Abstract
Context: Cucumis prophetarum Linn. (Cucurbitaceae) fruit is used for inflammatory-related problems and is proved to be possessing anticancer and hepatoprotective effects.
Objective: The present investigation was to study the effect of different fractions of C. prophetarum on antidiabetic and antioxidant activity.
Materials and methods: Aqueous crude extract (CE) of C. prophetarum fruits was fractionated into water soluble fraction 1 (F1), chloroform fraction 2 (F2), basic fraction 3 (F3), and neutral fraction 4 (F4) by acid–base extraction. CE and its fractions at different doses (0.02–0.1 mg/mL) were subjected to antidiabetic (α-amylase and α-glucosidase inhibition assays) and antioxidant (DPPH, superoxide radical scavenging (SO) and metal chelation) evaluation.
Results: F1 exhibited effective antidiabetic activity (p < 0.05) with an IC50 value of 20.6 and 59.9 µg/mL. The activity decreased in the order of CE > F4 >F3 > F2, according to α-amylase assay, which were the same, with the exception of the rank order of F4 and CE, as the α-glucosidase assay. Furthermore, F1 (IC50 = 73 µg/mL) showed better reducing ability than CE >F4 >F2 > F3 (IC50 = 78–272 µg/mL), according to the DPPH assay. In SO and metal chelation assays, F1 showed the highest activity (IC50 = 101 and 147 µg/mL), respectively; the activity decreased in the order of CE >F4 >F3 > F2 (IC50 = 126–469 µg/mL) for SO and 194–944 µg/mL for metal chelation assay.
Conclusion: The results indicate that F1 possesses potent in vitro antidiabetic and antioxidant activities.
Introduction
Cucumis prophetarum L. (Cucurbitaceae), commonly known as wild cucumber native to Asia and Africa, is mostly a prostrate or climbing monoecious herb. The fruits are ellipsoid, echinate, and green with white strips. It is distributed in the Western Ghats of Coorg region (Karnataka, India). The family includes about 130 genera and 800 species of plants and a few active compounds have been investigated for their hepatoprotective, anti-inflammatory, cardiovascular, and cytotoxic effects (Dhiman et al., Citation2012).
Diabetes mellitus (DM) is a heterogeneous metabolic disorder characterized by chronic hyperglycemia with disturbances in carbohydrate, fat, and protein metabolism resulting from defects in insulin secretion, insulin action, or both (WHO, Citation1999). The International Diabetes Federation predicts a worldwide increase from 8.3 to 10.1% by the year 2035 (IDF, Citation2013), of which 80% could be type 2 diabetic cases. The conventional approach for treating diabetes includes oral hypoglycemic agents such as sulfonylureas, biguanides, thiazolidinediones, meglinitides and, α-amylase and α-glucosidase inhibitors (Rang et al., Citation2003).
α-Amylase and α-glucosidase, involved in the digestive system, are responsible for hydrolysis of starch to glucose, which on absorption enters the blood. The activity of these enzymes correlates to an increase in post-prandial hyperglycemia (PPHG) and control of this PPHG is an important aspect in the treatment of type 2 diabetes. Compounds like acarbose, miglitol, and voglibose inhibit these enzymes causing a delay in carbohydrate digestion, thereby reducing the rate of glucose absorption and consequently, blunting the post-prandial plasma glucose rise (Conforti et al., Citation2005). Also, these inhibitors exhibit some gastrointestinal side effects.
In DM, free radicals are formed adversely through glucose oxidation, non-enzymatic glycation of proteins, and from the respiratory chain as a result of occasional leakage. This leads to lipid peroxidation and oxidative degradation of glycated proteins (Maritime et al., Citation2003), generating more reactive oxygen species (ROS). Most of the ROS are scavenged by the endogenous defense system such as catalase, superoxide dismutase, and the peroxidase-glutathione system. However, inefficiency of this system to scavenge free radicals relies on exogenous antioxidants from natural sources. Antioxidants from plant source are known to reduce oxidative stress and protect the body against free radical-mediated toxicities (Olga et al., Citation2003).
Plant species evaluated for antioxidant activity are known to exhibit antidiabetic effect. Indeed, it is estimated that more than 400 herbal or plant-derived products are used for the management of type 2 diabetes across the globe (Balley & Day, Citation1989). The hypoglycemic effect of some plant extracts has been confirmed in human and animal models of type 2 diabetes (Manisha et al., Citation2007). The WHO Expert Committee on diabetes has recommended that medicinal plants be further investigated. Therefore, the study was to determine the in vitro antidiabetic and antioxidant properties of different fractions obtained from aqueous extract of C. prophetarum fruit by using standard methods.
Materials and methods
Materials
Chemicals such as DNSA, ascorbic acid, α-tocopherol, citric acid, ferrous chloride, ferrozine, trichloroacetic acid, ferric chloride, Tris, NBT, NADH, PMS, and methanol were purchased from Himedia Laboratories, Mumbai, India. DPPH was obtained from Sigma-Aldrich (St. Louis, MO). α-Amylase, α-glucosidase, soluble starch, and maltose were procured from SRL Pvt Ltd, Mumbai, India. Glucose assay kit was purchased from Agappe Diagnostic Pvt Ltd, Kerala, India. All other chemicals and reagents used were of analytical grade.
Plant material
The fruits of C. prophetarum were collected during July and August 2011 from Western Ghats of Coorg region (Karnataka, India) and were authenticated by Prof. M. B. Shivanna. The herbarium with voucher no. KU/BL/MK/105 has been deposited in Applied Botany Department, Kuvempu University, Shimoga, India.
Extract preparation
Fresh fruits were washed, cleaned, and air-dried. Whole fruits (300 g) were homogenized without water in a commercial blender. The fresh juice was filtered using muslin cloth and the filtrate was centrifuged at 23 000 × g for 10 min, the supernatant was lyophilized and stored at −20 °C until use. The extract was used at the required concentrations.
Qualitative preliminary phytochemical group test
The tests for carbohydrates, reducing sugars, proteins, amino acids, saponins, sterols, alkaloids, tannins, glycosides, and flavonoids content of aqueous extract were performed by standard methods (Brain & Turner, Citation1975; Evans, 1996).
Preparation of different fractions
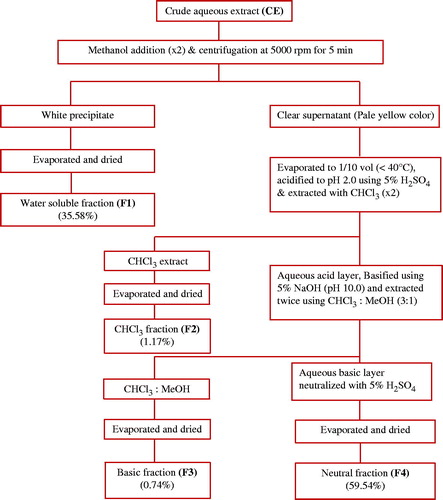
Four different fractions were obtained from aqueous crude extract of C. prophetarum fruit as shown in .
Antidiabetic activity determination
Two methods, α-amylase and α-glucosidase inhibition assays, were used based on the interaction of different fractions with the enzymes leading to decreases in the activity.
Pancreatic α-amylase inhibition assay
The inhibition assay was performed using the chromogenic DNSA method as described by Miller (1959), with slight modification. The assay mixture composed of 500 µL of 0.02 M sodium phosphate buffer (pH 6.9 containing 6 mM sodium chloride), 0.25 units of PPA solution, and different test samples at 0.02, 0.04, 0.06, 0.08, and 0.1 mg/mL (w/v) concentration were pre-incubated at 37 °C for 10 min. To the above buffer, 500 µL of 1% (w/v) starch solution was added to each tube and incubated at 37 °C for 15 min. The reaction was terminated with 1.0 mL DNSA reagent, placed in a boiling water bath for 5 min, cooled to room temperature, diluted, and the absorbance was measured at 540 nm. The control without test sample represented 100% enzyme activity. The absorbance produced by CE and its fractions was eliminated by including appropriate sample control in the reaction mixture except for the enzyme and starch. The known PPA inhibitor acarbose was used as a positive control.
α-Glucosidase inhibition assay
This was done according to the method of Andrade–Cetto with slight modifications (Andrade-Cetto et al., Citation2008). The assay was carried out by incubating a solution of starch substrate (2% w/v maltose) 1 mL of 0.2 M Tris buffer pH 8.0 and various concentrations of different test samples for 5 min at 37 °C. The reaction was initiated by adding 1 mL of α-glucosidase enzyme (1 U/mL) followed by incubation for 10 min at 37 °C. The reaction mixture was heated for 2 min in a boiling water bath to stop the reaction. The quantity of glucose liberated was measured using GOD-POD kit (Sigma-Aldrich, St. Louis, MO). The control represented 100% enzyme activity and did not contain any test sample. The absorbance produced by test sample was eliminated by including appropriate sample controls in the reaction mixture except for the enzyme and substrate. The α-glucosidase inhibitor, acarbose, was used as a positive control.
Calculation of IC50 value of antidiabetic assay
The IC50 values were defined as the concentration of the extract, containing the enzyme inhibitor that inhibited the activity by 50%.
The % of inhibition was calculated as follows:
where EC is the enzyme activity of control, ET is the enzyme activity of test, and TC is the test control.
One unit of enzyme activity is defined as the amount of enzyme required to release one micromole of product from substrate per min under the assay conditions.
Antioxidant activity determination
Three methods, DPPH, superoxide radical scavenging, and metal chelation, were used based on the reaction with electron-donating or metal chelating antioxidants.
DPPH assay
The DPPH free radical-scavenging activity of each sample was determined (Lai et al., Citation2001). An aliquot of 200 µL of each sample with different concentrations (0.02, 0.04, 0.06, 0.08, and 0.1 mg/mL) were mixed with 100 mM Tris-HCl buffer (800 µL, pH 7.4) and then added to 1 mL of 500 mM freshly prepared DPPH solution in ethanol (a final concentration of 250 µM). The mixture after shaking vigorously was allowed to stand for 20 min at room temperature in dark and the absorbance was measured at 517 nm. Ascorbic acid was used as a standard.
Superoxide radical-scavenging assay
The activity was measured as described by Robak and Gryglewski (Citation1998). The superoxide anion radicals were generated in 3.0 mL of Tris-HCl buffer (16 mM, pH 8.0), containing 0.5 mL NBT (0.3 mM), 0.5 mL NADH (0.936 mM) solution, 1.0 mL extract, and 0.5 mL Tris-HCl buffer (16 mM, pH 8.0). The reaction was started by adding 0.5 mL PMS solution (0.12 mM) to the mixture, incubated at 25 °C for 5 min, and the absorbance was read at 560 nm against a blank. Ascorbic acid was used as a positive control.
Metal chelation assay
The effect of chelation of ferrous ions on each sample was estimated using the method of Dinis et al. (Citation1994). To 0.1 mL of the extract, a solution of 0.5 mL ferrous chloride (0.2 mM) was added. The reaction was initiated by adding 0.2 mL of ferrozine (5 mM), incubated at room temperature for 10 min, and the absorbance was measured at 562 nm. Citric acid was used as a positive control.
Calculation of IC50 value of antioxidant assay
The concentration of sample required for scavenging 50% of the radicals/to chelate metal ions was calculated as follows.
where “Acont” is the absorbance of control, and “Asamp” is the absorbance of sample. IC50 values denote the concentration of a sample required to decrease the absorbance by 50%.
Statistical analysis
Values in tables and graphs were means ± standard deviations (n = 3). The IC50 values were calculated from linear regression analysis. Statistical comparisons were performed by one-way analysis of variance (ANOVA) followed by Student’s t-test to determine the significant difference between samples with 95% confidence limit.
Results and discussions
Preparation of crude extract and its fractions from C. prophetarum and screening of phytochemicals
The crude extract was fractionated to obtain four fractions. Fraction 1 (F1) was isolated by precipitation and an acid–base extraction was carried out to obtain fraction 2 (F2), fraction 3 (F3), and fraction 4 (F4). The separation was based on pH with the use of different solvents that influence their solubility in either of one solvent based on the acidic, basic, or neutral nature. The total recovery from four fractions was 97.03% and the residual 2.97% was lost in extraction process. The recoveries of F1, F2, F3, and F4 were 35.58, 1.17, 0.74, and 59.54%, respectively. Phytochemical analysis of crude extract revealed the presence of carbohydrates, reducing sugars, amino acids, saponins, and sterols. F1 showed positive result for amino acid test and glycosides.
Antidiabetic activity
DM is probably the fastest growing metabolic disease, wherein type 2 diabetes accounts for 9% of deaths worldwide and as knowledge about the heterogeneous nature of the disease increases, so does the need for more challenging and appropriate therapies. The aim of oral therapy is to reach normoglycemia to prevent later complications. Among glucose-lowering medications, α-amylase and α-glucosidase inhibitors delay the absorption of ingested carbohydrates, reducing the postprandial glucose and insulin peaks. Polysaccharide starch and disaccharides such as sucrose and lactose are the main source of glucose in human diet. Starch is initially broken down to oligosaccharides by α-amylase present in the saliva and pancreatic juice. The pancreatic form released into the small intestine is more potent than the salivary enzyme, and contact with these enzymes results in almost total conversion of starch to disaccharide maltose and other very small glucose oligomers before it leaves the duodenum (Guyton & Hall, Citation2000). α-Glucosidase is a collective term referring to membrane-bound enzymes of the small intestinal villi involved in the breakdown of α-linkages of oligosaccharides and disaccharides into glucose. Monosaccharides; glucose, fructose, and galactose are the final products of carbohydrate digestion. Normally, monosaccharides released by digestion are rapidly absorbed in the first half of the small intestine. However, digestion occurs throughout the small intestine, in the presence of inhibitors, resulting in slower absorption of monosaccharides and blunting of the postprandial glucose rise (Rhabasa-Lhoret & Chiasson, Citation2004). A search for compounds that can inhibit these intestinal enzymes is therefore regarded as one of the therapeutic approaches for developing novel antidiabetic agents (Lebovitz, Citation1992).
Recent advances in understanding the activity of these intestinal enzymes have led to the development of newer pharmacological agents. In this study, in vitro inhibitory effect of different fractions of C. prophetarum on porcine pancreatic amylase and intestinal α-glucosidase was evaluated based on chromogenic DNSA and glucose oxidase peroxidase methods, respectively (). F1 exhibited effective inhibition of α-amylase and α-glucosidase with an IC50 value of 20.6 and 59.9 µg/mL, respectively, and showed a dose-dependent effect on increasing the concentration. Acarbose used as a positive control showed an IC50 value of 32.98 µg/mL for α-amylase and 33.6 µg/mL for α-glucosidase, under similar assay conditions. F4 (IC50 = 95 µg/mL) showed less inhibition of α-amylase when compared with CE (IC50 = 85 µg/mL). Whereas, for α-glucosidase, F4 with an IC50 value of 78 µg/mL showed better inhibition than CE (IC50 = 132 µg/mL). In both the assays, F2 showed the least inhibition when compared with F3 ().
Figure 2. Antidiabetic activity of crude extract and its fractions from C. prophetarum as determined by α-amylase inhibition assay (A) and α-glucosidase inhibition assay (B).
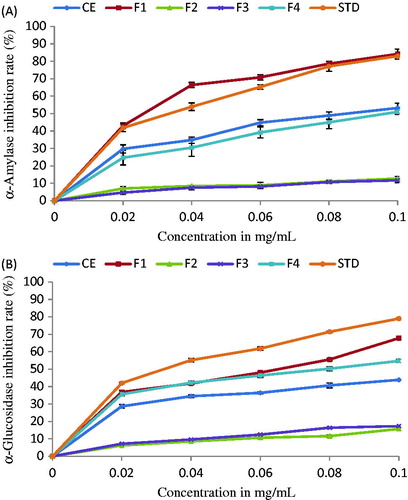
Table 1. Antidiabetic activity of CE and its fractions of C. prophetarum as determined by α-amylase and α-glucosidase inhibition assay.
Antioxidant activity
Recent experiments and clinical studies have uncovered new insights into the role of oxidative stress in diabetic complications and these reports have demonstrated different and innovative approaches to employ natural, antioxidant therapies. Various plant species have been evaluated for antioxidant activities which have also shown to exhibit antidiabetic activity (Kavishankar et al., Citation2011).
Antioxidants are compounds that prevent the oxidation of essential biological macromolecules by inhibiting the propagation of the oxidizing chain reaction. Keeping in mind the adverse effects of synthetic antioxidants, researchers have channeled their interest in isolating natural antioxidants (Kuo et al., Citation2005), which are very effective to control the oxidative stress and hence prevent the initiation of disease propagation. One important mechanism of antioxidation is scavenging of reactive oxygen species (ROS). ROS have various forms of activated oxygen, which include free radicals such as superoxide anion radicals (), hydroxyl radicals (
), non-free radicals (H2O2), and singlet oxygen (Halliwell, Citation1995). The stable radical DPPH has been used widely for the determination of primary antioxidant activity (Goncalves et al., Citation2005).
DPPH is one of the compounds that possess a proton free radical with a characteristic absorption, which decreases significantly on exposure to proton radical scavengers and gets converted to 1,1-diphenyl-2-picryl hydrazine. In the present investigation, the effect of CE and its fractions are shown in . Five different concentrations were used for each sample. F1 exhibited higher scavenging activity (IC50 = 73 µg/mL) than CE, F4, F2, and F3, where the IC50 values ranged from 78 to 272 µg/mL (), but was less compared with ascorbic acid that showed maximum activity with an IC50 value of 59.95 µg/mL. The free radical scavenging activity of crude extract was much more than F4, as this could be due to the presence of F1 in it. The greater free radical-scavenging activity corresponds to lower IC50 values.
Figure 3. Antioxidant activity of crude extract and its fractions from C. prophetarum as determined by the DPPH free radical-scavenging assay (A), superoxide radical scavenging assay (B), and metal chelation assay (C).
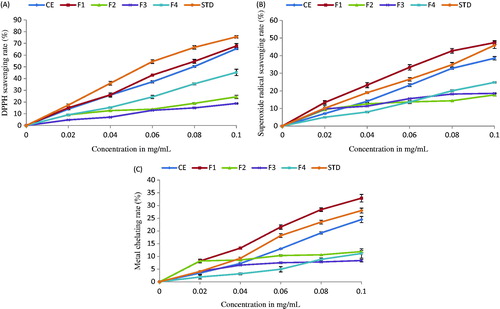
Table 2. Antioxidant activity of CE and its fractions of C. prophetarum as determined by DPPH, SO, and metal chelation assays.
Superoxides are radicals in which the unpaired electron is located on oxygen. They can generate more highly reactive hydroxyl radicals and protonated form of superoxide (Halliwell & Chirico, Citation1993). The strongest reactive oxygen species are derived from free amino groups that can react with hydroxyl radical or the NH2+ groups can form NH3+ subsequently reacting with hydroxyl radical through addition reaction (Xue et al., Citation1998). The more free amino groups present, higher will be the scavenging effect towards DPPH radicals and superoxide anion radicals. In the study, the IC50 values of CE, its fractions and standards were determined; ascorbic acid and citric acid were compared, which revealed F1 to be more potent () with an IC50 value of 101 and 147 µg/mL for superoxide radical scavenging and metal chelation assay, respectively. Whereas IC50 values of standards, ascorbic acid and citric acid, CE, F4, F3, and F2 were in the range of 112–469 µg/mL for SO, in which, F2 showed least inhibition (). Superoxide anion, hydrogen peroxide, and hydroxyl radical contribute to the pathogenesis of a variety of diseases. They are generated in vivo by mechanisms such as, respiratory redox chain in mitochondria, the respiratory burst of phagocytes, and the activities of various oxidases. Although superoxide anion is by itself a weak oxidant, it gives rise to the powerful and dangerous hydroxyl radicals as well as singlet oxygen both of which contribute to oxidative stress (Dahl & Richardson, Citation1978; Meyer & Isaksen, Citation1995; Pietta, Citation2000). Therefore, superoxide radical scavenging by antioxidants has physiological implications.
Ferrozine quantitatively complexes with Fe2+. This complex formation is disrupted in the presence of chelating agents resulting in decrease of red color complex formation. Therefore, the color reduction estimates the chelating activity of the coexisting chelator. The antioxidant abilities of CE and its fractions determined by the metal chelation method are shown in . The chelating effect on Fe2+ and ferrozine complex formation is shown in . The highest metal chelating rate was found for F1 (IC50 = 147 µg/mL), when compared with standard citric acid (IC50 = 168 µg/mL). F1 interfered with the complex formation, suggesting that it has chelating activity and complexes with ferrous ion before ferrozine to overcome ROS generation. Here, F4 showed less chelation compared with CE, which was followed by F2 that showed the least activity with an IC50 value ranging from 194 to 944 µg/mL.
Conclusions
In vitro antidiabetic activity of CE and its four fractions of water extract were determined by α-amylase and α-glucosidase inhibition assays and their antioxidant activities were assessed by DPPH, SO, and metal chelation methods. It was found that F1 showed potent antidiabetic and antioxidant activities. The results provide useful information on pharmacological activities of this plant associated with diabetes.
Declaration of interest
The authors have declared no conflict of interest. The investigators are thankful for financial assistance provided by University Grants Commission, New Delhi, India, to carry out this research work.
References
- Andrade-Cetto A, Becerra-Jimenez J, Cardenas-Vazquez R. (2008). α-Glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol 116:27–32
- Balley CJ, Day C. (1989). Traditional plant medicines as treatments for diabetes. Diabetes Care 12:553–64
- Brain KR, Turner TD. (1975). The Practical Evaluation of Phytopharmaceuticals, 2nd ed. Bristol: Wright Science technica, 81–2
- Conforti F, Statti G, Loizzo MR, et al. (2005). In vitro antioxidant effect and inhibition of α-amylase of two varieties of Amaranthus caudatus seeds. Biol Pharm Bull 28:1098–02
- Dahl MK, Richardson T. (1978). Photogeneration of superoxide anion in serum of bovine milk and in model systems containing riboflavin and amino acids. J Dairy Sci 61:400–7
- Dhiman K, Gupta A, Sharma DK, et al. (2012). A review on the medicinally plants of the family Cucurbitaceae. Asian Am J Clin Nutr 4:16–26
- Dinis TCP, Madeira VMC, Almeida LM. (1994). Action of phenolic derivatives (acetoaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxy radical scavengers. Arch Biochem Biophys 315:161–9
- Evans WC. (1996). Trease and Evans Pharmacognosy, 14th ed. London, England: W.B. Sounders Company Limited, 545–6
- Goncalves C, Dinis T, Batista MT. (2005). Antioxidant properties of pro-anthocyanidins of Uncaria tomentosa bark decotion: A mechanism for anti-inflammatory activity. Phytochemistry 66:89–98
- Guyton AC, Hall JE, eds. (2000). Digestion and absorption in the gastrointestinal tract. In: Textbook of Medical Physiology, 10th ed. UK: WB Saunders Company, 753–63
- Halliwell B. (1995). How to characterize an antioxidant: An update. Biochem Soc Symp 61:73–101
- Halliwell B, Chirico S. (1993). Lipid peroxidation: Its mechanism, measurement, and significance. Am J Clin Nutr 57:715–25S
- International Diabetes Federation (2013). IDF Diabetes Atlas, 6th ed. Brussels, Belgium: International Diabetes Federation. http://www.idf.org/diabetesatlas [last accessed on 12 May 2014]
- Kavishankar GB, Lakshmidevi N, Mahadeva Murthy S, et al. (2011). Diabetes and medicinal plants – A review. Int J Pharm Biomed Sci 2:65–80
- Kuo PC, Damu AG, Cherng CY, et al. (2005). Isolation of a natural antioxidant, dehydrozingerone from Zingiber officinale and synthesis of its analogues for recognition of effective antioxidant and antityrosinase agents. Arch Pharm Res 28:518–28
- Lai LS, Chou ST, Chao WW. (2001). Studies on the antioxidative activities of Hsian-tsao (Mesona procumbens Hemsl) leaf gum. J Agric Food Chem 49:963–8
- Lebovitz H. (1992). Oral antidiabetic agents: The emergence of α-glucosidase inhibitors. Drugs 44:21–8
- Meyer AS, Isaksen A. (1995). Application of enzymes as food antioxidants. Trends Food Sci Tech 6:300–4
- Manisha M, Priyanjali D, Jayant L, et al. (2007). Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr 40:163–73
- Maritime AC, Sanders RA, Watkins JB. (2003). Diabetes, oxidation stress and antioxidants: A review. J Biochem Mol Toxic 17:24–38
- Miller GL. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–8
- Olga B, Eija V, Kurt V. (2003). Antioxidants, oxidative damage & oxygen deprivation stress: A review. Ann Bot 91:179–94
- Pietta PG. (2000). Flavonoids as antioxidants. J Nat Prod 63:1035–42
- Rang HP, Dale MM, Ritter JM, Moore PK. (2003). Pharmocology, 5th ed. London: Churchill Livingstone, 382
- Rhabasa-Lhoret R, Chiasson JL. (2004). α-Glucosidase inhibitors. In: Defronzo RA, Ferrannini E, Keen H, Zimmet P, eds. International Textbook of Diabetes Mellitus, 3rd ed. UK: John Wiley & Sons Ltd, 901–14
- Robak J, Gryglewski RJ. (1988). Flavonoids are scavengers of superoxide anions. Biochem Pharm 37:837–41
- World Health Organization Consultation. (1999). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Report of a WHO Consultation Geneva. WHO/NCD/NCS/99.2
- Xue C, Yu G, Hirata T, et al. (1998). Antioxidative activities of several marine polysaccharides evaluated in a phosphatidylcholine-liposomal suspension and organic solvents. Biosci Biotechnol Biochem 62:206–9