Abstract
Context: Geniposidic acid, one of the main active ingredients in Gardenia jasminoides J. Ellis (Rubiaceae), may also possess important pharmacological activities for cardiovascular disorders similar to other derivatives, such as geniposide.
Objective: To evaluate its anti-atherosclerosis (anti-AS) effect, the related pharmacological activities and possible cellular mechanisms were studied.
Materials and methods: Thirty rabbits were randomly divided into normal control group, model control group, and geniposidic acid subgroups. In the AS model, its effects on the intima/media thickness ratio and aortic morphology were observed. In the study of primary cultured endothelial cells (ECs) and human umbilical artery smooth muscle cells (HUASMCs), its activities on both ECs and HUASMCs proliferation, HUASMCs' migration were also studied.
Results: Compared with the model control group, the plaque area, intima/media thickness ratio, and intimal foam cells number in geniposidic acid (80, 160, and 240 mg/kg) subgroups were significantly improved (p < 0.05). By HE staining, the activities of geniposidic acid on relieving ECs shedding and improving aortic morphology disorders were also demonstrated. From the results of CCK-8 testing, only 100 μg/ml geniposidic acid performed significant inhibition on SMC proliferation. The relative IC50 of geniposidic acid on SMC inhibition was 87.73 μg/ml. Geniposide acid also showed promotion effect on ECs proliferation, and the related ED50 of geniposidic acid was 86.05 μg/ml. Besides, only 50 and 100 μg/ml geniposidic acid showed obvious inhibition on SMC migration from the upper chamber (p < 0.05).
Discussion and conclusion: The effects of geniposidic acid on protecting vascular endothelium and reversing plaque formation in an atherosclerotic model were demonstrated.
Introduction
Geniposidic acid is one of the main active ingredients of Gardenia jasminoides J. Ellis (Rubiaceae), both of which were traditional Chinese medicines. Many studies had showed that geniposidic acid had effects on blood pressure regulation, lowering blood pressure (Koo & Li, Citation1977), repairing soft tissue injury (Yao et al., Citation1991), anti-inflammatory (Carrillo-Ocampo et al., Citation2013), cancer prevention (Li et al., Citation2000), osteoporosis treatment (Ha et al., Citation2003), etc. As one of its derivative, geniposide also indicated a similar wide range of pharmacological activities, such as anti-thrombosis (Zhang et al., Citation2013), anti-platelet aggregation (Liu et al., Citation2013), etc. In recent years, some studies reported that Gardeniae extract exhibited a positive effect on promoting endothelial cell (EC) proliferation, protecting endothelial function injured by a variety of factors, and meanwhile, showed no obvious effect on smooth muscle cells (SMCs) (Hayashi et al., Citation1992; Kaji et al., Citation1991; Metzner et al., Citation2007). Finally, it might also play some role on cerebral ischemia–reperfusion injury mitigation and other pharmacological effects. The main dynamic factors of atherosclerosis (AS) at the beginning were vascular endothelial injury and a large accumulation of oxidized low-density lipoprotein. However, the specific role of geniposidic acid in the total pharmacological activities of Gardeniae extract was still unknown as there was no associated study on it yet. Therefore, this study intended to start from the animal experiment to evaluate whether geniposidic acid has anti-AS effects, and then to the associated mechanisms in cellular level for further clarification of the possible role of geniposidic acid in the development of AS process.
Materials and methods
Animals
Healthy male New Zealand rabbits in general grade with body mass of 2.0–2.5 kg were obtained from the Experimental Animal Center of Henan Province in China. Rabbits were housed in natural light with a laboratory temperature of 24 ± 1 °C and a humidity of 50–60% under the condition of ventilation. They were provided a fixed amount of food with free access to drink water. After the adaptive feeding for 7 d, animals were randomly divided into five groups: normal control group, model control group, and geniposidic acid subgroups (80, 160, and 240 mg/kg), keeping six rabbits in each group. This study obtained the approval of local ethics committee and followed the basic principles of the Declaration of Helsinki.
Drugs, chemicals, and instruments
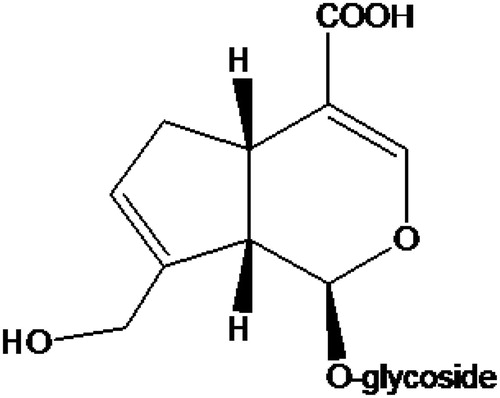
Geniposidic acid (purity 95%) was purchased from Linchuan Zhixin Biotechnology Co., Ltd. (Jiangxi, China). The chemical structure is shown in . The other reagents included were the following: DMEM/F12 medium, M199 medium, and fetal bovine serum (Hyclone Co., Ltd., Logan, UT); collagenase II and trypsin (Invitrogen Corporation, Grand Island, NY); CCK-8 kit (Dojindo Laboratories, Kyushu, Japan); crystal violet (Sigma Chemical Co., St. Louis, MO), saline (for infusion, Kelun Co., Ltd., Sichuan, China), and neonatal umbilical cord (The Fifth Affiliated Hospital of Zhengzhou University, Henan, China). The rabbit basal diet consisted of protein (21.1%), crude fiber (10.3%), ash (7.4%), water (9%), calcium (1.3%), and phosphorus (0.73%). The rabbit high-cholesterol diet was composed of basal diet, egg yolk powder, lard, cholesterol, and starch (83:10:3:1:3) from the Experimental Animal Center of Zhengzhou University (Henan, China).
The instruments included were the following: CO2 incubator (Binder Company, Tuttlingen, Germany), microplate reader (Bio-tek Co., Ltd., Winooski, VT), clean benches (Suzhou Ronghai Purification Technology Co., Ltd., Jiangsu, China), and optical microscope (Olympus Corporation, Tokyo, Japan).
Measurements of HE staining, aortic plaque area, and intima/media thickness ratio in high-fat diet-induced atherosclerosis rabbit model
Rabbits in the normal control group were fed with basal diet, while those in the model subgroups were fed with high-cholesterol diet. Each rabbit was given a quantitative diet of 120 g/d, in which all were basal diet for the normal control group but they were composed of 40 g/d high-cholesterol diet and 80 g/d basic diet for model subgroups. The rabbits in the geniposidic acid subgroups were also fed with different concentrations of geniposidic acid (80, 160, and 240 mg/kg). During the feeding for 12 weeks, the high-cholesterol diet was given firstly, and then basal diet was provided as a supplement. All rabbits had free to access to water.
After feeding, rabbits were killed via ear vein injection of 20 ml air. The chest was quickly opened, and the aorta segment between aortic arch and iliac artery bifurcation was separated. The residual vessels of arterial branches and adventitia fat were also cleared. Subsequently, the anterior wall of the aorta was cut along the median carefully using ophthalmic scissors. After blood clots were washed away, the cut artery was tiled on cardboard, fixed by placing into 10% neutral formalin, then washed with water 48 h later, dehydrated by 85% ethanol for 4 h, placed into the dye solution of Sudan III for impregnation for 30 min, followed with differentiation in 70% ethanol for 2 min, and then impregnated in the dye Sudan IV for 30 min. After that, it was differentiated secondly in 70% ethanol for 30 min, washed with water for 30 min, and finally reserved in the solution of 10% formalin.
For HE staining, paraffin sections were prepared after conventional dehydration. The relative pathological changes of intima and adventitia under optical microscopy were recorded.
The measurements of aortic plaque area and intima/media thickness ratio were completed through taking pictures with a camera. The plaque area was calculated with the software of Osiris 4 (The area identified by software did not mean the actual area, and could not be translated to actual area. But the data identified by the same software within the group and between groups were comparable). The thickest cross-section of aortic intima between the free edge of intimal cavity surface and internal elastic lamina was selected and identified as the thickness of intima. The medial thickness of aortic vessel was measured between internal elastic lamina and external elastic lamina. Therefore, the ratio of intimal thickness/medial thickness was obtained.
Under the 40 × 10 magnification condition, eyepiece micrometer and hand control counters were used to calculate foam cells number of five small lattices, taking the average value of all the slices to calculate foam cells number of each 1 mm2, which was foam cell number per unit area of intima.
ECs in primary culture
Human umbilical vein ECs (HUVECs) were isolated by enzymatic digestion (Wakabayashi et al., Citation2013). Umbilical cord was obtained aseptically from normal childbirth or caesarean newborn. Two ends of the umbilical vein were inserted by the needles with cannula and pinched by a hemostatic clamp. After the lumen was flushed with saline, 0.1% collagenase II was added for digestion for 15 min. Then digested solution was collected and added into the M199 medium containing serum. The supernatant was discarded after centrifugation. The culture fluid was added to mix the cells and finally they were placed into culture flasks for cell grow. After cell fusion, 0.25% trypsin was utilized to digest cells for subculture. The second–third generations were used for the following experiments.
SMCs in primary culture
Human umbilical artery smooth muscle cells (HUASMCs) were obtained by the tissue adherent method (Artwohl et al., Citation2009; Ilagan & Amsden, Citation2009). Saline was applied to rinse the umbilical cord. After the umbilical arteries were stripped, the umbilical cord was cut into small pieces affixed to the culture bottle wall and cultured by DMEM/F 12 culture solution containing serum. After the cells were grown and migrated out of the tissue block, 0.25% trypsin was utilized to digest cells for the subculture. The third–fifth generations were used for the experiments.
The effects of geniposidic acid on EC and HUASMCs proliferation
EC was cultured in drug-containing medium for 12, 24, and 48 h, while HUASMC was for 12, 18, and 24 h. Each dose subgroup was given five sub-holes. About 10 μl CCK-8 reagent was added into each hole 2 h before the termination of culture. The absorbance of cell supernatant was detected at a wavelength of 450 nm, and the cell culture medium was used as a blank control of zero absorbance. The original cell was still cultivated in the cultural medium and the absorbance values of supernatant were tested in different time points with the same method.
EC and HUASMC all were seeded in 96-well plates at a density of 5 × 103 cells/well. After 24 h incubation, adherent cells were randomly divided into five groups: (1) control group: the culture medium (containing 20% fetal bovine serum); (2) subgroups with different concentrations intervention of geniposidic acid (25, 50, and 100 μg/ml) in cultured cells. Drug-containing medium was prepared immediately by weighing and dissolving different masses of geniposidic acid in the culture medium before use.
SMCs migration ability to detect
Transwell migration chambers (8 μm pore size) were applied to determine migration ability of HUASMC. HUAMSMC under logarithmic phase were selected and utilized to prepare a cell suspension at the density of 1.5 × 107 cells with a cultural medium containing 0.1% fetal bovine serum (FBS). After mixing evenly, 200 μl were carefully added into the upper chamber of transwell chambers, while 500 μl culture medium containing 0.1% FBS were added into the lower chamber. About 50 μl of geniposidic acid (25, 50, and 100 μg/ml) were added into each upper chamber of the Transwell chambers with two sub-wells for each dose group. They were cultured in an incubator thermostat with the condition of 5% CO2 and saturated humidity for 24 h. Then the chambers were moved out and rinsed with phosphate-buffered saline, wiping off the cells upon the surface of filter membrane. The cells that invaded into porous membrane and attached under the porous membrane would be fixed by 4% paraformaldehyde for 10 min, stained by crystal violet, and randomly selected under a fluorescence microscope with the magnification of 200 × to count the number of cells migrating to the bottom of porous membrane. The migration abilities of HUASMC in each group were estimated.
Statistical analysis
Results were expressed in the form of mean ± SEM. Data were analyzed by a one-way ANOVA, followed with Student's two-tailed t-test for comparison between two groups. p < 0.05 means statistically significant.
Result
HE-staining results
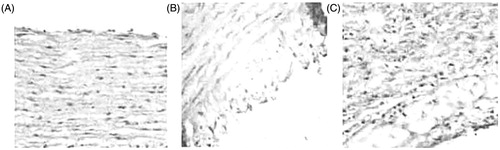
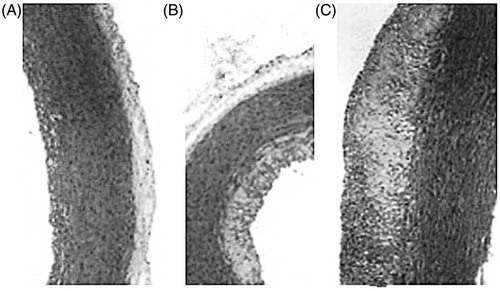
Aortic elastic membrane in the normal diet group was integral. Endothelium was close to the internal elastic membrane and was organized in neat rows with parallel smooth muscle and middle elastic membrane. In the model control group, aortic intima thickened significantly with a large accumulation of foam cells. ECs fell off or loosely attached to the surface of membrane themselves. Intimal lesions always went along with the extensively pathological changes of collagen fiber glass. Elastic fibers were ruptured and disappeared as well. In geniposidic acid subgroups, compared with the thickening degree of aortic intima in the model control group, sub-endothelial gap was increased with visible foam cells aggregation. But foam cell number was significantly less than that of the model control group. The structure of medial membrane was basically integral with SMCs in the same pole, as it shown in (200 ×) and (100 ×).
The effects of geniposidic acid on rabbit aortic plaque area, intimal thickness/medial thickness, and foam cells number
By visual observation on the normal diet group, rabbit aortic intima surface was smooth and flat with the color of milky white. There was no plaque and the result of Sudan staining was negative. In the high-cholesterol-induced rabbit AS model, aortic intimal surface was rough with different sizes of white plaques, which merged together and covered almost the entire arterial wall. The result of Sudan staining was positive, especially on the branches entrance of aortic arch, thoracic aorta, and abdominal aorta. In geniposidic acid treatment subgroups (160 and 240 mg/kg), aortic intima became smooth, and the average thickness of plaques on aortic arch was significantly thinner compared with the model control group. Lipid depositions in other parts were also scattered. As shown in , the plaque area, intimal/medial thickness ratio, and the intimal foam cells number of rabbits in the AS model were significantly reduced in geniposidic acid treatment subgroups.
Table 1. The effects of geniposidic acid on rabbit aortic plaque area, intimal/medial thickness ratio, and the foam cell number in the AS model.
The effect of geniposidic acid on EC proliferation
ECs were cultured in a drug-containing medium for 12, 24, and 48 h. After that, the OD value of culture supernatants in each group was recorded. The results showed that geniposidic acid with the concentrations of 50 and 100 μg/ml could promote ECs proliferation, as shown in . According to the increased rate of EC in each subgroup of geniposidic acid at 48 h, the related ED50 of geniposidic acid was 86.05 μg/ml.
Table 2. The effects of geniposidic acid on EC proliferation.
The effects of geniposidic acid on HUASMCs proliferation and migration
After treatment for 12, 18, and 24 h, OD values of culture supernatants were determined. The results showed that geniposidic acid with all different concentrations could inhibit HUASMCs proliferation to a certain degree. But only 100 μg/ml geniposidic acid showed significant effects compared with the control group. In the Transwell chamber experiments, 50 and 100 μg/ml geniposidic acid resulted in obvious activities on SMCs migration, as it is shown in and . According to the inhibition rate of SMCs in each subgroup of geniposidic acid at 48 h, the IC50 of geniposidic acid was 87.73 μg/ml.
Figure 4. The crystal violet staining of HUASMCs on each group: (A) 100 μg/ml geniposidic acid group and (B) control group.
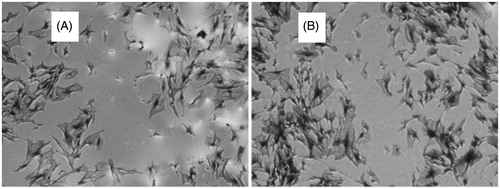
Table 3. The effects of geniposidic acid on HUASMCs proliferation and migration.
Discussion
Many vascular cells are involved in the early formation and development of AS, which was a kind of chronic inflammatory disorder. And inflammatory mediators also play an important role in the occurrence and development process of AS. This study evaluated the effect of geniposidic acid on AS via high-cholesterol-induced rabbit AS model. The results showed that after the administration of geniposidic acid, both high blood cholesterol-induced rabbit aortic plaque area, intimal/medial thickness ratio and the intimal foam cells number were markedly reduced in the AS model. According to the results of HE staining via light microscope, geniposidic acid could significantly reduce or relieve the thickening degree of aortic intima, subendothelial space, ECs number loss, and the accumulation number of foam cells, maintaining the basic integrity of the membrane structure and polarity consistency of SMCs. From the phenomenon above, it could explain that geniposidic acid might have a protective effect on vascular tissues to some degree. This study evaluated the effects of geniposidic acid on both HUASMC and EC proliferation by primary culture. The results found that it was also effective on improving EC proliferation in different concentrations (25, 50, and 100 μg/ml). Although geniposidic acid in low concentration could not obviously suppress HUASMC proliferation, it still showed remarkable inhibition on HUASMCs migration and proliferation in high concentrations (100 μg/ml). Considering that HUASMCs exposed to plasma could promote atherosclerotic plaque formation through its own migration, geniposidic acid might be effective on weakening further expansion of arterial plaque.
In short, geniposidic acid might play important roles on preventing and reversing blood vessel injury in the early AS state by inhibiting foam cell formation and SMCs proliferation, protecting ECs and promoting its proliferation. They all resulted in the anti-AS effect of geniposidic acid at the cellular level and in an animal model. Further studies with more animal models with different injury degrees of blood vessels are still warranted.
Declaration of interest
There is no conflict of interest. The authors alone are responsible for the content and writing of the paper. This work was supported by finances from Science and Technology Program Project of TaiZhou (Grant no. 1301ky19, Zhejiang, China).
References
- Artwohl M, Lindenmair A, Roden M, et al. (2009). Fatty acids induce apoptosis in human smooth muscle cells depending on chain length, saturation, and duration of exposure. Atherosclerosis 202:351–62
- Carrillo-Ocampo D, Bazaldza-Gzaldzald Bazald D, Bazaldosis Aburto-Amar R, et al. (2013). Anti-Inflammatory activity of iridoids and verbascoside isolated from Castilleja tenuiflora. Molecules 18:12109–18
- Ha H, Ho J, Shin S, et al. (2003). Effects of Eucommiae Cortex on osteoblast-like cell proliferation and osleoclast inhibition. Arch Pharm Res 26:929–36
- Hayashi T, Kaji T, Takebayashi M, et al. (1992). Stimulants from Gardeniae fructus for cultured endothelial cell proliferation. Chem Pharm Bull (Tokyo) 40:942–5
- Ilagan BG, Amsden BG. (2009). Surface modifications of photocrosslinked biodegradable elastomers and their influence on smooth muscle cell adhesion and proliferation. Acta Biomater 5:2429–40
- Kaji T, Hayashi T, Miezi N, et al. (1991). Gardenia fruit extract does not stimulate the proliferation of cultured vascular smooth muscle cells, A10. Chem Pharm Bull (Tokyo) 39:1312–4
- Koo A, Li KM. (1977). Phytochemical properties and hypotensive mechanism of extracts from Gardenia jasminoides seeds. Am J Chin Med (Gard City N Y) 5:31–7
- Li Y, Kamo S, Metori K, et al. (2000). The promoting effect of eucommiol from Eucommiae Cortex on collagen synthesis. Biol Pharm Bull 23:54–9
- Liu H, Chen YF, Li F, Zhang HY. (2013). Fructus Gardenia (Gardenia jasminoides J. Ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. J Asian Nat Prod Res 15:94–110
- Metzner J, Popp L, Marian C, et al. (2007). The effects of COX-2 selective and non-selective NSAIDs on the initiation and progression of atherosclerosis in ApoE–/– mice. J Mol Med (Berl) 85:623–33
- Wakabayashi T, Naito H, Takara K, et al. (2013). Identification of vascular endothelial side population cells in the choroidal vessels and their potential role in age-related macular degeneration. Invest Ophthalmol Vis Sci 54:6686–93
- Yao Q, Zhou G, Zhu Y, et al. (1991). Screening studies on anti-inflammatory function of traditional Chinese herb Gardenia jasminoides Ellis and its possibility in treating soft tissue injuries in animals. Zhongguo Zhong Yao Za Zhi 16:489–93
- Zhang HY, Liu H, Yang M, Wei SF. (2013). Antithrombotic activities of aqueous extract from Gardenia jasminoides and its main constituent. Pharm Biol 51:221–5