Abstract
Context: Portulacerebroside A (PCA) is a novel cerebroside compound isolated from Portulaca oleracea L. (Portulacaceae), an edible and medicinal plant distributed in the temperate and tropical zones worldwide.
Objective: This study investigates the effects of PCA in human liver cancer HCCLM3 cells on metastasis and invasion.
Materials and methods: After the cells were treated with PCA (2.5, 5, and 10 μg/ml) for 6, 12, 24, or 48 h, adhesion, transwell invasion, and scratch tests were conducted and cell functions were evaluated. Western blot and FQ-RT-PCR assays explored the mechanism of PCA-inhibited invasion and metastasis in the cells.
Results: The adhesion rate of the cells was suppressed at 0.5 h (79.4 ± 1.0, 68.7 ± 1.3, and 58.1 ± 1.3%, versus 100 ± 1.5% in the control), 1 h (78.2 ± 1.2, 70.9 ± 1.6, and 55.4 ± 1.9%, versus 100 ± 1.2% in the control), and 1.5 h (71.6 ± 1.1, 62.3 ± 0.9, and 50.4 ± 0.9%, versus 100 ± 1.1% in the control). The 24 h invasion ability was decreased (356.6 ± 11.2, 204.0 ± 17.6, and 113.0 ± 9.5%, versus 443.6 ± 15.4% in the control). The migration capability was also restrained by PCA for 24 h (324.8 ± 25.4, 250.4 ± 21.0, and 126.3 ± 10.1, versus 381.6 ± 30.6 in the control) and 48 h (470.3 ± 34.3, 404.0 ± 19.7, and 201.0 ± 15.4, versus 752.0 ± 63.6 in the control). There was an increase in the mRNA and protein expression levels of TIMP-2 and nm23-H1, inhibition in the mRNA expression of MTA1, MMP-2, and MMP-9, and suppression in the protein expression of MTA1, RhoA, Rac1/Cdc42, MMP-2, but not RhoC and MMP-9.
Conclusion: PCA suppresses the invasion and metastasis of HCCLM3 cells possibly by modulation of the mRNA and protein expression of related parameters. This is the first study to reveal a new potential therapeutic application of PCA in antimetastatic therapy for liver cancer.
Introduction
Portulaca oleracea L. (Portulacaceae) is an edible and medicinal plant widely distributed in the temperate and tropical zones of the world. Its aerial part (Chinese name Ma-Chi-Xian) has a long history of use in China for the prevention and treatment of diarrhea, urinary tract infection, and diabetes. It is also used for clearing away heat and toxic materials from blood to stop bleeding and for relieving the symptoms of dysentery.
Portulaca oleracea also displays a wide range of pharmacological properties, such as regulating lipidemia, anti-aging, neuroprotective, antidiabetic, antimicrobial, antioxidative, anti-inflammatory, antiulcerogenic, hepatoprotective, and analgesic activities (Xin et al., Citation2008; Yan et al., Citation2012). Several fractions obtained from this plant have been reported to display antitumor effects (Li et al., Citation2009). For example, polysaccharides isolated from this plant have evidently scavenged accumulating free radicals and modulated immunity functions of rats with ovarian cancer (Chen et al., Citation2009). Three homoisoflavonoids present in this plant display selectively cytotoxic activities towards four human cancer cell lines, especially 2,2′-dihydroxy-4′,6′-dimethoxychalcone against gastric carcinoma SGC-7901 cells with an IC50 of 1.6 μg/ml, which is more potent than the reference compound mitomycin C which has an IC50 of 13.0 μg/ml (Yan et al., Citation2012). Alkaloids, N-trans-feruloyltyramine, (7′R)-N-feruloylnormetanephrine, 1,5-dimethyl-6-phenyl-1,2-dihydro-1,2,4-triazin-3(2H)-one, and (3R)-3,5-bis(3-methoxy-4-hydroxyphenyl)-2,3-dihydro-2(1H)-pyridinone, revealed weak bioactivities against K562 with IC50 values of 222.77, 66.94, 90.09, and 41.52 μM, respectively, and moderate bioactivities against A549 with IC50 values of 28.80, 21.76, 24.54, and 37.20 μM, respectively (Tian et al., Citation2014).
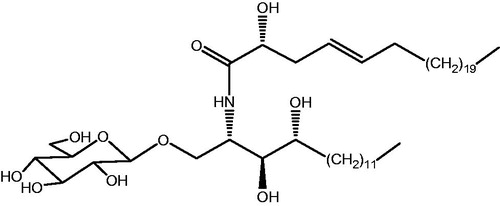
We have previously isolated a novel cerebroside compound named portulacerebroside A (PCA, ) from Portulaca oleracea (Xin et al., Citation2008), which markedly inhibited cell viability, induced chromatin aggregation and fragmented nuclei, and enhanced the apoptotic percentage of human liver cancer HCCLM3 cells. Furthermore, PCA remarkably increased the protein phosphorylation of p38 MAPK and JNK, disrupted mitochondrial membrane penetrability, and triggered the release of mitochondrial cytochrome C and AIF to cytosol in the cells. PCA also evidently activated caspase-9 and caspase-3, but not caspase-8. These results suggest that PCA-induced apoptosis is associated with the activation of the p38 MAPK- and JNK-triggered mitochondrial death pathway (Zheng et al., Citation2014). In the present study, we investigated the effects of PCA on invasive and metastasis in HCCLM3 cells and the possible underlying mechanism.
Materials and methods
PCA
PCA was isolated and purified from the aerial parts of Portulaca oleracea L. as reported previously (Xin et al., Citation2008) and appears as a white powder (Molecular formula: C48H93NO10; molecular weight: 843; purity: 98.1%). The appropriate amount of dimethylsulfoxide (DMSO) was used to dissolve PCA, and the final concentration of DMSO was kept below 0.1%. PCA was diluted to the desired concentrations before the application and no differences were observed in cell growth between the cells treated with or without DMSO, indicating that DMEM containing 0.1% DMSO had no effects on cell growth.
Cell culture
The human liver cancer HCCLM3 cells were obtained from JRDUN Biotechnology (Shanghai) Co., Ltd. (Shanghai, China), and were grown in DMEM containing 10% FBS and 1% penicillin/streptomycin as described by our previous literature (Zheng et al., Citation2014). The cells were pre-incubated at 37 °C in a humid atmosphere containing 5% CO2.
Adhesion test
A 96-well plate was used and 30 μl of BD Matrigel was added to each well and left to dry for 1 h. The plate was then rinsed two-times with PBS and 100 μl of PBS containing 1% fetal bovine serum (FBS) was then added to each well, incubated for 1 h in an incubator at 37 °C followed by a further wash with 2 × 200 μl of PBS. The cells were pre-treated with PCA at concentrations 2.5, 5, and 10 μg/ml for 12 h and were then suspended in serum-free DMEM to give a concentration of 1 × 105/ml followed by the addition of 100 μl/well of this suspension and the plate was finally incubated in an incubator for 0.5, 1, and 1.5 h, respectively. The medium was removed from each well by aspiration and the cells were further washed two times with PBS and 200 μl of DMEM containing 2% (v/v) FBS was added to each well. The OD value of each well was then measured using a CCK-8 assay and the results are presented as % of control.
Transwell invasion test
The cells were suspended in serum-free DMEM and pretreated with PCA at a concentration of 2.5, 5, and 10 μg/ml, for 6 h. A 24-well plate with transwell cabinets was soaked in PBS for 5 min prior to cell seeding and the bottom of cabinets was precoated with matrigel to form a genuine reconstituted basement membrane. After digestion with pancreatic enzymes and washing with serum-free DMEM, the cells were suspended in DMEM containing 1% (v/v) FBS and seeded in transwell cabinets with 0.5 ml/cabinet at the density of 2 × 105/ml. This was followed by the addition of 0.75 ml of DMEM containing 10% (v/v) FBS and the plate was placed in an incubator at 37 °C for 24 h. Subsequently 1 ml of 4% formaldehyde solution was added to each well and the cells were fixed for 10 min at room temperature. The excess stationary formaldehyde was then removed by aspiration and 1 ml of 0.5% crystal violet solution was added to each well and the cells were stained for 30 min. Finally the cells were washed three-times with PBS, dried and images were taken by an Olympus inverted microscope (Olympus, Tokyo, Japan) (magnification, × 20) and invaded cells were quantified by manual counting.
Migration test
The HCCLM3 cells were seeded in a 6-well plate until 95% confluence was reached. The monolayer cells were subjected to a cross line wound by a sterile pipette tip and washed with three times PBS to remove cell debris. The cells were then incubated at 37 °C with PCA concentrations of 2.5, 5, and 10 μg/ml for 24 and 48 h. An inverted microscope was used to observe cell morphology and digital images (×10) were obtained and then the cells that migrated to the wound were quantified by manual counting. The experiment was repeated three times.
Fluorescent quantitative reverse transcription-PCR (FQ-RT-PCR)
After 24 h of incubation in a 6-well plate, the cells were treated with various concentrations of PCA for 6 h before being harvested. Total RNA was extracted using a TRIzol® Plus RNA Purification Kit (Invitrogen, Beijing, China), which provides a simple, reliable, and a rapid method for isolating high-quality total RNA from cells. The isolated RNA was treated with RNase-free DNase (Promega, Shanghai, China).
Reverse transcription was carried out using a cDNA synthesis kit (Thermo, Shanghai, China) in accordance with the manufacturer's instructions and as reported previously (Paul et al., Citation2013; Zhang et al., Citation2012a). PCR amplification was performed using a SYBR Green PCR kit (Thermo, Shanghai, China). Primer pairs for human genes were designed using the Primer Express Software (Applied Biosystems, Shanghai, China) and are listed in . Relative expression of mRNA (%) = 2−ΔCT(1,2,3,4,5) × 100%, where CT represents threshold cycle, ΔCT1 = CT(nm23-H1)−CT(GAPDH), ΔCT2 = CT(MTA1)−CT(GAPDH), ΔCT3 =CT(MMP-2)−CT(GAPDH), ΔCT4 = CT(MMP-9)−CT(GAPDH), ΔCT5 = CT(TIMP-2)−CT(GAPDH).
Table 1. Primers used in FQ-RT-PCR analysis.
Western blot assay
After incubation with the designated concentrations of PCA for 24 h, the cells were washed and suspended at 4 °C for 20 min. Following centrifugation, the supernatant was transferred to a microtube and the protein concentration was measured using a BCA protein assay kit (Thermo, Shanghai, China). Protein expression was detected by Western blot hybridization in accordance with the manufacturer’s instructions and as reported previously (Zhang et al., Citation2012b).
Statistical analysis
All results are presented as the mean ± SD and the data were analyzed using a SPSS 13.0 statistical package (SPSS Inc., Chicago, IL). Data for multiple comparisons were subjected to one-way ANOVA followed by Dunnett’s test and a value of p < 0.05 was considered statistically significant.
Results
PCA depresses the adhesion of HCCLM3 cells
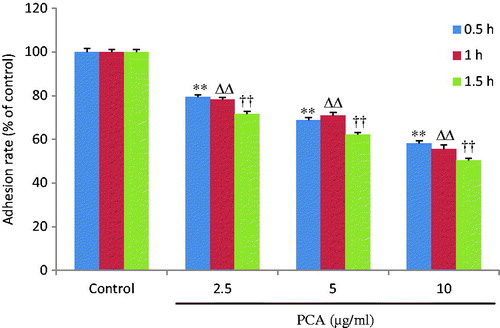
As shown in , the adhesive rate of cells descended in a time- and dose-dependent manner after 12 h of pre-treatment with PCA (2.5, 5, and 10 μg/ml) at 0.5 h (79.4 ± 1.0, 68.7 ± 1.3, and 58.1 ± 1.3%, respectively, versus 100 ± 1.5% in the control group; all p < 0.01), 1 h (78.2 ± 1.2, 70.9 ± 1.6, and 55.4 ± 1.9%, respectively, versus 100 ± 1.2% in the control group; all p < 0.01), and 1.5 h (71.6 ± 1.1, 62.3 ± 0.9, and 50.4 ± 0.9%, respectively, versus 100 ± 1.1% in the control group; all p < 0.01). The PCA treatment significantly reduced the adhesive ability of HCCLM3 cells when compared with the control cells (untreated cells).
Figure 2. Effects of portulacerebroside A on adhesive ability of HCCLM3 cells. The cells were pre-treated with various concentrations of portulacerebroside A (PCA) for 12 h in a 96-well plate and the adhesion rate of the cells was determined at 0.5 h, 1 h, and 1.5 h. **,ΔΔ, ††p < 0.01 compared with the control group. Data are expressed as the mean ± SD, n = 6.
PCA inhibits the invasion of HCCLM3 cells
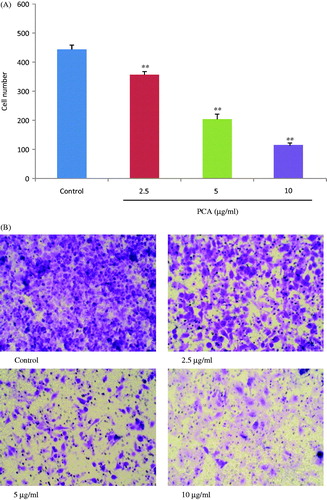
For pervasion to distant organs, the invasion of tumor cells occurs via cell-secreted proteolytic degradation of the cellular basement membrane, which is the leading cause of cancer death. The transwell invasion test results are presented in . Pretreatment with PCA (2.5, 5, and 10 μg/ml) for 6 h significantly suppressed the 24 h invasion ability of cells in a dose-dependent manner when compared with the control group of untreated cells (356.6 ± 11.2, 204.0 ± 17.6, and 113.0 ± 9.5%, respectively, versus 443.6 ± 15.4% in the control group; all p < 0.01).
Figure 3. Effects of portulacerebroside A on invasive ability of HCCLM3 cells. After 6 h of pretreatment with designated concentrations of portulacerebroside A (PCA), the cells were incubated in transwell cabinets for a further 24 h, and the invasive cell number (A) was counted. (B) Representative images (×20) for each group. **p < 0.01 compared with the control group. Data are expressed as the mean ± SD, n = 6.
PCA prevents HCCLM3 cells from migration
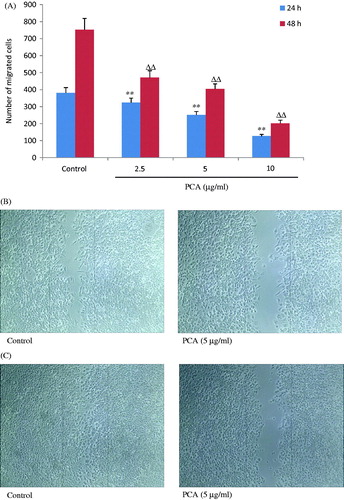
The effects of PCA on chemotactic motility in cells were evaluated by the scratch test. As displayed in , the migrated cells decreased dramatically and dose dependently after treatment with designated concentrations of PCA (2.5, 5, and 10 μg/ml) for 24 h (324.8 ± 25.4, 250.4 ± 21.0, and 126.3 ± 10.1, respectively, versus 381.6 ± 30.6 in the control group; all p < 0.01) and 48 h (470.3 ± 34.3, 404.0 ± 19.7, and 201.0 ± 15.4, respectively, versus 752.0 ± 63.6 in the control group; all p < 0.01), respectively. Typical images of PCA inhibiting cell migration are presented in .
Figure 4. Effects of portulacerebroside A on migration ability of HCCLM3 cells. The cells were subjected to a cross line wound by a sterile pipette tip in a 6-well plate and were then incubated with designated concentrations of portulacerebroside A (PCA) for 24 h and 48 h. The migration cells were then counted (A). (B) and (C) display typical images (×10) of cells treated with PCA. **ΔΔp < 0.01 compared with the control group. Data are expressed as the mean ± SD. n = 6.
PCA regulates the mRNA expression of metastasis-related parameters in HCCLM3 cells
The effects of PCA on the mRNA expression of nm23-H1, MTA1, MMP-2, MMP-9, and TIMP-2, which are closely related to metastasis of tumor cells, were also investigated and the results are presented in . As a result, 6 h of PCA treatment significantly increased the expression of nm23-H1 and TIMP-2 and reduced the expression of MTA1, MMP-2, and MMP-9 in a dose-dependent manner.
Table 2. Effects of PCA on the mRNA expression of metastasis-related parameters.
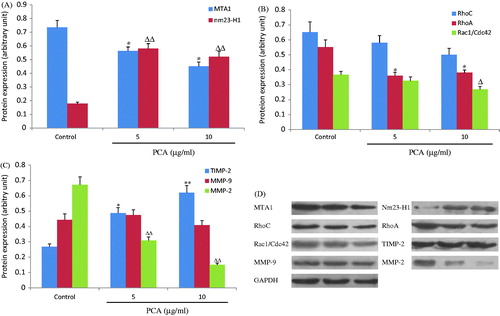
PCA adjusts the metastasis-related protein expression in HCCLM3 cells
After 24 h of PCA treatment, the metastasis-related protein expression in cells was analyzed by western blot. As shown in , PCA (5 and 10 μg/ml) significantly increased the expression levels of nm23-H1 (0.58 ± 0.04 and 0.52 ± 0.04, respectively, versus 0.17 ± 0.01 in the control group; all p < 0.01) and TIMP-2 (0.49 ± 0.04 and 0.62 ± 0.05, respectively, versus 0.26 ± 0.02 in the control group; p < 0.05 or p < 0.01), decreased the expression levels of MTA1 (0.56 ± 0.03 and 0.45 ± 0.03, respectively, versus 0.74 ± 0.05 in the control group; all p < 0.05), RhoA (0.36 ± 0.02 and 0.38 ± 0.02, respectively, versus 0.55 ± 0.04 in the control group; all p < 0.05), Rac/Cdc42 (0.32 ± 0.03 (p > 0.05) and 0.26 ± 0.01 (p < 0.05), respectively, versus 0.26 ± 0.02 in the control group), and MMP-2 (0.30 ± 0.02 and 0.15 ± 0.01, respectively, versus 0.67 ± 0.05 in the control group; all p < 0.01). However, no effect on MMP-9 expression was evident (0.47 ± 0.04 and 0.41 ± 0.03, respectively, versus 0.44 ± 0.04 in the control group; all p > 0.05). Although RhoC expression was suppressed by PCA, this was not statistically significant (0.58 ± 0.04 and 0.50 ± 0.04, respectively, versus 0.65 ± 0.07 in the control group; all p > 0.05).
Figure 5. Effects of portulacerebroside A on the protein expression of metastasis-related parameters. After 24 h of treatment with portulacerebroside A (PCA), the protein expression of MTA1 (A), nm23-H1 (A), RhoC (B), RhoA (B), Rac1/Cdc42 (B), MMP-2 (C), MMP-9 (C), and TIMP-2 (C) was detected by western blot. (D) Representative western blot images of HCCLM3 cells treated with PCA. *, Δ p < 0.05; **, ΔΔ p < 0.01 compared with the control group. Data are expressed as the mean ± SD, n = 3.
Discussion
A leading cause of death in cancer patients is tumor metastasis, due to failure of complete removal of tumor tissue during surgery and radiotherapy. Although there are numerous chemical drugs of choice used in chemotherapy, too many side effects or uncertain actions impede their clinical applications hence it is necessary to search for novel anti-tumor agents. PCA, a novel natural compound isolated from Portulaca oleracea, is reported to significantly elicit HCCLM3 cell apoptosis (Zheng et al., Citation2014), although the PCA-modulated tumor migration and invasion was not investigated. In the present study, PCA significantly inhibited the adhesion, invasion, and migration of HCCLM3 cells at doses of 2.5, 5, and 10 μg/ml in a dose-dependent manner. This suggests that PCA not only induces apoptosis of tumor cells but also prevents them from metastasis, subsequently in this paper we report the possible mechanisms involved.
Metastasis-associated gene 1 (MTA1), an integral part of the nucleosome remodeling and histone deacetylation (NuRD) complex, can inhibit the transcription of target genes by recruiting histone deacetylases into the promoter regions of target genes thus inducing histone deacetylation (Li et al., Citation2013). MTA1 is up-regulated in human tumors (Hofer et al., Citation2006) and increases the metastatic and invasive potential of carcinoma cells (Hofer et al., Citation2004; Kumar et al., Citation2003; Toh et al., Citation2004; Zhang et al., Citation2014). In this study, PCA evidently inhibited the mRNA and protein expression of MTA1.
Many biological functions are associated with nm23-H1, which can inhibit tumor metastasis (Novak et al., Citation2011) and is also capable of binding to DNA leading to the regulation of gene expression (Choudhuri et al., Citation2010; Thakur et al., Citation2009). The exogenous expression of nm23-H1 in cells without endogenous nm23-H1 expression not only suppresses migration and invasion but also decreases cell proliferation and anchorage-independent growth (Jung et al., Citation2006; McDermott et al., Citation2008). Moreover, nm23-H1 protein inhibits telomerase activity (Kar et al., Citation2012) and increases cell–cell adhesion (Bago et al., Citation2009), cell-cycle arrest, and apoptosis (Cervoni et al., Citation2006). This suggests that nm23-H1 plays an important role in the process of tumor formation and metastasis. Therefore, we further analyzed the mRNA and protein expression of nm23-H1, both of which were markedly increased by PCA treatment.
The extracellular matrix (ECM) regulates cell attachment, motility, invasion, and metastasis (Kumar et al., Citation2010). Degradation of ECM is performed by matrix metalloproteinases (MMPs), especially MMP-2 and MMP-9, which play a key role in degrading basement membranes and cancer invasion and metastasis (Gialeli et al., Citation2011; Iguchi, Citation2012), but are regulated by TIMPs, the endogenous inhibitors of the zinc-dependent endopeptidases of MMPs (Rubaci et al., Citation2012). In the present study, PCA raised the mRNA and protein expression of TIMP-2, suppressed the protein expression of MMP-2 and the mRNA expression of MMP-2 and MMP-9; however, it did not repress the protein expression of MMP-9 in HCCLM3 cells. These findings indicate that the anti-metastatic effects of PCA in tumor cells are associated with regulation of the MMP/TIMP balance.
RhoA and RhoC, are GTPases and are part of the extensive Ras superfamily, have 92% amino acid identity and interact with various regulators and effectors with modulation activity to regulate invasion, metastasis, actin cytoskeleton, cell proliferation, oncogenesis, and survival of cells (Mardilovich et al., Citation2012; Rathinam et al., Citation2011). RhoA and RhoC have been extensively documented for migration and invasion (Faried et al., Citation2007; Liu et al., Citation2012; Struckhoff et al., Citation2011). RhoA expression is increased in human breast cancers due to miRNAmiR-31 suppression, and metastasis in mouse models has also been reported (Valastyan et al., Citation2009). Deletion of RhoC does not affect breast cancer growth in a mouse model, but metastasis is significantly impaired (Hakem et al., Citation2005). RhoC mRNA is one of the targets of miRNA miR-493 and miR-138, both of which decrease the migration of tumor cells (Jiang et al., Citation2010; Ueno et al., Citation2012). Rac1 and Cdc42 also belong to GTPases and take part in the control of cell migration (Zins et al., Citation2013), activation of which is required for HER2/ErbB2-elicited migration and invasion of breast cancer cells (Johnson et al., Citation2010). Therefore, we investigated the protein expression levels of RhoA, RhoC, and Rac1/Cdc42 in the cells. As a result, treatment with PCA for 24 h remarkably depressed the expression of RhoA and Rac1/Cdc42 but not RhoC, indicating that PCA-induced migration and invasion had no direct involvement in RhoC.
In conclusion, our investigation has demonstrated for the first time the inhibitory effects of PCA on the metastatic and invasive capabilities of human hepatoma HCCLM3 cells. The possible mechanisms involved are that PCA increases the expression of TIMP-2 and decreases the expression of MMP-2, RhoA, RhoC, and Rac1/Cdc42 through modulation of the metastasis promoting gene MTA1 and metastasis suppressor gene nm23-H1. These findings reveal a new potential therapeutic application of PCA in anti-metastatic therapy for liver cancer.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by the National Natural Science Foundation of China (No. 81102774).
References
- Bago R, Pavelic J, Maravic Vlahovicek G, Bosnar MH. (2009). Nm23-H1 promotes adhesion of CAL 27 cells in vitro. Mol Carcinog 48:779–89
- Cervoni L, Egistelli L, Eufemi M, et al. (2006). DNA sequences acting as binding sites for NM23/NDPK proteins in melanoma M14 cells. J Cell Biochem 98:421–8
- Chen YG, Shen ZJ, Chen XP. (2009). Evaluation of free radicals scavenging and immunity-modulatory activities of Purslane polysaccharides. Int J Biol Macromol 45:448–52
- Choudhuri T, Murakami M, Kaul R, et al. (2010). Nm23-H1 can induce cell cycle arrest and apoptosis in B cells. Cancer Biol Ther 9:1065–78
- Faried A, Faried LS, Usman N, et al. (2007). Clinical and prognostic significance of RhoA and RhoC gene expression in esophageal squamous cell carcinoma. Ann Surg Oncol 14:3593–601
- Gialeli C, Theocharis AD, Karamanos NK. (2011). Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278:16–27
- Hakem A, Sanchez-Sweatman O, You-Ten A, et al. (2005). RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev 19:1974–9
- Hofer MD, Kuefer R, Varambally S, et al. (2004). The role of metastasis-associated protein 1 in prostate cancer progression. Cancer Res 64:825–9
- Hofer MD, Tapia C, Browne TJ, et al. (2006). Comprehensive analysis of the expression of the metastasis-associated gene 1 in human neoplastic tissue. Arch Pathol Lab Med 130:989–96
- Iguchi K. (2012). Effect of bisphosphonates on anticancer activity in prostate cancer cells. Yakugaku Zasshi 132:1025–30
- Jiang L, Liu X, Kolokythas A, et al. (2010). Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. Int J Cancer 127:505–12
- Johnson E, Seachrist DD, DeLeon-Rodriguez CM, et al. (2010). HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42. J Biol Chem 285:29491–501
- Jung S, Paek YW, Moon KS, et al. (2006). Expression of Nm23 in gliomas and its effect on migration and invasion in vitro. Anticancer Res 26:249–58
- Kar A, Saha D, Purohit G, et al. (2012). Metastases suppressor NME2 associates with telomere ends and telomerase and reduces telomerase activity within cells. Nucleic Acids Res 40:2554–65
- Kumar A, El-Osta A, Hussain AA, Marshall J. (2010). Increased sequestration of matrix metalloproteinases in ageing human Bruch's membrane: Implications for ECM turnover. Invest Ophthalmol Vis Sci 51:2664–70
- Kumar R, Wang RA, Bagheri-Yarmand R. (2003). Emerging roles of MTA family members in human cancers. Semin Oncol 30:30–7
- Li Y, Chao Y, Fang Y, et al. (2013). MTA1 promotes the invasion and migration of non-small cell lung cancer cells by downregulating miR-125b. J Exp Clin Cancer Res 32:33. doi:10.1186/1756-9966-32-33
- Li YP, Zeng XW, Ye J, et al. (2009). Screening antitumor effect of active constituents from Portulaca oleracea L. in vitro and in vivo. Lishizhen Med Mater Med Res 20:2726–8
- Liu M, Bi F, Zhou X, Zheng Y. (2012). Rho GTPase regulation by miRNAs and covalent modifications. Trends Cell Biol 22:365–73
- Mardilovich K, Olson MF, Baugh M. (2012). Targeting Rho GTPase signaling for cancer therapy. Future Oncol 8:165–77
- McDermott WG, Boissan M, Lacombe ML, et al. (2008). Nm23-H1 homologs suppress tumor cell motility and anchorage independent growth. Clin Exp Metastasis 25:131–8
- Novak M, Jarrett SG, McCorkle JR, et al. (2011). Multiple mechanisms underlie metastasis suppressor function of NM23-H1 in melanoma. Naunyn Schmiedebergs Arch Pharmacol 384:433–8
- Paul A, Das S, Das J, et al. (2013). Diarylheptanoid-myricanone isolated from ethanolic extract of Myrica cerifera shows anticancer effectson HeLa and PC3 cell lines: Signalling pathway and drug-DNA interaction. J Integr Med 11:405–15
- Rathinam R, Berrier A, Alahari SK. (2011). Role of Rho GTPases and their regulators in cancer progression. Front Biosci 16:2561–71
- Rubaci AH, Kazancioglu HO, Olgac V, Ak G. (2012). The roles of matrix metalloproteinases-2, -7, -10 and tissue inhibitor of metalloproteinase-1 in the pathogenesis of oral lichen planus. J Oral Pathol Med 41:689–96
- Struckhoff AP, Rana MK, Worthylake RA. (2011). RhoA can lead the way in tumor cell invasion and metastasis. Front Biosci 16:1915–26
- Thakur RK, Kumar P, Halder K, et al. (2009). Metastases suppressor NM23-H2 interaction with G-quadruplex DNA within c-MYC promoter nuclease hypersensitive element induces c-MYC expression. Nucleic Acids Res 37:172–83
- Tian JL, Liang X, Gao PY, et al. (2014). Two new alkaloids from Portulaca oleracea and their cytotoxic activities. J Asian Nat Prod Res 16:259–64
- Toh Y, Ohga T, Endo K, et al. (2004). Expression of the metastasis-associated MTA1 protein and its relationship to deacetylation of the histone H4 in esophageal squamous cell carcinomas. Int J Cancer 110:362–7
- Ueno K, Hirata H, Majid S, et al. (2012). Tumor suppressor microRNA-493 decreases cell motility and migration ability in human bladder cancer cells by downregulating RhoC and FZD4. Mol Cancer Ther 11:244–53
- Valastyan S, Reinhardt F, Benaich N, et al. (2009). A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell 137:1032–46
- Xin HL, Hou YH, Xu YF, et al. (2008). Portulacerebroside A: New cerebroside from Portulaca oleracea L. Chin J Nat Med 6:401–3
- Yan J, Sun LR, Zhou ZY, et al. (2012). Homoisoflavonoids from the medicinal plant Portulaca olerace. Phytochemistry 80:37–41
- Zhang H, Yang D, Wang H, et al. (2014). Metastasis-associated gene 1 promotes invasion and migration potential of laryngeal squamous cell carcinoma cells. Oncol Lett 7:399–404
- Zhang H, Yu CH, Jiang YP, et al. (2012a). Protective effects of polydatin from Polygonum cuspidatum against carbon tetrachloride-induced liver injury in mice. PLoS One 7:e46574
- Zhang H, Zhang Y, Jiang YP, et al. (2012b). Curative effects of oleanolic acid on formed hypertrophic scars in the rabbit ear model. Evid-Based Compl Alt 2012:837581. doi: 10.1155/2012/837581
- Zheng GY, Qu LP, Yue XQ, et al. (2014). Portulacerebroside A induces apoptosis via activation of the mitochondrial death pathway in human liver cancer HCCLM3 cells. Phytochem Lett 7:77–84
- Zins K, Lucas T, Reichl P, et al. (2013). A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice. PLoS One 8:e74924