Abstract
Context: Tragopogon graminifolius DC. (Compositae) (TG) has been proposed as an efficacious remedy for gastrointestinal ulcers in Iranian traditional medicine.
Objective: The present study evaluates the efficacy of TG on experimental colitis and the responsible mechanisms.
Materials and methods: After induction of IBD by 2,4,6-trinitrobenzenesulfonic acid (TNBS), rats received standardized ethanol extract of TG aerial part at 20, 30, or 50 mg/kg/d orally. After 12 d, the rats were sacrificed and the colon was removed and assessed for macroscopic and microscopic changes. Also, tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), total antioxidant capacity, myeloperoxidase (MPO), and lipid peroxidation (LPO) were measured in the colon homogenate.
Result: TG extract significantly reduced macroscopic and microscopic scores of colitis with ED50 values of 23 and 39 mg/kg, respectively. MPO was significantly reduced in all plant extract groups with an ED50 value of 41 mg/kg. The ED50 values of extract for inhibition of TNF-α and LPO were 44 and 93 mg/kg, respectively. IL-1β significantly decreased by 50 mg/kg of TG extract (ED50 = 57 mg/kg). Total antioxidant power markedly increased by 50 mg/kg group (ED50 = 43 mg/kg).
Discussion: TG exhibited efficacy on TNBS-induced colitis via anti-inflammatory, immunomodulatory, antioxidant, and mucosal healing properties.
Conclusion: TG possesses promising healing function on colitis. Clinical trials are warranted to prove its efficacy and tolerability in IBD.
Introduction
Chronic or relapsing activation of the immune system and also inflammation of the gastrointestinal (GI) tract called inflammatory bowel disease (IBD) is a prevalent disorder in the world with unknown etiology. Crohn's disease (CD) and ulcerative colitis (UC) are the main types of IBD (Allocca et al., Citation2013; Rahimi et al., Citation2013a). Imbalance in the immune system (Wallace et al., Citation2014), oxidative stress (Jena et al., Citation2012), microflora of GI (De Greef et al., Citation2013), toxic stress (Abdolghaffari et al., Citation2010), abnormality in the epithelial mucosa (Rahimi et al., Citation2013a), and irregularity of inflammatory agents (Pedersen et al., Citation2014) are the most important factors that seem to be involved in the etiology of IBD. Imbalance in the production of pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-1β which play a pivotal role in the signaling cascade of inflammatory pathway, are considered therapeutic targets in the management of IBD (Pedersen et al., Citation2014; Wallace et al., Citation2014). Although a wide range of chemical drugs has been considered useful in the management of IBD, serious side effects, probability of disease recurrence and drug interaction has resulted in a comprehensive trend to herbal resources with a long history of efficacious and safe usage in the traditional medicine of different nations (Rahimi et al., Citation2008, Citation2010). Many medicinal plants in Iranian folk and traditional medicine sources have been proposed for the management of IBD (Rahimi et al., Citation2010, Citation2013b). One of them is Tragopogon graminifolius DC. (Compositae), known as “lahiat-o-tis” or “sheng”, with various therapeutic effects including antibacterial, poison eliminating, antidiarrheal, wound healing, and antihemorrhage activity and is assumed to be useful for the treatment of gastrointestinal disorders such as peptic ulcer and IBD (Farzaei et al., Citation2013a,Citationb). TG is widely used as a green vegetable or as an ingredient of some meals in Kermanshah province, the west of Iran.
The main constituents of Trogopogon genus are phenolic compounds including gallic acid, ferulic acid, caffeic acid, rutin, sinapic acid, resveratrol, apigenin, luteolin, quercetin, vitexin, isovitexin, vicenin-1and 2, swertisin, orientin, isoorientin, and lucenin (Kroschewsky et al., Citation1969; Kucekova et al., Citation2011). Teriterpene saponins, bibenzyls, and dihydroisocoumarins have been found in the Tragopogon genus (Miyase et al., Citation1992; Zidorn et al., Citation2005). Additionally, some vitamins such as C, K, and E were recognized in Trogopogon species (Vardavas et al., Citation2006).
The present study was conducted to investigate the efficacy of a standardized extract from TG in experimental model of colitis and demonstrate related mechanisms.
Materials and methods
Chemicals
2,4,6-Trinitrobenzene sulphonic acid (TNBS) (Sigma-Aldrich, Steinheim, Germany), ethanol, methanol, ethyl acetate, n-hexane, thiobarbituric acid (TBA), trichloroacetic acid (TCA), n-butanol, hexadecyltrimethyl ammonium bromide (HETAB), 2,4,6-tri(2-pyridyl)-S-triazine (TPTZ), hydrochloric acid (HCl), malondialdehyde (MDA), ethylene diamine tetra acetic acid (EDTA), toluene, dichloromethane, O-dianisidine hydrochloride, hydrogen peroxide, acetic acid, sodium acetate, Coomassie reagent, bovine serum albumin (BSA), ferric chloride (FeCl3–6H2O), sodium sulfate (Na2SO4), acetone, rhodanine, gallic acid standard, potassium hydroxide (KOH), sulfuric acid (H2SO4), phosphoric acid (H3PO4), potassium dihydrogen phosphate (KH2PO4), potassium hydrogen diphosphate (K2HPO4), hydrogen peroxide (H2O2), sodium carbonate (Na2CO3), sodium bicarbonate (NaHCO3), Na–K–tartarate, cupric sulfate (CuSO4–5H2O) from Merck (Germany), and rat-specific tumor necrosis factor-α (TNF-α), and interlukin-1β (IL-1β) enzyme-linked immunosorbent assay (ELISA) kits from Bender Med Systems GmbH (Vienna, Austria) were used in this study.
Plant material
TG was collected from the west of Iran (Kermanshah province) in April and authenticated by Dr. F. Attar (Department of Biology, Faculty of Sciences, University of Tehran), and a voucher specimen No. 43603 was deposited in the central herbarium of Tehran University of Medical Sciences (TUMS).
Standardized extract preparation
As described previously, plant aerial parts were ground and then 100 g of powder were extracted three-times with 70% ethanol. The extract was filtered and evaporated at reduced pressure to yield residues about 29.45% on the basis of dry plant material. The standardization of the extract was performed using HPLC analysis and five phenolic components were quantified against validated analytical reference standards (Farzaei et al., Citation2014a).
Animals
Male Wistar-albino rats, weighing (180–200 g) were kept under standard conditions of temperature (23 ± 1 °C), relative humidity (55 ± 10%), and 12 h dark and 12 h light cycle, with standard pellet feeding diet and water ad libitum. Ethical rules of the investigation on animals were considered carefully and the experimental protocol was approved by the ethical committee of Tehran University of Medical Sciences (TUMS) with code number 91-04-33-20198.
Experimental design
Rats were randomly divided into six groups each containing seven animals. Induction of colitis was performed by instillation of TNBS in all groups except group 1. According to Rahimi et al. (Citation2013), 36 h fasted rats were anesthetized with intraperitoneal administration of 50 mg/kg pentobarbital sodium, and the animals were positioned on their right side. Afterward, 0.3 ml of a mixture containing six volumes of TNBS 5% w/v in H2O (equal to 15 mg TNBS) plus four volume of ethanol (99%) was instilled through rectum using a rubber cannula (8 cm long). Subsequent to instillation of TNBS, rats were maintained in a supine Trendelenburg position for anal leakage of TNBS prevention. The groups were (1) normal control which received normal saline orally; (2) TG-20 which received 20 mg/kg/d of TG extract; (3) TG-30 which received 30 mg/kg/d of TG extract; (4) TG-50 which received 50 mg/kg/d of TG extract; (5) control which received vehicle water orally; and (6) Dexa which received dexamethasone (1 mg/kg/d, intraperitoneally). Standardized TG extract was dissolved in water and administered orally by gavage. Treatment was administered to the animals for 12 d as described above. On the 13th day, the animals were sacrificed by an overdose of ether inhalation. The abdomen was then rapidly dissected, and colon was removed. The pieces of colons were cut open in an ice bath, cleansed gently with normal saline, and observed normally for macroscopic changes and scored as described later. Subsequently, the samples were sliced into two pieces, one piece for histopathology assessment (fixed in 5 ml formalin 10%) and the other for measuring biomarkers that were weighed and maintained in −20 °C for 24 h. The colonic samples were then homogenized in 10-volume ice cold potassium phosphate buffer (50 mM, pH 7.4), sonicated and centrifuged for 30 min at 3500 ×g. The supernatants were transformed into several microtubes for separate biochemical assays and all were kept at −80 °C until analyses (Rahimi et al., Citation2013).
Macroscopic and microscopic assessment of colonic damage
The macroscopic damage was determined according to the criteria described by Abdolghaffari et al. (Citation2010). In order to perform microscopic evaluation, the fixed segments in formalin 10% were embedded in paraffin and stained with hematoxylin and eosin. The scoring was performed by one who was blind to the treated groups. The microscopic scoring was assessed according to criteria explained previously by Abdolghaffari et al. (Citation2010). The criteria used for assessing macroscopic and microscopic damage are shown in .
Table 1. Criteria for scoring macroscopic and microscopic colonic damage.
Determination of TNF-α and IL-1β
TNF-α and IL-1β levels in colon tissues were detected using an enzyme-linked immunosorbent assay (ELISA) kit. The absorbance of the final colored product was measured in 450 nm as the primary wavelength and 620 nm as the reference wavelength. TNF-α and IL-1β levels were expressed as pg/mg protein of tissue as described previously (Ebrahimi et al., Citation2008).
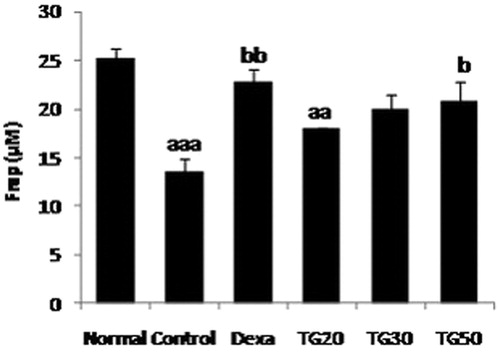
Total ferric reducing antioxidant power (FRAP) assay
Total antioxidant power of colon was evaluated by measuring the ability to reduce Fe3+ to Fe2+. Interaction of tripyridyltriazine (TPTZ) with Fe2+ results in the formation of a blue color, with a maximum absorbance at 593 nm. Data were expressed as mM ferric ions reduced to ferrous per mg of protein as described previously (Hasani et al., Citation2007).
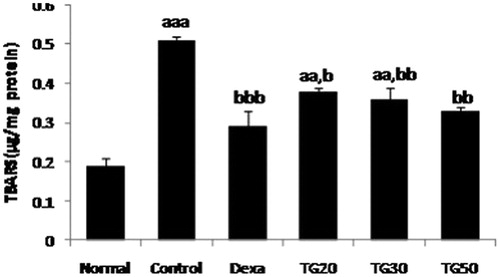
TBA-reactive substances (TBARS) assay
Lipid peroxidation (LPO) activity in colon tissue was assessed by TBARS assay as previously described (Amini-Shirazi et al., Citation2009). Data were shown as µg per mg of protein.
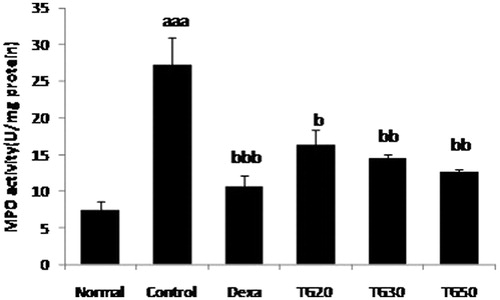
Myeloperoxidase (MPO) activity determination
According to Ghazanfari et al. (Citation2006), the supernatant of the samples were combined with O-dianisidine and H2O2 (0.0005%) which resulted in an absorbance at 460 nm that was measured for 3 min. One unit of MPO activity is described as the change in absorbance per min at room temperature, in the final reaction.
Total protein of colon homogenate
Total protein of colon tissue was assessed according to the Bradford method, using BSA as the standard (Bradford, Citation1976). Results were reported as mg of protein per ml of homogenized tissue.
Statistical analysis
Data were analyzed by Stats Direct ver. 2.7.9 (SAS Inc., Cary, NC). One-way ANOVA followed by Newman–Keul's post hoc test for multiple comparisons were used. The p values less than 0.05 were considered significant. Results are expressed as mean ± standard error of the mean (SEM).
Results
Macroscopic and microscopic assessment of colonic damage
Results of macroscopic and microscopic scores are shown in . TNBS administration to the control group exhibited severe ulceration, wall thickening, adhesions, and inflammation in comparison with the normal group. In rats which received dexamethasone, improvement in macroscopic scores was significant compared with control. The best healing activity among plant group belonged to TG-50 (0.43 ± 0.53) followed by TG-30 (1.71 ± 0.49), which were significantly different from control. Although TG-50 score was less than that of Dexa, the difference was not significant. The median effective dose (ED50) value was 23 mg/kg. Microscopic evaluation of the control group showed severe ulcer, necrosis, mucosal, and submucosal polymorphonuclear leucocytes (PMN) infiltration with crypt abscess, whereas in the sham group, features of colons were within normal limits. Treatment with TG-50 showed mild inflammation of submucosa beside PMN cells infiltration. In the TG-30 group, diffuse destruction of crypts was observed. In the TG-20 group, animals showed severe inflammation of mucosa and submucosa, profuse PMN infiltration, and almost complete necrosis of crypts (). The lowest microscopic score was seen in the Dexa group (1.57 ± 0.29) followed by TG-50 (1.67 ± 0.52). The ED50 value was 39 mg/kg. The macroscopic and microscopic scores in all TG groups were significantly less than that of the control group.
Figure 1. Histological images of colon tissues obtained from control and experimental groups. Microscopic evaluation of the control group showed intense transmural inflammation and/or diffuse necrosis (A) and severe crypt destruction (B). In histological examination of the dexamethasone group, minimal mucosal inflammation was observed (C). In the TG-20 group, almost complete necrosis of crypts (D) and severe inflammation of mucosa and submucosa were observed (E). In the TG-30 group, diffuse destruction of crypts was observed (F). In the TG-50 group, no necrosis in crypt (G) and mild inflammation of mucosa (H) were observed.
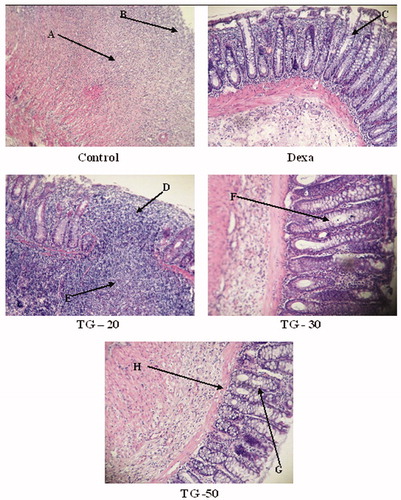
Table 2. Macroscopic and microscopic scores as criteria for assessing colonic damage.
Colonic TNF-α
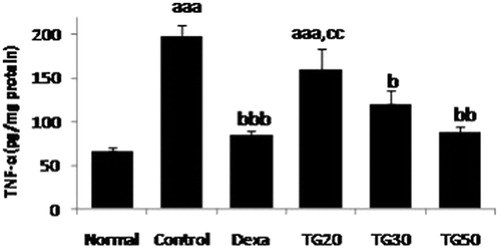
TNF-α was significantly low in TG-30 and TG-50 groups in comparison with control, and among the extract groups, the lowest amount belonged to TG-50 (87.60 ± 7.49), followed by the TG-30 group (120.54 ± 15.56) with an ED50 of 44 mg/kg. The amount of TNF-α in the control group was significantly different (p < 0.001) from the Normal group ().
Figure 2. Tumor necrosis factor-alpha (TNF-α) level in colon. Values are mean ± SEM. Dexa, dexamethasone; TG20, T. graminifolius at dose of 20 mg/kg; TG30, T. graminifolius at dose of 30 mg/kg; TG50, T. graminifolius at dose of 50 mg/kg. aSignificantly different from the Normal group at p < 0.05. bSignificantly different from the control group at p < 0.05. cSignificantly different from the Dexa group at p < 0.05. aaSignificantly different from the Normal group at p < 0.01. bbSignificantly different from the control group at p < 0.01. ccSignificantly different from the Dexa group at p < 0.01. aaaSignificantly different from the Normal group at p < 0.001. bbbSignificantly different from the control group at p < 0.001. cccSignificantly different from the Dexa group at p < 0.001.
Colonic IL-1β levels
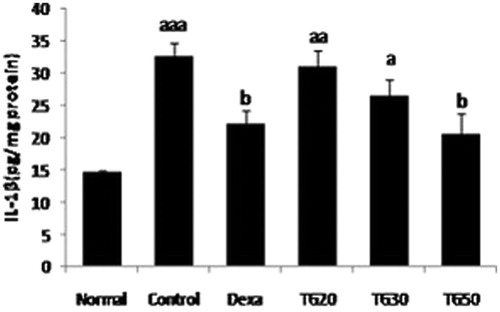
IL-1β in all extract groups were lower than control, these values only in Dexa and TG-50 were significant in comparison with control. TG-50 (20.47 ± 3.4) demonstrated the lowest amount of IL-1β among other groups. This value was lower than Dexa (22.12 ± 2.2) not in a significant manner. IL-1β amount in control was significantly higher than Sham (p < 0.001) (). The ED50 value was 57 mg/kg.
Figure 3. Interleukin 1-beta (IL-1β) level in colon. Values are mean ± SEM. Dexa, dexamethasone; TG20, T. graminifolius at dose of 20 mg/kg; TG30, T. graminifolius at dose of 30 mg/kg; TG50, T. graminifolius at dose of 50 mg/kg. aSignificantly different from the Normal group at p < 0.05. bSignificantly different from the control group at p < 0.05. cSignificantly different from the Dexa group at p < 0.05. aaSignificantly different from the Normal group at p < 0.01. bbSignificantly different from the control group at p < 0.01. ccSignificantly different from the Dexa group at p < 0.01. aaaSignificantly different from the Normal group at p < 0.001. bbbSignificantly different from the control group at p < 0.001. cccSignificantly different from the Dexa group at p < 0.001.
Colonic total antioxidant power as FRAP
The highest FRAP antioxidant activity belonged to Dexa (22.75 ± 1.4), followed by TG-50 (20.9 ± 1.9). These values were lower than normal, but the difference was not significant. The control group was significantly lower than normal (p < 0.001) (). The ED50 value was 43 mg/kg.
Figure 4. Total antioxidant power as ferric-reducing antioxidant power (FRAP) level in colon. Values are mean ± SEM. Dexa, dexamethasone; TG20, T. graminifolius at dose of 20 mg/kg; TG30, T. graminifolius at dose of 30 mg/kg; TG50, T. graminifolius at dose of 50 mg/kg. aSignificantly different from the Normal group at p < 0.05. bSignificantly different from the control group at p < 0.05. cSignificantly different from the Dexa group at p < 0.05. aaSignificantly different from the Normal group at p < 0.01. bbSignificantly different from the control group at p < 0.01. ccSignificantly different from the Dexa group at p < 0.01. aaaSignificantly different from the Normal group at p < 0.001. bbbSignificantly different from the control group at p < 0.001. cccSignificantly different from the Dexa group at p < 0.001.
Colonic LPO level as TBARS
TG groups in 20, 30, and 50 mg/kg doses demonstrated significant reduction in the colonic LPO level compared with control. Dexa (0.29 ± 0.04) showed the lowest LPO level followed by TG-50 (0.33 ± 0.009). The TBARS value of the control group was significantly different from normal (p < 0.001) (). The ED50 value was 93 mg/kg.
Figure 5. Lipid peroxidation (LPO) levels as thiobarbituric acid reacting substances (TBARS) in colon. Values are mean ± SEM. Dexa, dexamethasone; TG20, T. graminifolius at dose of 20 mg/kg; TG30, T. graminifolius at dose of 30 mg/kg; TG50, T. graminifolius at dose of 50 mg/kg. aSignificantly different from the Normal group at p < 0.05. bSignificantly different from the control group at p < 0.05. cSignificantly different from the Dexa group at p < 0.05. aaSignificantly different from the Normal group at p < 0.01. bbSignificantly different from the control group at p < 0.01. ccSignificantly different from the Dexa group at p < 0.01. aaaSignificantly different from the Normal group at p < 0.001. bbbSignificantly different from the control group at p < 0.001. cccSignificantly different from the Dexa group at p < 0.001.
Colonic MPO activity
Tragopogon graminifolius in all doses demonstrated significant reduction in the value of MPO in comparison with the control group. Among plant groups, TG-50 (12.66 ± 0.32) showed the highest influence on MPO, followed by TG-30 (14.45 ± 0.5) (). The ED50 value was 41 mg/kg.
Figure 6. Myeloperoxidase activity (MPO) in colon. Values are mean ± SEM. Dexa, dexamethasone; TG20, T. graminifolius at dose of 20 mg/kg; TG30, T. graminifolius at dose of 30 mg/kg; TG50, T. graminifolius at dose of 50 mg/kg. aSignificantly different from the Normal group at p < 0.05. bSignificantly different from the control group at p < 0.05. cSignificantly different from the Dexa group at p < 0.05. aa Significantly different from the Normal group at p < 0.01. bbSignificantly different from the control group at p < 0.01. ccSignificantly different from the Dexa group at p < 0.01. aaaSignificantly different from the Normal group at p < 0.001. bbbSignificantly different from the control group at p < 0.001. cccSignificantly different from the Dexa group at p < 0.001.
Discussion
In the present study, the efficacy of T. graminifolius and possible pharmacological mechanisms in IBD were evaluated using TNBS-induced colitis. Tragopogon graminifolius caused improvement of macroscopic and microscopic damages dose dependently with best healing activity at 50 mg/kg. Colonic TNF-α, IL-1β, and MPO activities decreased by all doses of TG extract especially by TG-50 mg/kg. As described previously, TNF-α and IL-1β play an important role in the pathophysiology of intestinal inflammation in IBD disease (Rahimi & Abdollahi, Citation2012; Rahimi et al., Citation2009). MPO is a marker for neutrophil infiltration which is directly related to intensity of inflammation (Miroliaee et al., Citation2011). Since oxidative stress is proposed as an important factor in pathophysiology of IBD (Rezaie et al., Citation2007), the effects of TG extract on LPO and antioxidant power of colon were evaluated. Results showed that all doses of extract especially 50 mg/kg decreased LPO and increased antioxidant power of colon. In addition, antioxidant potential of TG extracts has been proven via various methods (Farzaei et al., Citation2014a,Citationb).
According to modern investigations, many medicinal plants encompass beneficial activity on IBD (Rahimi et al., Citation2013a). Herbal therapies have shown their effectiveness in managing IBD through several mechanisms including modulating immune system and proinflammatory cytokines, inhibition of leukotriene B4, cyclooxygenase (Cox) 1&2, nitric oxide (NO) and inducible isoform of nitric oxide synthase (iNOS), nuclear factor-kappa B (NF-κB), and antioxidant and antimicrobial activities (Rahimi et al., Citation2009).
In Iranian folk and traditional medicine, a wide range of natural remedies have been used for the treatment of various gastrointestinal disorders like peptic ulcer (Farzaei et al., Citation2013a,Citationc), Hemorrhoids (Rahimi & Abdollahi, Citation2013), irritable bowel syndrome (Rahimi & Abdollahi, Citation2012), and IBD (Rahimi et al., Citation2013b). TG is one of these remedies with a high potential activity on gastrointestinal diseases. Previous study on the acute toxicity of 70% ethanol extract from aerial parts of TG demonstrated that TG was safe up to a dose of 2000 mg/kg after oral administration. Moreover, this extract showed gastroprotective activity against ethanol-induced ulcer in the rat (Farzaei et al., Citation2013b).
Phenolic compounds are one of the main constituents of Tragopogon species. Our previous study showed high content of total phenol (560.67 ± 18.85 mg/g gallic acid equivalents) in ethanol extract from TG aerial part (Farzaei et al., Citation2014b). As shown in , our previous study for the determination of phenolic components from ethanolic extract of TG using HPLC analysis resulted in detection and quantification of five phenolic compounds including gallic acid (0.873 ± 0.027 mg g extract dry weight−1), catechin (1.079 ± 0.033 mg g extract dry weight−1), caffeic acid (0.326 ± 0.021 mg g extract dry weight−1), ρ-coumaric acid (6.357 ± 0.014 mg g extract dry weight−1), and ferulic acid (1.24 ± 0.018 mg g extract dry weight−1) (Farzaei et al., Citation2014a). Determination of these phenolic components with HPLC analysis was performed for standardization of this medicinal product. Various biological studies confirm the efficacy of polyphenols in the management of IBD via wide range of molecular mechanisms including suppression of pro-inflammatory cytokines [e.g., TNF-α, IL-1β, IL-10, NF-κB, and interferon (IFN)-γ], modulating inflammatory cell signaling pathways, suppression of oxidative stress, and lipid oxidation products and antioxidant properties (Biasi et al., Citation2011). In addition, investigations demonstrated the beneficial effects of gallic acid – one of the main phenolic compounds of TG – in colitis through antioxidant and anti-inflammatory functions (Yen et al., Citation2002). Its anti-inflammatory activity has been confirmed via suppressing polymorphonuclear leukocytes (PMNs), superoxide free radical scavenging, and MPO inhibition (Kroes et al., Citation1992). Other phenolic compounds identified in TG have also shown favorable effects. Caffeic acid demonstrated healing activity on colitis through suppression of colonic mRNA expression of IL-17 and iNOS and enhancement of IL-4 and CYP4B1 mRNA in dextran sulfate sodium induce-colitis mice (Ye et al., Citation2009). It also exhibited an anti-inflammatory activity by MPO, malondialdehyde (MDA), and nitrite suppression in rats (Mehrotra et al., Citation2011). Wound healing activity of catechin has been proved in in vitro model (Schmidt et al., Citation2010). It exhibited anti-inflammatory activity in mice (Iijima et al., Citation2009). Moreover, ferulic acid has wound healing activity via enhancement of oxidative defense (heme oxygenase-1 and heat shock protein-70) and suppression of protein and lipid oxidation in vitro in human dermal fibroblasts (Calabrese et al., Citation2008). Furthermore, ρ-coumaric acid showed anti-inflammatory function via reduction of cell-mediated immune responses, macrophage phagocyte function and TNF-α expression (Pragasam et al., Citation2013).
Conclusion
In conclusion, standardized extract of TG exhibited a strong healing activity on TNBS induced colitis dose dependently. Phenolic compounds particularly gallic acid, catechin, caffeic acid, ρ-coumaric acid, and ferulic acid may be responsible for this activity. Therefore, it can be assumed that in an optimum dose (50 mg/kg), the phenolic compounds are free from their phytochemical complexes and can perform the most efficacious activity. The mechanisms of action of TG in IBD may be attributed to its antioxidant, anti-inflammatory, and immunomodulating activities as well as mucosal healing properties. The present study confirmed the use of TG in folk and traditional medicine as dietary supplement and complementary medicine for the management of IBD. Clinical trials are recommended for obtaining more conclusive results about the efficacy and tolerability of TG in IBD.
Acknowledgements
The authors thank the assistance of NEF and INSF.
Declaration of interest
The authors have no conflict of interest. Authors thank TUMS for partial support of the study.
References
- Abdolghaffari A, Baghaei A, Moayer F, et al. (2010). On the benefit of Teucrium in murine colitis through improvement of toxic inflammatory mediators. Hum Exp Toxicol 29:287–95
- Allocca M, Jovani M, Fiorino G, et al. (2013). Anti-IL-6 treatment for inflammatory bowel diseases: Next cytokine, next target. Curr Drug Targets 14:1508–21
- Amini-Shirazi N, Hoseini A, Ranjbar A, et al. (2009). Inhibition of tumor necrosis factor and nitrosative/oxidative stresses by ziziphora clinopoides (kahlioti): A molecular mechanism of protection against dextran sodium sulfate-induced colitis in mice. Toxicol Mech Meth 19:183–9
- Biasi F, Astegiano M, Maina M, et al. (2011). Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr Med Chem 18:4851–65
- Bradford MM. (1976). A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–54
- Calabrese V, Calafato S, Puleo E, et al. (2008). Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: Role of vitagenes. Clin Dermatol 26:358–63
- De Greef E, Vandenplas Y, Hauser B, et al. (2013). Probiotics and IBD. Acta Gastroenterol Belg 76:15–19
- Ebrahimi F, Esmaily H, Baeeri M, et al. (2008). Molecular evidences on the benefit of N-acetylcysteine in experimental colitis. Cent Eur J Biol 3:135–2
- Farzaei MH, Rahimi R, Abasabadi Z, Abdollahi M. (2013a). An evidence-based review on medicinal plants used for the treatment of peptic ulcer in traditional Iranian medicine. Int J Pharm 9:108–24
- Farzaei MH, Khazaei M, Abasabadi Z, et al. (2013b). Protective effect of Trogopogon graminifolius DC. against ethanol induced gastric ulcer in rat. Iranian Red Cresc Med J 15:813–16
- Farzaei MH, Khanavi M, Moghaddam G, et al. (2014a). Standardization of Tragopogon graminifolius DC. extracts based on phenolic compounds and antioxidant activity. J Chem 2014:425965
- Farzaei MH, Shams-Ardekani MR, Abbasabadi Z, Rahimi R. (2013c). Scientific evaluation of edible fruits and spices used for the treatment of peptic ulcer in traditional Iranian medicine. ISRN Gastroenterol 2013:Article ID 136932, 12 pages
- Farzaei MH, Rahimi R, Attar F, et al. (2014b). Chemical composition, antioxidant and antimicrobial activity of essential oil and extracts of Tragopogon graminifolius DC., a medicinal herb from Iran. Nat Prod Comm 9:121–4
- Ghazanfari G, Minaie B, Yasa N, et al. (2006). Biochemical and histopathological evidences for beneficial effects of Satureja khuzestanica Jamzad essential oil on the mouse model of inflammatory bowel diseases. Toxicol Mech Meth 16:365–72
- Hasani P, Yasa N, Vosough-Ghanbari S, et al. (2007). In vivo antioxidant potential of Teucrium polium, as compared to α-tocopherol. Acta Pharm 57:123–9
- Iijima T, Mohri Y, Hattori Y, et al. (2009). Synthesis of (−)-epicatechin 3-(3-O-methylgallate) and (+)-catechin 3-(3-O-methylgallate), and their anti-inflammatory activity. Chem Biodivers 6:520–6
- Jena G, Trivedi PP, Sandala B. (2012). Oxidative stress in ulcerative colitis: An old concept but a new concern. Free Radic Res 46:1339–45
- Kroes BH, Berg AJ, Ufford HCQ, et al. (1992). Anti-inflammatory activity of gallic acid. Planta Med 58:499–504
- Kroschewsky JR, Mabry TJ, Markham KR, Alston RE. (1969). Flavonoids from the genus Tragopogon (Compositae). Phytochemistry 8:1495–8
- Kucekova Z, Mlcek J, Humpolicek P, et al. (2011). Phenolic compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and their antiproliferative effects. Molecules 16:9207–17
- Mehrotra A, Shanbhag R, Chamallamudi MR, et al. (2011). Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur J Pharmacol 666:80–6
- Miroliaee AE, Esmaily H, Vaziri-Bami A, et al. (2011). Amelioration of experimental colitis by a novel nanoselenium–silymarin mixture. Toxicol Mech Methods 21:200–8
- Miyase T, Kohsaka H, Akira U. (1992). Tragopogon A-I, oleanaesaponins from Tragopogon pratensis. Phytochemistry 31:2087–91
- Pedersen J, Coskun M, Soendergaard C, et al. (2014). Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol 20:64–77
- Pragasam SJ, Venkatesan V, Rasool M. (2013). Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 36:169–76
- Rahimi R, Abdollahi M. (2012). Herbal medicines for the management of irritable bowel syndrome: A comprehensive review. World J Gastroenterol 18:589–600
- Rahimi R, Abdollahi M. (2013). Evidence-based review of medicinal plants used for the treatment of hemorrhoids. Int J Pharmacol 9:1–11
- Rahimi R, Nikfar S, Abdollahi M. (2013a). Induction of clinical response and remission of inflammatory bowel disease by use of herbal medicines: A meta-analysis. World J Gastroenterol 19:5738–49
- Rahimi R, Baghaei A, Baeeri M, et al. (2013b). Promising effect of Magliasa, a traditional Iranian formula, on experimental colitis on the basis of biochemical and cellular findings. World J Gastroenterol 19:1901–11
- Rahimi R, Mozaffari S, Abdollahi M. (2009). On the use of herbal medicines in management of inflammatory bowel diseases: A systematic review of animal and human studies. Dig Dis Sci 54:471–80
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. (2008). Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: A meta-analysis. Reprod Toxicol 25:271–5
- Rahimi R, Shams-Ardekani MR, Abdollahi M. (2010). A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol 16:4504–14
- Rezaie A, Parker RD, Abdollahi M. (2007). Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig Dis Sci 52:2015–21
- Schmidt CA, Murillo R, Bruhn T, et al. (2010). Catechin derivatives from Parapiptadenia rigida with in vitro wound-healing properties. J Nat Prod 73:2035–41
- Vardavas CI, Majchrzak D, Wagner KH, et al. (2006). The antioxidant and phylloquinone content of wildly grown greens in Crete. Food Chem 99:813–21
- Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. (2014). Immunopathology of inflammatory bowel disease. World J Gastroenterol 20:6–21
- Ye Z, Liu Z, Henderson A, et al. (2009). Increased CYP4B1 mRNA is associated with the inhibition of dextran sulfate sodium-induced colitis by caffeic acid in mice. Exp Biol Med, 234:605–16
- Yen GC, Duh PD, Tsai HL. (2002). Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem 79:307–13
- Zidorn C, Lohwasser U, Pschorr S, et al. (2005). Bibenzyls and dihydroisocoumarins from white salsify (Tragopogon porrifolius subsp. porrifolius). Phytochemistry 66:1691–7