports that the activity of membrane-bound enzymes, including some enzymes that synthesize cell wall polymers, is also dependent on the content of ergosterol and the native conformation of fungal cell membrane (Martinez-Rossi et al., Citation2008).
Conclusions
It is worth realizing that the decrease of content of ergosterol interferes with the integrity and functionality of the cell membrane of T. rubrum cells. The findings of the present study, particularly the possible mechanisms of action, indicate that the drugs act by a mechanism that seems to involve inhibition of the ergosterol biosynthesis. Based on this, we are first reporting observations on the way that geraniol and citronellol act on cell wall and cell membrane of T. rubrum. In conclusion, these observations might be useful for further investigation aiming clinical applications of geraniol and citronellol in the management of dermatophytosis, especially in T. rubrum-infections.
Acknowledgements
The authors conducted the experiments at the Universidade Federal de Paraíba. We thank Wellington L. Navarro for technical support and Dr. A. Leyva for English editing of the manuscript.
Declaration of interest
The authors are grateful to Brazilian agencies CNPq and CAPES for financial support. The authors report no conflicts of interest.
References
- Aoudou Y, Léopold TN, Michel JDP, et al. (2010). Antifungal properties of essential oils and some constituents to reduce foodborne pathogen. J Yeast Fungal Res 1:001–8
- Arthington-Skaggs BA, Warnock DW, Morrison CJ. (1999). Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in murine model of invasive candidiasis. Antimicrob Agents Chemother 44:2081–5
- Barros MES, Santos DA, Hamdan JS. (2006). In vitro methods for antifungal susceptibility testing of Trichophyton spp. Mycol Res 110:1355–60
- Brand A. (2012). Hyphal growth in human fungal pathogens and its role in virulence. Int J Microbiol 2012: Article ID 517529, 11 pages
- Brito RG, Guimarães AG, Quintans JSS, et al. (2012). Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. J Nat Med 66:637–44
- Da Silva BCM, Paula CR, Auler ME, et al. (2014). Dermatophytosis and immunovirological status of HIV-infected and AIDS patients from Sao Paulo city, Brazil. Mycoses. Jan 13
- Di Pasqua R, Betts G, Hoskins N, et al. (2007). Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem 55:4863–70
- Escalante A, Gattuso M, Pérez P, Zacchino S. (2008). Evidence for the mechanism of action of the antifungal phytolaccoside B isolated from Phytolacca tetramera Hauman. J Nat Prod 71:1720–5
- Freire MM, Jham GN, Dhingra OD, et al. (2012). Composition, antifungal activity and main fungitoxic components of the essential oil of Mentha piperita L. J Food Saf 32:29–36
- Ghahfarokhi MS, Goodarzi M, Abyaneh MR, et al. (2004). Morphological evidence for onion-induced growth inhibition of Trichophyton rubrum and Trichophyton mentagrophytes. Fitoterapia 75:645–655
- Gunji S, Arima K, Beppu T. (1983). Screening of antifungal antibiotics according to activities inducing morphological abnormalities. Agric Biol Chem 47:2061–9
- Hierro I, Valero A, Perez P, et al. (2004). Action of different monoterpenic compounds against Anisakis simplex s.1. L3 larvae. Phytomedicine 11:77–82
- Hyldgaard M, Mygind T, Meyer RL. (2012). Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front Microbiol 3:1–24
- Jeon JH, Lee CH, Lee HS. (2009). Food protective effect of geraniol and its congeners against stored food mites. J Food Prot 72:1468–71
- Khan MSA, Ahmad I. (2011). In vitro antifungal, anti-elastase and anti-keratinase activity of essential oils of Cinnamomum-, Syzygium- and Cymbopogon-species against Aspergillus fumigatus and Trichophyton rubrum. Phytomedicine 19:48–55
- Kim E, Park II-K. (2012). Fumigant antifungal activity of Myrtaceae essential oils and constituents from Leptospermum petersonii against three Aspergillus species. Molecules 17:10459–69
- Levitz SM. (2010). Innate recognition of fungal cell walls. PLoS Pathog 6:e1000758
- Lin X, Heitman J. (2005). Chlamydospore formation during hyphal growth in Cryptococcus neoformans. Eukaryotic Cell 4:1746–54
- Liu T, Zhang Q, Wang L, et al. (2007). The use of global transcriptional analysis to reveal the biological and cellular events involved in distinct development phases of Trichophyton rubrum conidial germination. BMC Genomics 8:100–13
- Lunde CS, Kubo I. (2000). Effect of polygodial on the mitochondrial ATPase of Saccharomyces cereviseae. Antimicrob Agents Chemother 44:1943–53
- Martinez-Rossi NM, Peres NTA, Rossi A. (2008). Antifungal resistance mechanisms in dermatophytes. Mycopathology 166:369–83
- Miceli MH, Díaz JA, Lee SA. (2011). Emerging opportunistic yeast infections. Lancet Infect Dis 11:142–51
- Oliveira WA, Pereira FO, Luna CGDG, et al. (2011). Antifungal activity of Cymbopogon winterianus Jowitt ex Bor against Candida albicans. Braz J Microbiol 42:433–41
- Peres NTA, Maranhão FCA, Rossi A, Martinez-Rossi NM. (2010). Dermatophytes: Host-pathogen interaction and antifungal resistance. An Bras Dermatol 85:657–67
- Santos DA, Hamdan JS. (2005). Evaluation of broth microdilution antifungal susceptibility testing conditions for Trichophyton rubrum. J Clin Microbiol 43:1917–20
- Sartoratto A, Machado ALM, Delarmelina C, et al. (2004). Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol 35:275–80
- Schaller M, Borelli C, Berger U, et al. (2009). Susceptibility testing of amorolfine, bifonazole and ciclopiroxolamine against Trichophyton rubrum in an in vitro model of dermatophyte nail infection. Med Mycol 47:753–8
- Seebacher C, Bouchara JP, Mignon B. (2008). Updates on the epidemiology of dermatophyte infections. Mycopathol 166:335–52
- Semmler M, Abdel-Ghaffar F, Schmidt J, Mehlhorn H. (2014). Evaluation of biological and chemical insect repellents and their potential adverse effects. Parasitol Res 113:185–8
- Sharma N, Tripathi A. (2008). Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol Res 163:337–44
- Shin S, Lim S. (2004). Antifungal effects of herbal essential oils alone and in combination with ketoconazole against Trichophyton spp. J Appl Microbiol 97:1289–96
- Silveira-Gomes F, Oliveira EF, Nepomuceno LB, et al. (2013). Dermatophytosis diagnosed at the Evandro Chagas Institute, Pará, Brazil. Braz J Microbiol 44:443–6
- Souza NAB, Lima EO, Guedes DN, et al. (2010). Efficacy of Origanum essential oils for inhibition of potentially pathogenic fungi. Braz J Pharm Sci 46:499–507
- Takasuka T. (2000). Amino acid- or protein-dependent growth of Trichophyton mentagrophytes and Trichophyton rubrum. FEMS Immunol Med Microbiol 29:241–5
- Van Minnebruggen G, François IEJA, Cammue BPA, et al. (2010). General overview on past, present and future antimycotics. Open Mycol J 4:22–32
- Van Zyl RL, Seatlholo ST, Van Vuuren SF, Viljoen AM. (2006). The biological activities of 20 nature identical essential oil constituents. J Essent Oil Res 18:129–33
- Wiseman DA, Werner SR, Crowell PL. (2007). Cell cycle arrest by the isoprenoids perillyl alcohol, geraniol, and farnesol is mediated by p21Cip1 and p27Kip1 in human pancreatic adenocarcinoma cells. J Pharmacol Exp Ther 320:1163–70
- Wu LC, Sun PL, Chang YT. (2013). Extensive deep dermatophytosis cause by Trichophyton rubrum in a patient with liver cirrhosis and chronic renal failure. Mycopathology 176:457–62
