Abstract
Context: Glechon spathulata Benth. and Glechon marifolia Benth. (Lamiaceae, Mentheae) are aromatic plants used in traditional medicine for the treatment of viral infections.
Objective: The chemical composition and antiviral and antifungal activities of Glechon spathulata and Glechon marifolia essential oils were investigated.
Materials and methods: The oils were obtained by hydrodistillation and analyzed by GC–FID and GC–MS. Anti-herpes virus (HSV-1) activity was examined in Vero cells by yield reduction assay, in doses of 0.0095% v/v and 0.039% v/v, for G. spathulata and G. marifolia oil, respectively. Antifungal activity was carried by the broth microdilution method, in oil concentrations that ranged from 5.2 to 500 µg/mL.
Results: β-Caryophyllene (14.2% and 32.2% for G. spathulata and G. marifolia, respectively) and bicyclogermacrene (17.1% and 16.5%, respectively) were the major components of both oils. At noncytotoxic concentrations of the essential oils, the viral titer was reduced by up to 2 log10 for KOS and VR-733 strains. The antifungal activity was observed against Trichophyton rubrum (MIC 10–83 µg/mL) and Epidermophyton floccosum (MIC 83–500 µg/mL). The oil of G. spathulata exhibited activity against the three strains tested (KOS, VR733, and 29-R), whereas G. marifolia oil was active against two strains, KOS and VR733.
Discussion and conclusion: The chemical composition for G. spathulata and G. marifolia essential oils is very similar. The oil of G. spathulata can be promising as a new antifungal agent against dermatophytes. The findings add important information to the biological activity of Glechon species essential oils, specifically its antiviral and antidermatophytic properties.
Introduction
Herpes simplex virus 1 (HSV-1) is among the most common viral pathogens of humans. Although usually causing mild disease or asymptomatic infections, eventually HSV-1 may be associated with serious health problems. The virus spreads mainly by person-to-person direct or indirect contact with lesions or infected secretions (White & Frank, Citation1994).
At present, efforts are being made to evaluate potential antiviral activities of natural products with the aim of identifying new compounds with potential therapeutic use. Among such substances, essential oils have been tested for antiviral activity. These low molecular weight (mostly below 300 g/mol) compounds easily diffuse across cell membranes to induce biological responses (Allahverdiyev et al., Citation2004; De Logu et al., Citation2000; Farag et al., Citation2004; Hayashi et al., Citation1995; Schuhmacher et al., Citation2003; Sökmen et al., Citation2004).
Dermatomycosis are mycotic diseases of skin caused by dermatophytes, the most common causative agents, and some opportunistic fungi (Seebacher et al., Citation2008). The last three decades have seen an increase in the incidence of dermatophytosis due to the widespread use of broad-spectrum antibacterial treatment and increasing numbers of patients with immunosuppression (Havlickova et al., Citation2008). Several new antifungal agents including azoles and allylamine derivatives were introduced in the therapy antidermatophytic; however, these antifungal agents are expensive, with side effect or ineffective due to acquired drug resistance during treatment (Martinez-Rossi et al., Citation2008).
Glechon (Lamiaceae, Mentheae) is a South American genus with about nine species distributed across southern Brazil, northeastern Argentina, the southern portions of Paraguay, and Uruguay (Mallo & Xifreda, Citation2004). Glechon spathulata Benth., known as “manjerona-do-campo”, is an aromatic plant popularly used as a diaphoretic or antitussive and for the treatment of chronic respiratory bronchitis and laryngitis, which are often associated with viral infections. It is described in the first edition of the Brazilian Pharmacopeia (1926) (Mentz et al., Citation1997). We reported previously the anti-herpes virus activity of aqueous and hydroalcoholic extracts of G. marifolia and G. spathulata (Montanha et al., Citation2004).
Several interesting applications such as the use of essential oils instead of synthetic drugs to circumvent the increasing resistance of some pathogens are been investigated that could be associated to virus and fungal. In this way, many studies have focused on the antiviral activity of essential oils (De Logu et al., Citation2000; Farag et al., Citation2004; Loizzo et al., Citation2008; Schuhmacher et al., Citation2003; Sökmen et al., Citation2004). In an attempt to identify anti-herpes virus and antifungal potential of Glechon species essential oils, we carried out a study of the antiviral and antidermatophytic activities of the essential oils and its chemical characterization.
Material and methods
Plant material
The aerial parts of G. marifolia were collected in Santa Cristina do Pinhal, and those of G. spathulata in Fontoura Xavier (state of Rio Grande do Sul, Southern Brazil). The botanical identification was made by Dr. Sérgio Bordignon and a voucher specimen of each was deposited in the herbarium of the Universidade Federal do Rio Grande do Sul (ICN/UFRGS), G. marifolia (number 129236) and G. spathulata (number 129237).
Essential oil obtention
Essential oils from leaves of G. marifolia (570 g) and G. spathulata (400 g) were hydrodistilled for 4 h using a Clevenger-type apparatus (Clevenger Apparatus, Ambala Cantt, India). The yield (v/w) of essential oil was determined based on the leaf dry weight. The obtained oils were stored in sample vials and kept frozen at −5 °C until analysis by gas chromatography (GC) and GC mass spectrometry (GC–MS).
In order to investigate the influence of exposure to natural light, the essential oils were stored in cryovials and exposed to sunlight for 20 d (temperature interval 20–25 °C). Subsequently these aliquots were resubmitted to GC–MS analysis and retested for antiviral activity.
GC and GC–MS conditions
Essential oils were quantitatively analyzed by GC using a Shimadzu GC-17A chromatograph (Shimadzu, Tokyo, Japan) equipped with the Shimadzu GC 10 software and flame ionization detector (FID). A DB-5 fused silica capillary column (30 m, 0.25 mm; film thickness 0.25 µm; Supelco) was used. The oven temperature was programmed from 60 °C to 300 °C at 3 °C/min, with helium as the carrier gas at an inlet pressure of 80 kPa (1 mL/min). Injector (split ratio 1:50) and detector temperatures were set at 220 °C and 250 °C, respectively. The percentage compositions were obtained from electronic integration measurements.
The samples were qualitatively analyzed by GC–MS in the same chromatographic conditions as described above, using a Shimadzu QP-5000-quadrupole MS system (Shimadzu, Kyoto, Japan), operating with ionization energy of 70 eV and an interface temperature of 250 °C. Scan time and mass range were 1 s and 40–400 m/z, respectively, and the injection volume was 1 µL. The proportion of the components of the oils samples was obtained from the GC peak areas in the total ion chromatogram (TIC), by internal normalization.
Identification of the essential oil constituents
Identification of essential oil volatile compounds was based on the calculation of their retention indices (RI) relative to (C8–C22) n-alkane standards with those of authentic compounds available in our laboratory. Further identification was made by comparison of their mass spectral fragmentation patterns with those stored in the NIST database (National Institute of Standards and Technology) mass spectral library of the GC–MS data system and other published mass spectra data (Adams, Citation2009).
Cells and viruses
African green monkey kidney cells (Vero cell line ATCC CCL-81) were grown in MEM supplemented with 10% fetal calf serum, 2 μg/mL of amphotericin B, and 10 µg/mL of enrofloxacin. A virus stock of herpes simplex virus type 1, strains KOS (University of Rennes, France), VR733 (ATCC), and ACVres (strain 29-R), an acyclovir-resistant strain, were multiplied in Vero cells infected at a low multiplicity of infection (0.01), incubated for 1–2 d, frozen/thawed, and centrifuged at low speed to remove cell debris. Virus stocks were maintained in liquid nitrogen until use. Virus titrations were carried out in 10-fold dilutions, in 96-well microplates. Infectious virus titers were calculated by the method of Kärber (Payment & Trudel, Citation1989) based on the presence of a cytopathic effect (CPE) in infected cultures. Titers were expressed as 50% tissue culture infectious doses (TCID50/50 µL). Typically, infectious virus titers of stocks were around 106 TCID50/50 µL for HSV-1 strain KOS, 104 TCID50/50 µL for HSV-1 strain VR733 (ATCC), and 105.5 TCID50/50 µL for HSV-1 strain 29-R (ACVres).
Acyclovir (ACV) was purchased from Sigma (St. Louis, MO) and the stock solution was prepared in phosphate-buffered saline (PBS; NaCl 145.4 mM, NaH2 PO4. H2O 1.67 mM, Na2 HPO4ċH2O 145.4, pH 7.4).
Evaluation of cytotoxicity
Cytotoxicity of the essential oils towards uninfected Vero cells was examined by seeding onto 96-well microplates at a concentration of 4.0 × 104 cells/well and incubating overnight at 37 °C in a 5% CO2 atmosphere. Two-fold dilutions of essential oils (from 10% to 0.019%; v/v) were prepared in MEM and added to monolayers. After 72 h of incubation at 37 °C, cytotoxicity was determined by microscopic examination of cell morphology in treated and untreated cultures. The maximum tolerated dilution (MTD) was determined as the highest dilution at which no apparent effect on growth and viability of cells was observed (compared with controls) (Fritz et al., Citation2007). The MTD was determined for each oil before proceeding to the antiviral activity assays. All assays were carried out in triplicate.
Antiviral activity
The anti-HSV-1 activity in vitro studies were based on a yield reduction assay according to the methodology described by Fritz et al. (Citation2007). Briefly, the essential oils were diluted in MEM at the previously determined MTD and added to confluent, 24 h old monolayers of Vero cells in 96-well cell culture microplates just before virus inoculation. One hundred TCID50 of HSV-1 strains KOS, VR733 (ATCC), and 29-R (ACVres) were added to each of the wells, in quadruplicate. Appropriate controls were run simultaneously. Plates were incubated for 72 h at 37 °C and examined for the presence of CPE. The contents of the four identical wells were harvested, mixed, and clarified by low-speed centrifugation, then titrated. The antiviral activity of each sample was determined as the viral titer reduction (log10) by comparison with untreated controls. All experiments were carried out in triplicate.
Effect of the essential oils addition after virus inoculation
Vero cell monolayers in 4-well culture plates were infected with HSV-1 at 100 TCID50/50 µL. After 60 min at 37 °C, the unadsorbed virus was removed, and the monolayers were washed twice with MEM before adding 200 µL of MEM containing an aliquot of each oil, at the predetermined MTD, to each well. After incubation for 18 h (one cycle of replication), the cultures were frozen and thawed, the cell debris removed by low-speed centrifugation and infectious virus titers determined (Montanha et al., Citation1995).
Virucidal activity
To test for possible virucidal activity, equal volumes of HSV-1 suspensions containing 50 µL of either HSV-1 strain KOS, or VR733 (ATCC) or 29-R (ACVres) were directly exposed to emulsions containing the essential oils under test, at the predetermined MTD, and incubated for 1 h at 37 °C. Subsequently, these mixtures were diluted 10-fold serially and infectious titers were compared with those obtained with untreated virus suspension (Montanha et al., Citation1995).
Data analysis results ( and ) are expressed as mean ± standard deviation of the reduction of virus titer (n = 3). Means and SD values were analyzed using Excel for Windows software.
Table 1. Antiviral activity of Glechon spathulata and Glechon marifolia essential oils on Vero cells expressed as reduction of virus titer (log10) by yield reduction assay.
Table 2. Effect of Glechon spathulata and Glechon marifolia essential oils addition after virus inoculation expressed as reduction of virus titer (log10).
Antifungal activity
The antifungal activity test was carried out only with the essential oils of G. spathulata because this species was found to present popular uses. A total of 29 clinical isolates, opportunistic yeast, and filamentous fungi (dermatophytes) were tested for the antifungal susceptibility test in triplicate. The yeasts and filamentous fungi tested were Candida albicans (ATCC90028), Candida krusei (CKR01), Candida parapsilosis (CPA05), Candida glabrata (CG04), Candida tropicalis (ATCC750), Candida guilliermondi (CG40039), Cryptococcus neoformans (R27), Trichosporon inkin (TBE01), Geotrichum candidum (GEO01), Rhodotorula sp. (RHO07), Trichophyton rubrum (TRU31, TRU09, TRU04, TRU23, and TRU13), Trichophyton mentagrophytes (TME22), Microsporum canis (MCA02), Microsporum gypseum (MGY09), Epidermophyton floccosum (EPF31, EPF01, EPF18, EPF07, and EPFW4), and Scytalidium dimidiatum (SCY04, SCY04, SCY09, SCY05, SCY08, and SCY07).
Inoculums of all opportunistic yeasts and dermatophytes fungi were prepared according the Clinical Laboratory and Standards Institute (CLSI, Citation2008a,Citationb). Minimal inhibitory concentration (MIC) of active components in the essential oil was determined by the broth microdilution method following M38-A2 and M27-A3 CLSI guidelines, with RPMI-MOPS (RPMI 1640 medium containing l-glutamine, without sodium bicarbonate (Sigma-Aldrich Co., St Louis, MO) buffered to pH 7.0 with 0.165 mol/L MOPS buffer (Sigma, St. Louis, MO). The concentrations of the oils tested ranged from 5.2 to 500 µg/mL and 100 µL aliquots were inoculated of a flat-bottom 96-well microtiter. The MIC was defined as the lowest concentration of compounds at which the microorganism tested did not demonstrate visible growth. Terbinafine and amphotericin B (Sigma-Aldrich Co., St Louis, MO) were used as positive inhibition control ranging from 0.25 to 32 µg/mL. Also, the values of MIC50 and MIC90 were observed. These values represent the drug concentration that inhibits the growth of 50% and 90% of the isolates, respectively.
The assessment of the fungistatic/fungicidal activity was also evaluated. The MIC aliquots and the positive control (10 µL) were transferred to plates containing Sabouraud-dextrose agar (Difco, Carrickmore, County Tyrone, UK) and incubated at 35 °C for 7 d. Microorganism growth indicates fungistatic activity while the absence of growth means fungicidal activity. The fungal growth of MIC aliquots was compared with that of the positive control. All the experiments were conducted in triplicate.
Results and discussion
Distillation of G. spathulata and G. marifolia leaves yielded about 4.9% and 1.4% (v/w) essential oil based on dry weight, respectively. For the identification and quantification of constituents, the essential oils obtained were analyzed in GC and GC coupled to MS. The chromatograms from each sample are shown in and . shows the compositions of the essential oils, with about 40 compounds identified in the aerial parts from the Glechon species. With regard to chemical groups, the volatile oils obtained from the fresh leaves of both species were characterized by the predominance of sesquiterpene hydrocarbons (40.1% and 84.5%, respectively, for G. spathulata and G. marifolia). The oils’ compositions were very similar presenting as the major constituents as β-caryophyllene (14.2% and 32.2%, respectively) and bicyclogermacrene (17.1% and 16.5%, respectively). Glechon marifolia oil also presented a considerable amount of another sesquiterpene, α-humulene (23.3%). In G. spathulata oil the monoterpene hydrocarbon fraction was also well represented by the presence of 8.5% of β-pinene and 6.9% of α-pinene, while in G. marifolia oil the monoterpene fraction was very small.
Figure 1. Chromatograms obtained by GC/MS for analysis of the chemical composition of essential oils of Glechon spathulata, before and after sunlight exposition. Note: Essential oil of G. spathulata before (A) and after (B) sunlight exposition. Peak 1 – α- pinene; 2 – β-pinene; 3 – β-caryophyllene; 4 – Bicyclogermacrene; 5 – Spathulenol.
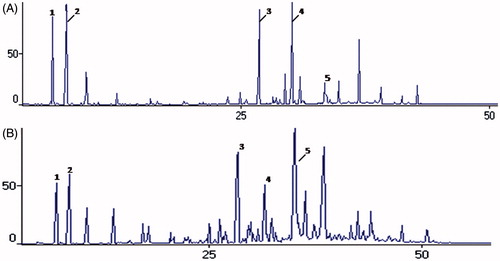
Figure 2. Chromatograms obtained by GC/MS for analysis of the chemical composition of essential oils of Glechon marifolia, before and after sunlight exposition. Note: Essential oil of G. marifolia before (A) and after (B) sunlight exposition. Peak 1 - β-caryophyllene; 2 – α-humuleme; 3 - Germacrene D; 4 – Bicyclogermacrene; 5 – Spathulenol.
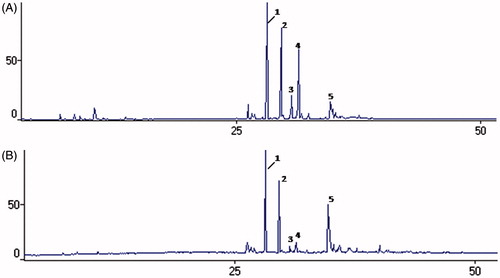
Table 3. Percentual composition of the essential oils from aerial parts of Glechon species, before and after sunlight exposition.
No information about the antiherpetic and antidermatophytic activities of Glechon is reported in the literature. As shown in , the extracted oil of G. spathulata exhibited activity against the three strains tested, whereas G. marifolia oil was active against two strains, KOS and VR733. HSV-1 was more susceptible to the oil of G. spathulata than that of G. marifolia.
Essential oils are complex and unstable mixture of different volatile compounds. It is known that due to this instability, these compounds may be altered during storage from various factors such as exposure to light and temperature (Turek & Stintzing, Citation2012). Therefore, they should be stored in tightly sealed glass container, protected from light and heat, to avoid changes in their chemical composition (Hassiotis & Lazari, Citation2010). Considering this, vials containing the essential oil of both species of Glechon were exposed to natural light for 20 d and then tested for antiviral activity. This experiment confirmed that the antiviral activity was lost after exposure to natural light (data not shown). Furthermore, GC-MS analysis of light-exposed samples revealed a marked decrease in the levels of monoterpenes (), with this fraction of G. spathulata essential oil decreasing to half of its total content before light exposure (28.9–14.1%), while in the G. marifolia sample exposed to light, the monoterpene fraction decayed to undetectable levels. In the sesquiterpene fraction, the major change was in the content of the main compound, bicyclogermacrene, with a sharp fall in its levels for both species, 17.1–5.9% in G. spathulata and 16.5–3.0% in G. marifolia oils. Although present in lower quantities, germacrene D also showed a notable decrease in its content (4.0–0.7% in G. spathulata and 4.3–1.9% in G. marifolia oils). At the same time, there was a marked increase in the content of the oxygenated sesquiterpenes fraction (15.1–47.1% and 7.9–28.4%, for G. spathulata and G. marifolia oils, respectively), especially for the oxygenated compounds spathulenol, caryophyllene oxide, humulene epoxide II, and γ-eudesmol (). Other studies have also demonstrated increased caryophyllene oxide during the oxidation of essential oils (Burt, Citation2004). This compound has been described as the main oxidation product of the β-caryophyllene (Misharina et al., Citation2003). The results of this work can be explained by the autoxidation of bicyclogermacrene and germacrene D in these compounds by the exposure to sunlight (Toyota et al., Citation1996). Therefore, these findings suggest that the monoterpenes are important for the antiviral activity, as well as the presence of germacrene D and bicyclogermacrene at their original levels, or at least the presence of a high percentage of oxygenated compounds compromises the biological response. This may also influence the results for antiviral activity, where monoterpenes exhibited positive effects since undegraded oils showed higher anti-HSV-1 activity than degraded oils.
When the viruses were directly exposed for 1 h at 37 °C, the two oils failed to inactivate HSV-1. Therefore, neither of the oils appears to have any virucidal effect against the HSV-1 strains under study. In contrast, the result of adding essential oils after virus inoculation indicated that the oils of G. spathulata and G. marifolia were effective at reducing infectivity after HSV-1 attachment (). These results suggest that the oils interfere with a subsequent stage of the virus replicative cycle within the cell.
Relative to antifungal activity, the oil was inactive against all opportunistic yeasts tested. Only two species of dermatophyte exhibited susceptibility (). The results indicate that G. spathulata essential oil was especially active against T. rubrum and E. floccosum strains, with MIC values ranging from 10.4 to 83.12 µg/mL and 83.12 to 500 µg/mL, respectively. These fungi are the most common agents of dermatomycoses, primarily causing tinea pedis, tinea corporis, tinea capitis, and onychomycosis (Havlickova et al., Citation2008; Seebacher et al., Citation2008). The isolates of T. rubrum shown to be more susceptible than isolates of E. floccosum, with MIC90 about three times lower.
Table 4. Antifungal activity of Glechon spathulata essential oil against clinical isolates of dermatophytes.
Several studies have reported antifungal and antiviral activities for essential oils, including oils whose main compounds are sesquiterpenes (Vieira et al., Citation2014). The oil from rhizomes of Zingiber nimmonii, for example, with predominance of caryophyllenes (α and β-caryophyllene), showed significant inhibitory activity against Candida glabrata, Candida albicans, and Aspergillus niger (Sabulal et al., Citation2006). This sesquiterpene is also associated with others biological activities, such as local anesthetic (Guelardini et al., Citation2001). Antimicrobial activity was also related with essential oils that presented bicyclogermacrene and spathulenol as major compounds (Fontenelle et al., Citation2008; Silva et al., Citation2013). Koch et al. (Citation2008) observed antiviral activity against herpes simplex virus type 2 (HSV-2) in vitro to oil from anise, hyssop, thyme, ginger, chamomile, and sandalwood and suggest that these oils can interfere with viral envelope structures.
The results of this work suggest that essential oil of G. spathulata can be used as a source of a new antifungal agent or adjuvant with antifungal agents in dermatophytosis, although the dermatophytes were less susceptible to it than to commercial antifungal agents, such as terbinafine and amphotericin B. No data of antifungal activity of Glechon species appear to have been published. Next steps include the antifungal investigation for G. marifolia essential oil.
Further studies including in vivo antiviral and antidermatophytic activity and isolation of pure constituents are necessary to identify the chemical substance(s) responsible for the antimicrobial effect of the essential oils investigated herein.
Declaration of interest
The authors report no declarations of interest. The authors are grateful to CNPq, Fapergs and Propesq/UFRGS for their financial support.
References
- Adams RP. (2009). Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Carol Stream (IL): Allured Publishing Corp
- Allahverdiyev A, Duranb N, Ozguvenc M, Koltasd S. (2004). Antiviral activity of volatile oils of Melissa officinalis L. against Herpes simplex virus type 2. Phytomedicine 11:657–61
- Burt S. (2004). Essential oils: Their antibacterial properties and potential applications in foods – A review. Int J Food Microbiol 94:223–53
- Clinical and Laboratory Standards Institute (CLSI). (2008a). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved standard CLSI M27-A3. Wayne, PA
- Clinical and Laboratory Standards Institute (CLSI). (2008b). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved standard CLSI M38-A2. Wayne, PA
- De Logu A, Loy G, Pellerano ML, et al. (2000). Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santonila insularis essential oil. Antiviral Res 48:177–85
- Farag RS, Shalaby AS, El-Baroty GA, et al. (2004). Chemical and biological evaluation of essential oils of different Malaleuca species. Phytother Res 18:30–5
- Fontenelle ROS, Morais SM, Brito EHS, et al. (2008). Antifungal activity of essential oil of Croton species from the Brazilian Caatinga biome. J App Microbiol 104:1383–90
- Fritz D, Venturi CR, Cargnin S, et al. (2007). Herpes virus inhibitory substances from Hypericum connatum Lam., a plant used in southern Brazil to treat oral lesions. J Ethnopharmacol 113:517–20
- Guelardini C, Galeotti N, Mannelli LDC, et al. (2001). Local anaesthetic activity of β-caryophyllene. Il Farmaco 56:387–9
- Hassiotis CN, Lazari DM. (2010). Decomposition process in the Mediterranean region. Chemical compounds and essential oil degradation from Myrtus communis. Int Biodeter Biodegr 64:356–62
- Havlickova B, Czaika VA, Friedrich M. (2008). Epidemiological trends in skin mycoses worldwide. Mycoses 51:2–15
- Hayashi K, Kamiya M, Hayashi T. (1995). Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, Influenza virus, and HIV. Planta Med 61:237–41
- Koch C, Reichling J, Schneele J, Schnitzler P. (2008). Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine 15:71–8
- Loizzo MR, Saab AM, Tundis R,et al. (2008). Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem Biodivers 5:461–70
- Mallo AC, Xifreda CC. (2004). Sobre dos espécies de Marsypianthes (Lamiaceae, Ocimeae) Del noreste argentino. Darwiniana 42:333–46
- Martinez-Rossi NM, Peres NT, Rossi A. (2008). Antifungal resistance mechanisms in dermatophytes. Mycopathologia 166:369–83
- Mentz LA, Lutzemberger LC, Schenkel EP. (1997). Da flora medicinal do Rio Grande do Sul: Notas sobre a obra de D’Ávila (1910). Cad Farm 13:25–48
- Misharina TA, Polshkov AN, Ruchkina EL, Medvedeva IB. (2003). Changes in the composition of the essential oil of Marjoram during storage. Appl Biochem Micro 39:311–16
- Montanha JA, Amoros M, Boustie J, Girre L. (1995). Anti-herpes virus activity of aporphine alkaloids. Planta Med 61:419–24
- Montanha JA, Moellerke P, Bordignon SAL, et al. (2004). Antiviral activity of Brazilian plant extracts. Acta Farm Bonaer 23:183–6
- Payment P, Trudel M. (1989). Manuel de techniques virologiques. Québec: Presses de l'Universitédu Québec
- Sabulal B, Dan M, John JA, et al. (2006). Caryophyllene-rich rizhome oil of Zingiber mimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 67:2469–73
- Schuhmacher A, Reichling J, Schnitzler P. (2003). Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 10:504–10
- Seebacher C, Bouchara JP, Mignon B. (2008). Updates on the epidemiology of dermatophyte infections. Mycopathologia 166:335–52
- Silva EBP, Soares MG, Mariane B, et al. (2013). The seasonal variation of the chemical composition of essential oils from Porceli macrocarpa R.E. Fries (Annonaceae) and their antimicrobial activity. Molecules 18:13574–87
- Sökmen M, Serkedjieva J, Daferera D, et al. (2004). In vitro antioxidant, antimicrobial and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum aciutidens. J Agr Food Chem 52:3309–12
- Toyota M, Koyama H, Mizutani M, Asakawa Y. (1996). (−)-ent-Spathulenol isolated from liverworts is an artifact. Phytochemistry 41:1347–50
- Turek C, Stintzing FC. (2012). Impact of different storage conditions on the quality of selected essential oils. Food Res Int 46:341–53
- Vieira PRN, Morais S.M, Bezerra FHQ, et al. (2014). Chemical composition and antifungal activity of essential oils from Ocimum species. Ind Crop Prod 55:267–71
- White DO, Frank JF. (1994). Medical Virology. San Diego: Academic Press
