Abstract
Context: The data concerning the influence of Plantaginaceae water extracts on bacterial growth are contradictory.
Objective: This study investigates the influence of Plantago maxima Juss. ex Jacq., Plantago lanceolata L., Plantago major L., Veronica teucrium L., Veronica spicata L., and Veronica incana L. aqueous extracts on growth of Escherichia coli K12 culture and the relation to antioxidant, reducing, and iron-binding activities.
Materials and methods: Aqueous extracts were prepared from the dried leaves with the final concentration of 1/10, 1/15, 1/20, 1/25, 1/30, 1/35, and 1/40 (w/w). Comparative analysis of total flavonoids, iridoids, and tannins in Plantaginaceae species was performed. Iron-binding, antioxidant, and reducing activities of plant extracts were analyzed spectrophotometrically. The influence of plant extracts on E. coli K12 growth was studied in vitro by estimating the bacterial growth in the extract-containing medium.
Results: Total tannin content in plant leaves positively correlated with iron-binding activity (r = 0.641), whereas total flavonoids correlated with antioxidant activity (r = 0.687). In an in vitro model, it is estimated that water extracts of studied Plantaginaceae species stimulated bacterial growth. Prebiotic activity significantly of 1/20 and 1/40 plant extracts positively correlated with antioxidant (r = 0.589; r = 0.576, respectively) and reducing activity (r = 0.721; r = 0.620, respectively) of plant aqueous extracts at 6–24 h. Negative correlation was observed between iron-binding activity and bacterial growth (r = −0.503 and r = −0.534 for 1/20 and 1/40 extracts, respectively).
Conclusion: Aqueous Plantaginaceae extracts possess prebiotic activity depending on the phytochemical content of plant leaves.
Introduction
From antiquity to nowadays, a number of plants were used in traditional medicine (Gurib-Fakim, Citation2006). The more recent development of novel methods of analysis and identification of phytochemicals with different activity spectra has significantly increased research of historic plant phytochemistry and their use in phytomedicine (Hostettmann & Marston, Citation2002).
Among those plants are Plantago and Veronica species, both traditionally used in folk medicine (Harput et al., Citation2004; Samuelsen, Citation2000). Recent studies have revealed phylogenetic proximity between these genera (Albach et al., Citation2005). This interrelationship between plant species determines a similar chemical content and biochemical activity.
Total antioxidant activity of Plantaginaceae preparations was widely studied (Beara et al., Citation2012; Harput et al., Citation2011; Živković et al., Citation2012). For instance, polyphenols were identified as potent antioxidant compounds (Pandey & Rizvi, Citation2009). It was also found that a number of polyphenolics have iron-binding (Khokhar & Owusu Apenten, Citation2003) and reducing properties (Pulido et al., Citation2000).
At the same time, a number of uncertainties remain. Specifically, the data concerning the influence of Plantaginaceae water extracts on bacterial growth are contradictory. A number of scholars have shown bactericidal activity of Plantago extracts (Sharifa et al., Citation2008; Stojković et al., Citation2013). Yet recent data have also demonstrated prebiotic activity of Plantago preparations (Elli et al., Citation2008).
Therefore, the primary objective of this study is to investigate the influence of Plantago and Veronica species on the bacterial growth of the standard Escherichia coli K12 culture and its relation to antioxidant, reducing and iron-binding activities.
Materials and methods
Plant preparation and total phytochemical content
Overground plant leaves of three Plantago (Plantago maxima Juss. ex Jacq., Plantago lanceolata L., Plantago major L.) and three Veronica (Veronica teucrium L., Veronica spicata L., Veronica incana L.) species were collected during flowering in different locations in the steppe zone of Southern Ural, with sharply continental climate.
Total iridoid content in the plant samples was determined photocolorimetrically (Groger & Simchen, Citation1967). The total tannins estimation in the Plantaginaceae leaves was performed using the Loewenthal method (Scalbert, Citation1992). The total flavonoid level was assessed colorimetrically with AlCl3 (Chang et al., Citation2002).
For the study of in vitro Plantaginaceae species activities, we used water extracts. Briefly, the dried leaves were minced and added to boiling sterile distilled water and steeped for 30 min. The obtained liquid was cooled to a room temperature and filtered under sterile conditions. The extracts were prepared in the following concentrations: 1/10, 1/15, 1/20, 1/25, 1/30, 1/35, and 1/40. All the above-mentioned plant extract concentrations were used for evaluation of iron-binding, antioxidant, and reducing activities. In order to estimate the influence of Plantaginaceae species on E. coli K12 growth, we used 1/20 and 1/40 extracts.
Chromatographic analysis
The chromatographic separation of biologically active substances in the plant extracts was performed by paper chromatography with an FN-1 Filtrak paper (Filtrak, Munich, Germany). For each compound, we have estimated the retardation factor (Rf).
The individual substances from iridoid fraction were analyzed in a complex solvent system containing n-butanol–acetic acid–water (BAAW; 4:1:2; v/v/v) in the ascending mode. The individual compounds were identified by means of exposure to visible and UV light before and after treatment with NH3 and the Trim–Hill reagent (Trim & Hill, Citation1952).
Polyphenolics (flavonoids and phenolcarboxylic acids) were analyzed using two-dimensional paper chromatography in two solvent systems (BAAW, 4:1:2 – system I; acetic acid–water, 15:85 (v/v) – system II), described earlier (Petrichenko et al., Citation2006). The spots were revealed by exposure to visible light and UV radiation before and after the treatment with NH3 and chromogenic substances, i.e. the Hepfner reagent and iron alum for phenolcarboxylic acids and 5% AlCl3 (in EtOH) for flavonoids. After that, characteristic fluorescence of the compounds was registered. The HPLC-grade standards of individual compounds analyzed (cynaroside, luteolin, apigenin, apigenin-7-glucuronide, neochlorogenic acid, chlorogenic acid, caffeic acid, quinic acid, ferulic acid, isoferulic acid, isocatalpol, catalpol, catalposide, aucubin, and aucubin acetate) were obtained from Perm State Pharmaceutical Academy (Perm, Russia).
Plants extract in vitro activities
Iron-binding activity of plant extracts was estimated using the ferrozine method (Kumaran & Karunakaran, Citation2007) with slight modifications. In order to evaluate the dose dependence of iron-binding activity, 20 μl of extracts were added to 0.4 ml of 0.25 mM iron solution ((NH4)2Fe(SO4)2·6H2O) and incubated for 15 min at room temperature with subsequent addition of 1.6 ml of 1.25 mM ferrozine. The probe was shaken and left standing at room temperature for 10 min. Afterwards the absorbance of the solution was measured spectrophotometrically at 562 nm. The percentage of iron binding was calculated according to the formula: [(A0 − A1)/A0]×100%, where A1 is the absorbance of the solution containing plant extract, whereas A0 is the absorbance of the control solution (20 μl of distilled water instead of plant extract). Time dependence of the iron-binding activity allowed the estimation of the speed of iron complexation. The above-mentioned method was used with slight modifications. The duration of incubation of the probe after addition of the plant extract was 0, 5, 10, 20, 40, and 60 min. Other manipulations remained the same.
Total antioxidant activity (Prieto et al., Citation1999) and reducing activity (Oyaizu, Citation1986) of the extracts were expressed in equivalents of 1 mM ascorbate activity. All spectrophotometric studies were conducted using a PD-303UV spectrophotometer (Apel, Japan).
Bacteriological analysis
The influence of plant extracts on E. coli K12 growth was estimated after incubation of the culture in the microtiter plates in the presence of extracts for 24 h. Briefly, 25 μl of bacterial culture (5 × 108 colony-forming units per ml) and 25 μl of plant extracts were added into every well of the plate containing 200 μl of beef-extract broth. Afterwards the plates were incubated at 37 °C. The bacterial growth was estimated by analyzing the absorbance of the culture at 492 nm using the Multiscan Accent microplate reader (Thermo Labsystems, Vantaa, Finland). In the current study, we evaluated the optical density of the bacterial culture at 0 (initial), 2, 4, 6, and 24 h of incubation. In order to estimate the dynamics of bacterial growth, we calculated the specific bacterial growth rate (Berney et al., Citation2006): μ=(ln At2–ln At1)/(T2 − T1), where μ is a specific bacterial growth rate, AT2 and AT1 are values of absorbance at time T2 and T1, respectively. The specific bacterial rate was estimated for time periods of 0–2, 2–4, 4–6, and 6–24 h.
Statistical analysis
The data are expressed as mean values of five measurements ± standard deviation (mean ± SD). The Mann–Whitney U-test was used for the group mean comparisons at the significance level p < 0.05 (*). Pearson’s correlation coefficient was used for correlation analysis (†). All statistical analyses are performed using Statistica 10 (StatSoft Inc., 2011, Tulsa, OK).
Results
Total phytochemical content in plant species
Our data () demonstrate that P. maxima leaves contained the maximal level of flavonoids, exceeding the levels obtained for P. lanceolata and P. major at least 2.5-fold. The total flavonoid content in the leaves of the Veronica species was the least in V. teucrium. At the same time, the total flavonoid content in V. spicata and V. incana leaves exceeded the values obtained for V. teucrium by 61 and 78%, respectively.
Table 1. Phytochemical content in Plantago and Veronica leaves (mg/g).
We observed the highest level of iridoids in P. maxima leaves. The level exceeded the values obtained for P. lanceolata and P. major more than seven- and nine-fold, respectively. As in the case of the flavonoid content, we observed the lowest level of iridoids between Veronica species for V. teucrium. The total iridoid content in V. spicata and V. incana leaves exceeded the similar values for V. teucrium by 28 and 53%, respectively. The maximal tannin level was found in P. maxima and P. lanceolata: the obtained values exceeded the values for all other studied Plantaginaceae species more than two-fold.
Chromatographic characterization of phytochemicals in plant species
The results of the chromatographic analysis show that P. maxima leaves contain 13 individual polyphenolics. Seven of them are estimated to be flavonoids (cynaroside, apigenin, and the not defined compounds), whereas the latter six are characterized as phenylcarboxylic acids including neochlorogenic, chlorogenic, and caffeic acids. Six chromatographic spots are related to iridoids (isocatalpol, catalpol, aucubin, aucubin acetate, and the undefined ones) ().
Table 2. Chromatographic characterization of flavonoids, phenylcarboxylic acids, and iridoids found in P. maxima leaves.
Twelve polyphenols are revealed during the chromatographic analysis of P. lanceolata leaves. Six of the compounds are related to flavonoids (cynaroside, luteolin, and the not defined ones), while the others are phenylcarboxylic acids (including neochlorogenic and chlorogenic acids). The chromatographic analysis of P. lanceolata iridoids revealed three individual compounds including catalpol and aucubin ().
Table 3. Chromatographic characterization of flavonoids, phenylcarboxylic acids, and iridoids found in P. lanceolata leaves.
The analysis of P. major leaves detected nine individual polyphenols including five flavonoids (cynaroside, luteolin, and the undefined ones) and four phenylcarboxylic acids (neochlorogenic, chlorogenic, ferulic acids, and one not defined compound). Iridoid analysis showed three individual compounds (catalpol, aucubin, and the not defined one) ().
Table 4. Chromatographic characterization of flavonoids, phenylcarboxylic acids, and iridoids found in P. major leaves.
Sixteen polyphenolic compounds were revealed during V. teucrium leaves analysis. Nine of these compounds are supposed to be flavonoids (including cynaroside and luteolin), while the others are phenylcarboxylic acids (caffeic, chlorogenic, neochlorogenic, quinic, and the undefined ones). Four chromatographic spots are related to individual iridoids (aucubin, catalposide, and the not defined compounds) ().
Table 5. Chromatographic characterization of flavonoids, phenylcarboxylic acids, and iridoids found in V. teucrium leaves.
The chromatographic analysis of V. spicata leaves revealed 23 individual polyphenols. According to the color and the Rf values 15 spots are related to flavonoids (luteolin, apigenin, apigenin-7-glucuronide, cynaroside, and the not defined ones). Eight polyphenolics are supposed to be phenylcarboxylic acids (including caffeic, ferulic, isoferulic, chlorogenic, and quinic acids). The chromatographic analysis of iridoids revealed five individual iridoids (isocatalpol, catalposide, aucubin, and the undefined compounds) ().
Table 6. Chromatographic characterization of flavonoids, phenylcarboxylic acids, and iridoids found in V. spicata leaves.
A total of 23 polyphenolics was detected during V. incana leaves analysis including 16 flavonoids (cynaroside, apigenin, apigenin-7-glucuronide, luteolin, and the undefined ones) and seven phenylcarboxylic acids. Five individual iridoids including catalpol, catalposide, and aucubin were revealed during the chromatographic analysis of iridoids in V. incana leaves ().
Table 7. Chromatographic characterization of flavonoids, phenylcarboxylic acids, and iridoids found in V. incana leaves.
Dose-dependence of iron-binding activity
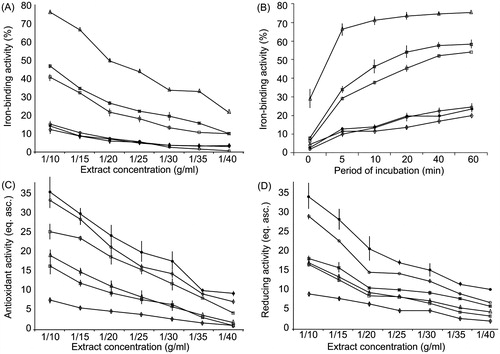
Our data () demonstrated that Fe-binding activity of 1/10 water extracts decreased in the following order: P. lanceolata > V. teucrium > P. maxima > V. incana > V. spicata > P. major. The described tendency persists for more diluted extracts. In all concentrations of the extracts, the Fe-binding activity of P. lanceolata exceeded the values obtained for P. maxima and P. major more than two-fold and six-fold, respectively. We registered the highest iron-binding activity in Veronica species for V. teucrium, which exceeded the values of V. spicata and V. incana more than three-fold.
Time-dependence of iron-binding activity
The time-dependence of iron binding () was in line with the values of dose-dependent iron-binding activity. At 0 min of incubation, the rate of iron binding was the following: P. lanceolata > V. teucrium > P. maxima > P. major > V. spicata > V. incana. With the increase of the period of incubation, the amount of chelated iron by the plant extracts increased. After a 60-min incubation, the iron-binding activity of the extracts was in the following order: P. lanceolata > V. teucrium > P. maxima > V. spicata > V. incana > P. major.
Total antioxidant activity
We observed the maximal values of total antioxidant activity of 1/10 extracts for V. spicata and V. incana (). The results exceeded those for V. teucrium more than two-fold. In the Plantago species, the highest activity of total antioxidant activity was registered in the P. maxima extracts. These values were significantly larger than the correspondent activity results for P. lanceolata (by 31%) and P. major (by more than 100%). This tendency was also registered for lower concentrations of extracts. Thus, the total antioxidant activity of plant extracts was as follows: V. spicata > V. incana > P. maxima > P. lanceolata > V. teucrium > P. major.
Reducing activity
The reducing activity of 1/10 extracts, generally, was higher in Veronica species (). The maximal level of reducing activity was observed in V. spicata, which surpassed the values of V. incana and V. teucrium by 18% and 90%, respectively. In the Plantago species, the highest reducing activity was observed in P. lanceolata and P. maxima, both exceeding the values of P. major nearly two-fold. Consequently, we classified the reducing activity of plant extracts in the following order: V. spicata > V. incana > V. teucrium > P. lanceolata > P. maxima > P. major.
Correlation between phytochemical content and observed activities
Statistical analysis shows that the iron-binding activity significantly and positively correlates only with the tannin content in the plant leaves (r = 0.641). The total antioxidant activity of extracts was characterized by a significant correlation with the total flavonoid content in the plant species (r = 0.687). It is important to note that the correlation analysis failed to reveal a significant relationship between the reducing activity and phytochemical content. However, a correlation coefficient of 0.418 was revealed for relationship between reducing activity and total flavonoids.
Influence of plant extracts on E. coli K12 growth
Our data demonstrate that the administration of 1/20 plant extracts into the nutrient medium significantly effected the E. coli K12 growth after 2 h of incubation (). Plantago maxima and P. major extracts elevated the optical density of the bacterial culture by 24 and 27%, respectively, in comparison with the control values. At the same time, the V. teucrium and V. spicata extracts increased the bacterial quantity by 31 and 27% when compared with the controls. After a 4-h incubation of the bacterial culture with a plant extracts, further stimulation of bacterial growth was observed. A similar tendency was observed after incubation of the bacterial culture for 6 h. After a 24-h incubation, the described above tendency changed. The V. teucrium, V. spicata and V. incana extracts elevated bacterial quantity by 45, 45, and 43% as compared with the control culture. After the incubation of the bacterial culture with the P. maxima, P. lanceolata and P. major extracts for 24 h, the optical density was increased by 26, 25, and 36% in comparison with the control culture.
Table 8. Influence of plant extracts on E. coli K12 growth (units of optical density at λ=492 nm).
The incubation of 1/40 plant extracts with the bacterial culture significantly increased the E. coli K12 growth (). Activation of bacterial growth after administration of Plantaginaceae water extracts was observed at 2, 4, and 6 h of incubation. As a result, after a 24-h incubation, 1/40 water extracts of the Veronica species demonstrated a higher rate of prebiotic activity. Administration of the V. teucrium, V. spicata and V. incana extracts into the incubation medium increased the bacterial density by 19, 15, and 22% when compared with the control cultures. The water extracts of Plantago species did not increase the bacterial growth significantly.
Influence of plant extracts on E. coli K12-specific growth rate
Our data () show that the P. maxima 1/20 extracts increased the specific bacterial growth rate in periods of 0–2 h and 2–4 h by 21% and 36% compared with the control values. A relative decrease in the growth rate was registered at later periods. The P. lanceolata extract did not significantly affect the E. coli K12-specific growth rate in the period between 0 and 2 h. At the same time, the extract significantly increased the specific growth rate by 51% in period of 2–4 h. However, in the period between 6 and 24 h, the P. lanceolata extract diminished the specific bacterial growth rate by 34% when compared with the control culture. The P. major extract also influenced the specific bacterial growth rate, although this effect was not significant during the first 6 h of incubation. In contrast, in the period of 6–24 h, the P. major extract decreased the E. coli growth rate by 30% in comparison with the controls. The influence of V. teucrium and V. spicata on the E. coli-specific growth rate was nearly identical. Specifically, in the period between 0 and 2 h, the extracts increased the bacterial growth rate by 25 and 23%, respectively. In the other observed periods, V. teucrium and V. spicata did not affect the studied parameter significantly. Administration of the V. incana extracts into the nutrient medium insignificantly increased the specific bacterial growth rate.
Table 9. Influence of plant extracts on E. coli K12-specific growth rate.
Similar to the 1/20 extracts, lower concentrations of the extracts produced an analogous effect (). Administration of the P. maxima and P. major 1/40 extracts into the nutrient medium insignificantly increased the specific bacterial growth rate in the following periods: 0–2 h and 2–4 h. Furthermore, a slight decrease in the bacterial growth rate in the period of 4–6 h was not statistically significant, whereas the P. maxima and P. major extracts reduced the specific bacterial growth rate in the period between 6 and 24 h by 20 and 36%, respectively. The P. lanceolata water extract significantly increased the specific bacterial growth rate in the period of 2–4 h by 32% compared with the control values. In P. lanceolata-treated bacterial cultures, specific growth rate did not significantly change in the period between 4 and 6 h. In the subsequent period of 6–24 h, however, the P. lanceolata extract diminished bacterial growth by 36% in comparison with the control culture. Extracts made from the Veronica species in concentration of 1/40 did not affect the specific bacterial growth rate significantly. At the same time, these extracts exhibited a slight stimulatory activity.
Correlation between phytochemical content and specific bacterial growth rate
Our statistical analysis () indicates the highest correlation coefficients for the total tannins and specific bacterial growth rate after administration of the plant extracts. We found a significant positive correlation during the period of 2–4 h. At later time periods (4–6 and 6–24 h), the correlation between the total tannin content and the specific growth rate was negative. The other studied phytochemicals correlated with the specific bacterial growth rate to a lesser extent. A significant negative correlation with the bacterial growth was observed for iridoids at the period of 4–6 h.
Table 10. Correlation between phytochemical content and specific bacterial growth rate.
Correlation between in vitro activity and specific bacterial growth rate
The specific bacterial growth rate significantly and negatively correlated with the iron-binding activity () of both the 1/20 and 1/40 extracts at the initial period of colony growth (0–2 h). At the same time, in the period of 2–4 h, the Fe-binding activity positively correlated with the growth rate. In the later periods of the bacterial growth, we observed a negative but non-significant correlation between the iron-binding activity and the specific growth rate.
Table 11. Correlation between plant extract in vitro activity and specific bacterial growth rate.
A significant correlation between the antioxidant activity of the plant extracts and the specific growth rate was detected only in the period of 2–4 h and for lower concentrations of extracts (1/40). Importantly, in the later periods of bacterial growth, a positive but not significant correlation was registered between the studied parameters.
The reducing activity of both 1/20 and 1/40 plant extracts significantly and negatively correlated with the bacterial growth rate in the period of 2–4 h. At the same time, we found a positive correlation between these parameters at the later periods of bacterial growth (4–6 and 6–24 h).
Discussion
The observed differences in the biological activity of the studied plant extracts are determined by the phytochemical content in plant leaves. Significant correlation between the iron-binding activity and the tannin content was in accordance with the earlier studies (Khokhar & Owusu, Citation2003). The correlation between the antioxidant activity and the flavonoid content is generally determined by the antioxidant and antiradical characteristics of flavonoids (Pandey & Rizvi, Citation2009).
Our data indicate the prebiotic activity in the studied plant extracts. The observed prebiotic activity positively correlated with the reducing and antioxidant activity of the plant extracts. The iron-binding activity negatively correlated with the prebiotic activity. Presumably, the iron binding by the plant-derived extracts prevents iron absorption by a bacterial cell leading to growth restriction (Freidank et al., Citation2001). At the same time, the reducing activity allows to maintain the pool of catalytically active intracellular iron by reducing Fe3+ into Fe2+ and thus stimulating iron recycling and preventing iron deficiency. The positive influence of plant extracts’ antioxidant activity is determined by the prevention of the development of the oxidative stress that can occur during realization of the iron prooxidant activity (Schützendübel & Polle, Citation2002).
The above-mentioned dependence among the antioxidant, reducing, and iron-binding activities was registered during the longer periods of incubation. In contrast, in a period of 2–4 h, an inverse dependence between a specific growth rate and the studied activities was detected. This paradox may be, ex hypothesis, due to a temporary change in the iron requirements of the bacterial culture that can be observed in several bacteria (Wilhelm et al., Citation1996). We fully acknowledge that this explanatory hypothesis needs to be verified by further experiments.
In conclusion, our results demonstrate that Plantaginaceae water extracts exhibit a significant reducing, antioxidant, and iron-binding properties. All Plantaginaceae plants showed a prebiotic activity with respect to E. coli K12. However, the chemical nature of this potential plant-derived prebiotic is to be investigated.
Acknowledgements
We would like to thank Prof. Yuliya Guseva, Ph.D. (Rutgers University, Piscataway Township, NJ) for correction of the English language.
Declaration of interest
The authors declare no conflict of interest.
References
- Albach DC, Meudt HM, Oxelman B. (2005). Piecing together the “new” Plantaginaceae. Am J Bot 92:297–315
- Beara IN, Lesjak MM, Orcic DZ, et al. (2012). Comparative analysis of phenolic profile, antioxidant, anti-inflammatory and cytotoxic activity of two closely-related Plantain species: Plantago altissima L. and Plantago lanceolata L. LWT-Food Sci Technol 47:4e70
- Berney M, Weilenmann HU, Ihssen J, et al. (2006). Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl Environ Microbiol 72:2586–93
- Chang C, Yang M, Wen H, Chern J. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–82
- Elli M, Cattivelli D, Soldi S, et al. (2008). Evaluation of prebiotic potential of refined psyllium (Plantago ovata) fiber in healthy women. J Clin Gastroenterol 42:174–6
- Freidank HM, Billing H, Wiedmann-Al-Ahmad M. (2001). Influence of iron restriction on Chlamydia pneumoniae and C. trachomatis. J Med Microbiol 50:223–7
- Groger D, Simchen P. (1967). Zur Kentnis iridoider Pflanzenstoffe. Pharmazie 22:315–17
- Gurib-Fakim A. (2006). Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med 27:1–93
- Harput US, Genç Y, Khan N, Saracoglu I. (2011). Radical scavenging effects of different Veronica species. Rec Nat Prod 5:100–7
- Harput US, Varel M, Nagatsu A, Saracoglu I. (2004). Acylated iridoid glucosides from Veronica anagallis-aquatica. Phytochemistry 65:2135–9
- Hostettmann K, Marston A. (2002). Twenty years of research into medicinal plants: Results and perspectives. Phytochem Rev 1:275–85
- Khokhar S, Owusu ARK. (2003). Iron binding characteristics of phenolic compounds: Some tentative structure–activity relations. Food Chem 81:133–40
- Kumaran A, Joel Karunakaran JR. (2007). In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci Technol 40:344–52
- Oyaizu M. (1986). Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–15
- Pandey KB, Rizvi SI. (2009). Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2:270–8
- Petrichenko VM, Sukhinina TV, Babiyan LK, Shramm NI. (2006). Chemical composition and antioxidant properties of biologically active compounds from Euphrasia brevipila. Pharm Chem J 40:312–16
- Prieto P, Pineda M, Aguilar M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 269:337–41
- Pulido R, Bravo L, Saura-Calixto F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48:3396–402
- Samuelsen AB. (2000). The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J Ethnopharmacol 71:1–21
- Scalbert A. (1992). Quantitative methods for the estimation of tannins in plant tissues. In: Hemingway RW, Laks PS, eds. Plant Polyphenols: Synthesis, Properties, Significance. New York: Plenum Press, 259–80
- Schützendübel A, Polle A. (2002). Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–65
- Sharifa AA, Neoh YL, Iswadi MI, et al. (2008). Effects of methanol, ethanol and aqueous extract of Plantago major on Gram positive bacteria, Gram negative bacteria and yeast. Ann Microsc 8:42–4
- Stojkovic DS, Zivkovic J, Sokovic M, et al. (2013). Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem Toxicol 55:209–13
- Trim AR, Hill R. (1952). The preparation and properties of aucubin, asperuloside and some related glycosides. Biochem J 50:310–19
- Wilhelm SW, Maxwell DP, Trick CG. (1996). Growth, iron requirements, and siderophore production in iron-limited Synechococcus PCC 7002. Limnol Oceanogr 41:89–97
- Živković J, Ćebović T, Maksimović Z. (2012). In vivo and in vitro antioxidant effects of three Veronica species. Cent Eur J Biol 7:559–68