Abstract
Context: Crataegus species are widely used as herbal medicines for preventing cardiovascular diseases (CVDs). However, there are no studies on the effects of Crataegus monogyna Jacq. (Rosaceae) and C. davisii Browicz on thrombosis, which is an important mechanism in CVDs.
Objective: The purpose of this study was to investigate the antithrombotic effects of ethanol extracts of Crataegus monogyna (CMEx) and C. davisii (CDEx) leaves by using the carrageenan-induced tail thrombosis model.
Materials and methods: The hind paw of each mouse was injected with 1% Type I carrageenan to induce thrombosis. CMEx was tested at the doses of 100, 200, and 300 mg/kg and CDEx at the dose of 50, 100, 200, and 300 mg/kg in comparison with heparin. The lengths of tail thrombosis were measured at the 24, 48, and 72 h.
Results: Does of 200 and 300 mg/kg CMEx showed significant effects (p < 0.01; p < 0.001) at 24 h when compared with the control group. The antithrombotic activity of 200 and 300 mg/kg CMEx showed a decrease at 48 and 72 h but the activity of 300 mg/kg dose of CMEx was still significant (p < 0.01). The activities of 50 and 100 mg/kg doses of CDEx were significant (p < 0.001; p < 0.01) between 24 and 72 h whereas 200 and 300 mg/kg CDEx did not show any significance.
Discussion and conclusions: CMEx and CDEx significantly inhibited the carrageenan-induced mouse tail thrombosis. Based on these results, it was concluded that CDEx and CMEx may potentially be used as therapeutic agents or complementary treatments against thrombosis.
Introduction
The formation of thrombosis in cardiovascular diseases (CVDs) seems to be one of the important mechanisms responsible for death and disability (Schussheim & Fuster, Citation1997). Intravascular arterial thrombosis causes multiple types of cardiovascular damage such as ischemia, myocardial infarction, and angina (Gross & Weitz, Citation2009; Kumaran et al., Citation2011; Lands, Citation2003). Venous thrombosis, which causes deep vein thrombosis and pulmonary embolism, is one of the most common vascular disorders after heart attack and stroke (Bahloul et al., Citation2011; Gross & Weitz, Citation2009). Antithrombotic agents have been extensively used to prevent or treat thrombus formation. Nowadays, although there are various antithrombotic drugs that have widespread clinical usage, CVDs based on the thrombosis continue to be a major cause of morbidity and mortality. Therefore, more effective therapies are still needed to cope with these disorders. Consequently, studies are on-going into new, more potent antithrombotic agents that have limited effects on hemostasis, and lower side-effects and drug-interactions (Gross & Weitz, Citation2009).
Hawthorn [Crataegus spp., (Rosaceae)] plant extract is used in traditional medicine for the management of CVDs. Hawthorn is used as a herbal medicine in Europe and China for various CVDs such as coronary heart disease, hypertension, arrhythmias, congestive heart failure, and hyperlipidemia (Salehi et al., Citation2008). Crataegus monogyna Jacq., known as common hawthorn, is one of the species highly recommended in the folk medicine (Barros et al., Citation2010). It has antioxidant and anti-inflammatory activities, vasorelaxant, hypotensive, cardiotonic, and negative chronotropic effects (Martino et al., Citation2008; Salehi et al., Citation2008) and also increases coronary blood flow activity (Taskov, Citation1977). However, in vivo antithrombotic effectiveness has not been shown. Crataegus davisii Browicz is an endemic plant in Turkey (Sozer et al., Citation2006). There are limited pharmacological studies about the C. davisii species in the literature.
The carrageenan-induced tail thrombosis model enables the observation and measurement of the progression of thrombosis visually, continuously, and accurately. Moreover, this is a non-invasive and easily applicable method that can be performed without killing small laboratory animals (Yan et al., Citation2009). Based on this knowledge and our previous study about the antithrombotic activity of Crataegus orientalis (Arslan et al., Citation2011), our purpose was to evaluate the similar activities of C. monogyna and C. davisii ethanol extracts by using this model.
Materials and methods
Chemicals
Carrageenan Type I, gallic acid (GA), chlorogenic acid (CA), (+)catechin (CAT), vitexin (VIT), quercetin (QUE), methanol, and formic acid (Sigma, St. Louis, MO), heparin (low molecular weight) (Sanofi-Aventis, Istanbul, Turkey), and DMSO (Merck, Darmstadt, Germany) were used for this study.
Plant materials
Crataegus monogyna leaves were collected from Balikesir in Turkey in July 2005. The plant sample was confirmed by Prof. Dr. Ali H. Mericli and a voucher specimen (ISTE: 62470) has been kept at the Herbarium of the Faculty of Pharmacy, Istanbul University, Istanbul, Turkey. Crataegus davisii leaves were collected from Hakkari in Turkey in October 2001. The plant sample was confirmed by Prof. Dr. Ali A. Donmez and a voucher specimen (AAD: 10326) has been kept at the Herbarium of the Faculty of Biology, Hacettepe University, Ankara, Turkey.
Preparation of extracts
The leaves of the plant were air-dried at room temperature. Powdered leaves were extracted in 50% ethanol by using a Soxhlet apparatus for 18 h. The ethanol extracts were lyophilized (11.51 g, 23.56% yield for C. monogyna and 9.74 g, 20.32% yield for C. davisii).
Determination of total phenolics and content analyses of extracts by HPLC
The total phenolic content of the extracts was determined using the Folin–Ciocalteu technique and the total phenolics were determined as GA equivalents (mgGAE/gextract) (Kupeli Akkol et al., Citation2008). H2O (6.0 mL) and sample (100 μL) were transferred to a 10.0 mL volumetric flask and afterwards 500 μL of the undiluted Folin–Ciocalteu reagent was added to the mixture. About 1.5 mL of a solution containing 20% (w/v) Na2CO3 was added and the volume was increased to 10.0 mL with H2O after 1 min. The mixture was incubated at 25 °C for 2 h. For the absorbance measurements, 760 nm was used and measurements were compared to a GA calibration curve. The data are presented as the averages of triplicate analyses.
For the HPLC analysis of extracts, stock solutions of GA, CA, (+) CAT, VIT, and QUE were prepared at 1 ppt concentration in methanol and the solutions of the calibration standards were diluted at 1–50 ppm with 50% methanol. In addition, extract samples were prepared with accurately weighted extract powder in methanol. The analysis of the compounds was performed with an Agilent 1260 series HPLC system (Waldbornn, Germany) consisting of a binary pump, an autosampler, a temperature-controlled column oven, and a diode-array detector. The analytical separation was performed using a C18 (100 mm, 3 mm i.d., and 3 μm particle size). A gradient elution was utilized for the separation of the compounds from the extracts. The elution included a two-solvent system: (A) methanol:water:formic acid (10:88:2 v/v); (B) methanol:water:formic acid (90:8:2 v/v), and the gradient program was as follows: 0–15 min 100% A; 15–20 min changed to 85% A; 20–30 min to 50%; 30–35 min to 0% A; 36–42 min back to 100% A. The flow rate was a 0.8 mL/min and the injection volume was 10 μL, the column oven was set to 40 °C. The analysis wavelength was scanned at 200–400 nm for the spectrum of the compounds, and for quantification of the compounds, signals were used at 280 nm for GA, CA, CAT, and 330 nm VIT and 256 nm QUE. The peak analysis and assignment were performed using the standard compounds, which were evaluated with retention times and UV spectrum of the compounds (Ozturk et al., Citation2007).
Animals
The experimental groups consisted of inbred Swiss albino female mice (30–35 g). They were provided from Anadolu University Experimental Animals Research Centre. They were placed in a quiet and temperature/humidity (22 °C ± 2 °C/60 ± 5%) controlled room, in which a 12 h light-dark cycle was maintained. Animal care and research protocols were based on the principles and guidelines adopted by the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised in 1985) and approved by the Local Ethics Committee of Anadolu University, Eskisehir.
Experimental groups
Animals were randomly divided into 10 groups (n = 7–11). Dry extracts were dissolved in 20% DMSO (80 mL saline water, 20 mL DMSO). The control group was injected with 20% DMSO; treatment groups were, injected with 100, 200, and 300 mg/kg C. monogyna and 50, 100, 200, and 300 mg/kg C. davisii, respectively. About 10 and 100 IU heparin sodium injected animals were used as reference groups. All injections were applied intraperitoneally (i.p.).
Carrageenan-induced mice tail thrombosis model
In order to induce thrombosis and test the thrombolytic activity of Crataegus extracts in vivo, the carrageenan-induced tail thrombosis model was utilized. The right hind paw of each mouse was injected subplantar with 40 µL 1% Type I carrageenan 40 min after each dose of Crataegus extracts (Yan et al., Citation2009). The tail-thrombus lengths were measured and photographed at 24, 48, and 72 h after carrageenan injections.
Data analysis
Statistical analyses were carried out using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Statistical differences were calculated by a one-way ANOVA, followed by Tukey's multiple comparison tests. The values were given as the mean ± S.E.M. p ≤ 0.05 was considered statistically significant.
Results
Total phenolic contents and content analyses of extracts by HPLC
The amount of total phenolics in the extracts was determined as 181.06 ± 6.5 mgGAE/gextract for C. monogyna, 99.91 ± 3.2 for C. davisii. The identification of the compounds in the extracts for quantification was performed by comparing with the retention times of the standards. The retention times of GA, CAT, CA, VIT, and QUE in the HPLC analysis were 1.9, 5.4, 6.7, 12.2, and 17.4 min, respectively. The calibration equations were constructed using the ratio of the peak area and the retention time (PN) versus ppm concentration of the calibration standards of the compounds by employing linear regression analysis. The calibration equations were calculated as GA: PN = 51.56 × C − 1.88, CAT: PN = 7.77 × C − 0.03, CA: PN = 13.02 × C + 0.26, VIT: PN = 5.20 × C − 0.01, QUE: PN = 9.51 × C − 0.74. PN is the peak normalization value, C is the concentration of the compound (ppm). Limit of quantification (LOQ) values of the compounds were derived from standard deviation of calibration data and estimated as [standard deviation of the regression equation (Sy.x)/slope of the calibration equation] by multiplying with 10. Calculated LOQ values were GA: 0.35, CAT: 0.12, CA: 0.16, VIT: 0.20, QUE: 0.45 ppm. shows the calculated concentrations of the compounds.
Table 1. The calculated concentrations of the compounds contained in CMEx and CDEx.
Formation of thrombosis
Thrombosis occurred as expected after subplantar injection of Type I carrageenan and 100% frequency of thrombosis was achieved in the control group animals. About 6 h after carrageenan injection, tail-thrombosis was visible by a red wine color which developed into serious necrosis after 24 h.
Antithrombotic activity of C. monogyna
The antithrombotic effect of ethanol extract of C. monogyna (CMEx) in carrageenan-induced tail thrombosis at 24, 48, and 72 h is shown in . The lengths of thrombosis in 100 IU heparin-treated group (p < 0.01) and CMEx-treated groups, except 100 mg/kg administrated group, were significant (200 mg/kg; p < 0.01 and 300 mg/kg; p < 0.001) at 24 hour when compared with the control group. The antithrombotic activity of 200 and 300 mg/kg CMEx showed a decrease at 48 and 72 h but the activity of 300 mg/kg dose of CMEx was still significant at the aforementioned time interval (p < 0.01). The treatment of 10 IU heparin did not show any significance within these periods of time when compared with the control group. When compared with 10 IU heparin, the antithrombotic activity of CMEx showed identical significant values in comparison with the control group. As seen in , inhibition of thrombosis (%) increased concurrent with the applied doses of CMEx. Photographs of the tail-thrombosis of each group at 48 h are shown in (photographs at 24 and 72 h not shown).
Figure 1. The effects of CMEx, CDEx, and heparin in carrageenan-induced mice tail thrombosis at 24, 48, and 72h. Values are presented as the mean ± S.E.M. aap < 0.01, aaap < 0.001 significant difference from the control; bp < 0.05, bbp < 0.01, bbbp < 0.001 significant difference from heparin 10 IU (n = 7–11).
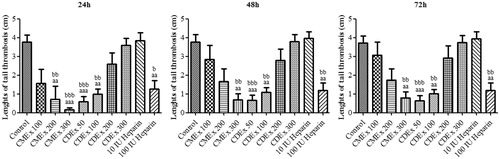
Figure 2. The effects of CMEx, CDEx, and heparin on carrageenan-induced mouse tail thrombus length (48 h after carrageenan injection). Group 1: 20% DMSO; Group 2: CDEx 50 mg/kg; Group 3: CDEx 100 mg/kg; Group 4: CDEx 200 mg/kg; Group 5: CDEx 300 mg/kg; Group 6: CMEx 100 mg/kg; Group 7: CMEx 200 mg/kg; Group 8: CMEx 300 mg/kg; Group 9: 10 IU heparin; Group 10: 100 IU heparin. Data represents two animals for each group.

Table 2. The inhibition activities of CMEx and CDEx against carrageenan-induced tail-thrombosis in mice.
Antithrombotic activity of C. davisii
The antithrombotic effect of ethanol extract of C. davisii (CDEx) in carrageenan-induced tail thrombosis at 24, 48, and 72 h is shown in . At first, 100, 200, and 300 mg/kg doses of CDEx were tested to evaluate antithrombotic activity as in the experiments performed for C. monogyna. However, interestingly, it was observed that the activity of CDEx was more obvious at lower doses. Therefore, it was decided that a 50 mg/kg dose of CDEx would be added to be certain of its efficacy at lower doses. The activities of 50 and 100 mg/kg doses of CDEx were significant (p < 0.001; p < 0.01) at the 24–72 h interval whereas 200 and 300 mg/kg CDEx did not show any significance when compared with the control group and 10 IU heparin. Inhibition of thrombosis (%), which decreased along with the injected doses of CDEx, was also calculated and shown in . Photographs of the tail-thrombosis of each group at 48 h are shown in (photographs at 24 and 72 h not shown).
Discussion
In the carrageenan-induced tail-thrombosis model, after administration of carrageenan, a red wine-colored part appears in the tail tip of the rodent, the length of thrombosis increases with the time elapsed, and then the pathogen region experiences typical dry necrosis. Since thrombus formation developed in the tail and has a clear border with the normal part, it is possible to detect the time it first appeared, the developing process, spreading range, and length of the formed thrombus in vivo (Yan et al., Citation2009). In our study, thrombosis occurred as expected through the subplantar injection of Type I carrageenan and 100% frequency of thrombosis was achieved in control group animals.
There are only a few studies about the effects of Crataegus species on thrombosis, which is an important mechanism in CVDs. In one of these studies, C. oxycantha tincture and flavonoids isolated from hawthorn have been demonstrated to suppress the formation of thromboxane A2 (TxA2) in vitro, which has a role in platelet aggregation and the inflammation process (Vibes et al., Citation1994). Another study is about the inhibition of balloon catheter-induced intimal hyperplasia by using Crataegus extract WS® 1442 in the carotid artery of rats, directly influencing platelet-derived growth factor receptor-(PDGFR)-β (Fürst et al., Citation2010). We recently used a carrageenan-induced tail thrombosis model to display the distinct antithrombotic effect of ethanol extract of C. orientalis (Arslan et al., Citation2011). In the present study, C. monogyna and C. davisii were evaluated for their antithrombotic activities and found to be promising. The findings obtained about CMEx indicate a similar pattern to ethanol extract of C. orientalis M. Bieb. ssp. szovitsii (Pojarkova), in which the activity was increased concurrent with the higher doses, whereas CDEx showed its efficacy at lower doses and lost the effectiveness as the dose increased. That is to say CDEx may present a U-shaped dose–response curve for antithrombotic effect. This dose–response curve is generally explained by drug actions on different systems (stimulant and depressant) with different thresholds of sensitivity to those drugs (Pereira et al., Citation2005). As C. monogyna and C. davisii are different species, they contain similar biologically active components. It is not surprising that different species show different activities due to variation of ingredients and in the amounts of the active substances.
We speculate that the antithrombotic activity of CDEx and CMEx might be via activation of Hageman factor, which is followed by intravascular coagulation but not by inducing platelet aggregation, since this is one explanation for the thrombotic activity of carrageenan, used for induced thrombosis in this study. The exact mechanism is uncertain for thrombosis induced by carrageenan (Yan et al., Citation2009). These speculations suggest the need for further investigations.
Flavonoids and oligomeric proanthocyanidins are the major constituents of hawthorn, and they are responsible for many pharmacological activities (Yao et al., Citation2008). In this study, it was shown that CMEx contains VIT, QUE, and clorogenic acid and CDEx contains VIT and CA. Pharmacological activity may be arising due to these phenolics. Prevalence of CVD may be reduced by herbs containing flavonoids. Some flavonoids are responsible for the inhibition of platelet aggregation (Tzeng et al., Citation1991). The possible mechanism postulated for the antithrombotic activity of flavonoids is their binding to cell receptors (Akyama et al., Citation1987; Polette et al., Citation1996). Thus, both adenosine receptors and von Willebrand factor binding to platelet glycoprotein Ibα have been recommended for blocking by flavones (Jacobson et al., Citation2002; Mruk et al., Citation2000). Some flavonoids such as apigenin, which is one of the main components of the Crataegus species, have been reported to inhibit TxA2-mediated responses (Guerrero et al., Citation2003). This reported finding also supports our speculation about the antithrombotic activities of Crataegus extracts. Since hawthorn also contains proanthocyanidins as major compounds rather than flavonoids, it is not appropriate to base their antithrombotic activity on the flavonoid content of Crataegus extracts. For instance, proanthocyanidins obtained from cocoa have been shown to suppress platelet function (Murphy et al., Citation2003).
Conclusion
Our results show the significant antithrombotic effects of ethanol extracts of C. monogyna and C. davisii for the first time. However, the exact mechanism(s) of the antithrombotic effects of these extracts remains unclear. Future studies looking at the effects of C. monogyna and C. davisii on platelet numbers, protrombin time, and activated partial thromboplastin time are needed to establish its mechanism(s) of action. It will eventually be worth considering the Crataegus species as potential therapeutic agents against thrombosis in complementary medicine and as good candidates for the development of new antithrombotic agents.
Acknowledgements
The authors are grateful to Prof. Dr. Ali A. Donmez from the Faculty of Science, Hacettepe University, Ankara, and Prof. Dr. Ali H. Mericli from the Faculty of Pharmacy, İstanbul University, İstanbul, Turkey, for the collection and identification of the plants.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Financial support for this work through the Scientific Research Projects Foundation of Anadolu University, Eskisehir, Turkey is gratefully acknowledged (Project code no: 090331).
References
- Akyama T, Ishida J, Nakagawa S, et al. (1987). Genistein, a specific inhibitor of tyrosine specific protein kinases. J Biol Chem 262:5592–5
- Arslan R, Bor Z, Bektas N, et al. (2011). Antithrombotic effects of ethanol extract of Crataegus orientalis in the carrageenan-induced mice tail thrombosis model. Thromb Res 127:210–13
- Bahloul M, Chaari A, Dammak H, et al. (2011). Post-traumatic pulmonary embolism in the intensive care unit. Ann Thorac Med 6:199–206
- Barros L, Carvalho AM, Ferreira IC. (2010). Comparing the composition and bioactivity of Crataegus monogyna flowers and fruits used in folk medicine. Phytochem Anal 22:181–8
- Fürst R, Zirrgiebel U, Totzke F, et al. (2010). The Crataegus extract WS® 1442 inhibits balloon catheter-induced intimal hyperplasia in the rat carotid artery by directly influencing PDGFR-β. Atherosclerosis 211:409–17
- Gross PL, Weitz JI. (2009). New antithrombotic drugs. Clin Pharmacol Ther 86:139–46
- Guerrero JA, Lozano ML, Castillo J, et al. (2003). Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J Thromb Haemost 5:369–76
- Jacobson KA, Moro S, Manthey JA, et al. (2002). Interactions of flavones and other phytochemicals with adenosine receptors. Adv Exp Med Biol 505:163–71
- Kumaran S, Palani P, Chellaram C, et al. (2011). Screening of fibrinolytic protease from south Indian isolates of Ganoderma lucidum. Inter J Pharm Biosci 2:419–31
- Kupeli Akkol E, Goger F, Kosar M, Baser KHC. (2008). Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem 108:942–9
- Lands WEM. (2003). Primary prevention in cardiovascular disease: Moving out of the shadows of the truth about death. Nutr Metab Cardiovasc Dis 13:154–64
- Martino E, Collina S, Rossi D, et al. (2008). Influence of the extraction mode on the yield of hyperoside, vitexin and vitexin 2′′-O-rhamnoside from Crataegus monogyna Jacq. (Hawthorn). Phytochem Anal 19:534–40
- Mruk JS, Webster MWI, Heras M, et al. (2000). Flavone-8-acetic acid (flavonoid) profoundly reduces platelet dependent thrombosis and vasoconstriction after deep arterial injury in vivo. Circulation 101:324–8
- Murphy KJ, Chronopoulos AK, Singh I, et al. (2003). Dietary flavanols and procyanidins oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr 77:1466–73
- Ozturk N, Tuncel M, Tuncel NB. (2007). Determination of phenolic acids by a modified HPLC: Its application to various plant materials. J Liq Chromatogr 30:587–96
- Pereira P, Tysca D, Oliveira P, et al. (2005). Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacol Res 52:199–203
- Polette A, Lemaitre D, Lagarde M, Véricel E. (1996). N-3 fatty acid-induced lipid peroxidation in human platelets is prevented by catechins. Thromb Haemost 75:945–9
- Salehi S, Long SR, Proteau PJ, Filtz TM. (2008). Hawthorn (Crataegus monogyna Jacq.) extract exhibits atropine-sensitive activity in a cultured cardiomyocyte assay. J Nat Med 63:1–8
- Schussheim AE, Fuster V. (1997). Thrombosis, antithrombotic agents, and the antithrombotic approach in cardiac disease. Prog Cardiovasc Dis 40:205–38
- Sozer U, Donmez AA, Mericli AH. (2006). Constituents from the leaves of Crataegus davisii Browicz. Sci Pharm 74:203–8
- Taskov M. (1977). On the coronary and cardiotonic action of crataemon. Acta Physiol Pharmacol Bulg 3:53–7
- Tzeng SH, Ko WC, Ko FN, Teng CM. (1991). Inhibition of platelet aggregation by some flavonoids. Thromb Res 64:91–100
- Vibes J, Lasserre J, Gleye J, Declume C. (1994). Inhibition of thromboxane A2 biosynthesis in vitro by the main components of Crataegus oxycantha (Hawthorn) flower heads. Prostaglandins Leukot Essent Fatty Acids 50:173–5
- Yan F, Yan J, Sun W, et al. (2009). Thrombolytic effect of subtilisin QK on carrageenan induced thrombosis model in mice. J Thromb Thrombolysis 28:444–8
- Yao M, Ritchie HE, Brown-Woodman PD. (2008). A reproductive screening test of hawthorn. J Ethnopharmacol 118:127–32
