Abstract
Context: Plants and most of the plant-derived compounds have long been known for their potential pharmaceutical effects. They are well known to play an important role in the treatment of several diseases from diabetes to various types of cancers. Today most of the clinically effective pharmaceuticals are developed from plant-derived ancestors in the history of medicine.
Objective: The aim of this study was to evaluate the free radical scavenging activity and total phenolic and flavonoid contents of methanol, ethanol, and acetone extracts from flowers and leaves of Onopordum acanthium L., Carduus acanthoides L., Cirsium arvense (L.) Scop., and Centaurea solstitialis L., all from the Asteraceae family, for investigating their potential medicinal values of biological targets that are participating in the antioxidant defense system such as catalase (CAT), glutathione S-transferase (GST), and glutathione peroxidase (GPx).
Materials and methods: In this study, free radical scavenging activity and total phenolic and flavonoid contents of the plant samples were assayed by DPPH, Folin–Ciocalteu, and aluminum chloride colorimetric methods. Also, the effects of extracts on CAT, GST, and GPx enzyme activities were investigated.
Results and discussion: The highest phenolic and flavonoid contents were detected in the acetone extract of C. acanthoides flowers, with 90.305 mg GAE/L and 185.43 mg Q/L values, respectively. The highest DPPH radical scavenging was observed with the methanol leaf extracts of C. arvense with an IC50 value of 366 ng/mL. The maximum GPx and GST enzyme inhibition activities were observed with acetone extracts from the flower of C. solstitialis with IC50 values of 79 and 232 ng/mL, respectively.
Introduction
Plant-derived compounds have played an important role in treating and preventing human diseases. These are important sources for new drugs and also good lead compounds suitable for further modification during drug development to discover new naturally occurring enzyme inhibitors (Ata et al., Citation2007). These enzyme inhibitors may be used as agents to improve chemotherapy or to treat various diseases. In recent years, the concern on chemotherapy has been raised mostly about anticancer drug resistance (Ata et al., Citation2007; Udenigwe et al., Citation2007), considering that the mechanisms of cytosolic enzymes in drug resistance during the course of cancer and parasitic disease treatments (Koehn et al., Citation2005; Nabekura, Citation2010; Tan et al., Citation2011). Different studies have indicated that glutathione S-transferases (GSTs) play an active role in this process. The GSTs (EC; 2.1.5.18) are major phase II detoxification enzymes and most of the isoenzymes are located in the cytosol. They catalyze the conjugation of glutathione (GSH) to a variety of exogenous and endogenous electrophilic compounds (Sheehan et al., Citation2001) and serve roles in the development of drug resistance as an inhibitor of the mitogen-activated protein (MAP) kinase pathway (Fan et al., Citation2001; Potapova et al., Citation1997; Townsend & Tew, Citation2003). Glutathione peroxidase (GPx; EC; 1.11.1.19) is an important enzyme which functions to protect the cell from oxidative damage by catalyzing the reduction of hydro peroxides, including hydrogen peroxide. The balance between activities and the intracellular levels of antioxidant enzymes are very important for the survival of organisms and their health. Previous studies have shown that chemotherapy causes several side effects on the biological system. During cancer therapy, some chemotherapeutic agents generate free radicals which cause apoptosis of target cells, but antioxidant enzymes may scavenge these radical molecules (Prasad et al., Citation2002; Simone et al., Citation2007) and may reduce the effectiveness of therapeutic agents. Thus, in this way, the inhibition of antioxidant enzymes may improve the treatment of proliferative diseases.
The Asteraceae is the largest family of flowering plants, comprising about 1100 genera and 20 000 species. Many members of Asteraceae are shown to have pharmacological activity which contained important phytochemical compounds such as polyphenols, flavonoids, and diterpenoids (Ertürk & Demirbağ Citation2003; Shing et al., Citation2002). Several studies demonstrated the antibacterial, antifungal, anti-inflammatory, insecticide, and antitumor capacities of Asteraceae species.
This paper reports the biological activities of acetone, ethanol, and methanol extracts of Onopordum acanthium L., Carduus acanthoides L., Cirsium arvense (L.) Scop., and Centaurea solstitialis L. to determine their effects on the GST and GPx and catalase (CAT) enzyme and free radicals scavenging activities.
Materials and methods
Chemicals
In this study, 4-aminoantipyrine (4-AP), hydrogen peroxide (H2O2), and sodium azide (NaN3) were purchased from (Acros (Fair Lawn, NJ), ascorbic acid, 3,5-dichloro-2-hydroxybenzenesulfonic acid (DHBS), ethylenediaminetetraacetic acid (EDTA), Folin–Ciocalteu reagent, reduced glutathione (GSH), glutathione reductase (GR), horseraddish peroxidase (HRP), CAT, and quercetin hydrate were supplied from Sigma Aldrich (Hamburg, Germany). Nicotine amide adenine dinucleotide phosphate reduced (NADPH) was purchased from Gerbu (Heidelberg, Germany). Also, 1,1-diphenyl-2-picrylhydrazyl (DPPH•) as a free radical form (90% purity) was obtained from Calbiochem (Darmstadt, Germany). All other chemicals used were of analytical grade.
Collection and identification of plant material
Plant materials were collected in June–July 2010 from Kastamonu, Turkey, at their flowering season. The plant samples were botanically identified by Associate Professor Dr. Ergin Murat Altuner, in Department of Biology, Kastamonu University.
Plant extract preparation
Flowers and leaves of each plant were washed with tap water and stored at −20 °C until they were used. For extraction, the previously reported procedure was used with some modifications (Coruh et al., Citation2007). Basically, the plant samples were grounded with liquid nitrogen to obtain fine powder of samples. Then, these samples were extracted with solvent acetone, ethanol, or methanol for 24 h at 4 °C, with a sample-to-solvent ratio of 1:10 (w/v). On the following day, the solvent was evaporated at 40 °C until they fully dried, the obtained product was dissolved in DMSO and kept at dark (4 °C) until they were used.
Determination of total phenolic content
The total phenolic contents of extracts were determined by employing the Folin–Ciocalteu assay (Slinkard & Singleton, Citation1977). Each extract solution (0.1 mL) was mixed with 1 mL of a 2% (w/v) sodium carbonate solution and vortexed strongly. After 5 min, 1 mL of Folin–Ciocalteu’s reagent (1:10 diluted with distilled water) was added and vortexed. After incubation for 1 h at room temperature, the absorbance of each mixture was measured at 750 nm. The total phenolic contents of extracts were calculated using the standard curve of gallic acid control where this curve was prepared with 0–250 mg/L of gallic acid (GA) in DMSO. The total polyphenol content (TPC) of extract was expressed as milligrams of gallic acid equivalent (GAE)/L of plant extract (mg GAE/L).
Determination of total flavonoid content
The total concentration of flavonoids in extracts was determined by employing the aluminum chloride colorimetric method which was previously described (Chang et al., Citation2002). Each plant extract (0.5 mL) was separately mixed with 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M sodium acetate, and 2.8 mL of DMSO. It was incubated at room temperature for 30 min and the absorbance was measured at 415 nm. The standard curve was prepared by quercetin solutions at concentrations 0–100 mg/L. The total flavonoid content of the extract was expressed milligrams of quercetin equivalent (QE)/L of plant extract (mg QE/L).
Free radical scavenging activity by DPPH assay
Radical scavenging activity against the stable radical DPPH was measured using the methods of Sharma and Bhat (Citation2009) with some modifications and miniaturized for microplate applications. The antioxidant activities of the plants extracts were determined on the basis of the radical scavenging effect of the stable DPPH-free radical. The assay mixture consisted of 6 μL plant extract solution in different concentrations, 50 μL DPPH methanol solution (200 μM) and 144 μL 99% methanol. After vortexing thoroughly and leaving for 25 min at room temperature, the absorbance was measured at 517 nm using a microplate reader spectrophotometer. The final concentration range of 0.01–3.75 µg/mL of ascorbic acid and quercetin, and 0.01–15 µg/mL of gallic acid was employed as a positive control. The DPPH radical scavenging activity of each sample was expressed as IC50 value and calculated from the dose–response inhibition curve.
Isolation of cytosol from bovine liver
The bovine liver was obtained from slaughterhouse of Kazan, Ankara, Turkey. The liver samples were homogenized in 10 mM potassium phosphate buffer (pH 7.0), containing 0.15 M KCl, 1 mM EDTA, and 1 mM of DTT, by using a glass Teflon homogenizer and centrifuged at 10 000× g for 20 min. The supernatant was filtered through cheesecloth and the filtrate was centrifuged at 30 000× g for 60 min. The collected supernatants were filtered again and the resultant filtrate was referred as a cytosol (Coruh et al., Citation2007). The prepared homogenates, containing 46.41 mg protein/mL, were kept in an ultralow freezer (−80 °C) until they were used. The total protein content was determined by the Lowry method (Lowry et al., Citation1951).
Inhibition of GST
GST activity was determined against the substrate, 1-chloro-2,4-dinitrobenzene (CDNB), by monitoring the thioether formation at 340 nm (Habig et al., Citation1974). The assay was miniaturized for microplate applications (Isgor & Isgor, Citation2012). Briefly described, the assay mixture consisted of plant extract solution (the final concentration was in the range of 2–285 ng/mL), 100 mM potassium phosphate buffer (pH 6.5) with 2.4 mM CDNB, and 3.2 mM GSH; and bovine liver cytosolic fractions at a final concentration of 0.982 mg protein/mL were prepared and used as the enzyme source to measure GST activity. GSH-CDNB conjugate formation was followed in 250 µL total volume assay by using a multimode microplate reader at 340 nm for 4 min. Initial rates of enzymatic reactions were determined as nanomoles of the conjugation product of GSH and reported as nmole/min/mg protein.
Inhibition of GPx
GPx activity was measured by the method of Paglia and Valentine (Citation1967) with some modifications and miniaturized for microplate applications (Isgor et al., Citation2008). The activity of the enzyme was defined as the amount of enzyme that converts 1 µmol of NADPH per minute in 1 mL, and expressed as U/mg of total protein. The assay mixture consisted of plant extract solution at a final concentration in the range of 2–285 ng/mL, 0.4 U/mL glutathione reductase, 0.2 mM NADPH, 1.6 mM GSH, 0.07 mM H2O2, and 50 mM TrisċHCl with pH 8.0. The mixture was incubated at room temperature for 2 min. Then, the reaction was initiated by adding bovine liver cytosolic fractions used as the GPx enzyme source at a final concentration of 0.982 mg protein/mL, and the change in absorbance was recorded at 340 nm for 5 min by using a multimode microplate reader. Enzymatic reactions were determined as micromoles of the conversion of NADPH and reported as µmole/min/mg protein.
Inhibition of CAT
CAT inhibition was determined by monitoring a red quinoneimine dye remaining hydrogen peroxide (Aebi, Citation1984; Bai et al., Citation1999; Fossati et al., Citation1980). The assay was miniaturized for microplate application (Isgor et al., Citation2008) and contained plant extraction solutions with a final concentration in the range of 2–285 ng/mL, 50 mM phosphate buffer (pH 7.0), 20 U/mL purified bovine liver catalase, and 0.0961 mM H2O2. The reaction was stopped by NaN3 and the mixture was incubated at room temperature for 5 min, followed by the incubation with chromogen at room temperature for 40 min. Then, the absorbance was read at 520 nm, the enzyme activity was calculated with respect to hydrogen peroxide remained which was determined by a calibration curve constructed in the range of 9.61–307.6 μM hydrogen peroxide.
Data analysis
The data analysis was applied with the Graphpad Prism 6.0 program (GraphPad Software, San Diego, CA). The kinetic results were expressed as inhibition of enzyme activity with respect to control (inhibition as % of control). IC50 values were obtained from dose–response curves that were constructed.
Results
Each extract was prepared by dissolving 2 g of dry samples in 20 mL organic solvents (acetone, ethanol, and methanol). The extraction yields of O. acanthium, C. acanthoides, C. Arvense, and C. solstitialis were in between 1.89 and 5.42% ().
Table 1. The percent (%) yield of extraction.
Total phenolic contents of extracts were ranging from 8.035 to 90.305 mg GAE/L of plant extract. According to the results in , the highest values of total phenolic contents were found in the acetone extract of the C. acanthoides’s flower with 90.305 mg GAE/L of plant extract. Total flavonoid contents varied from 18.031 to 185.437 mg QE/L of plant extract (). The best flavonoid contents were achieved by acetone extract of C. acanthoides’s flower with 185.437 mg QE/L of plant extract.
Table 2. The total phenolics content of plant extracts (mg GAE/La).
Table 3. The total flavonoid content of plant extracts (mgQE/La).
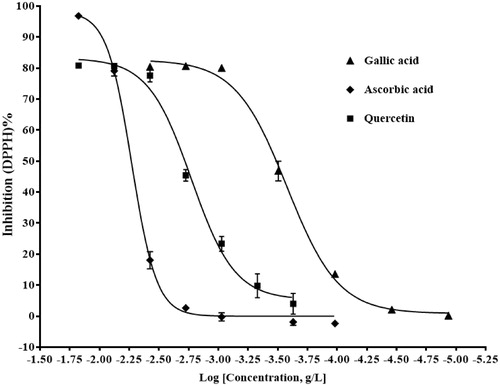
Free radical scavenging activity of extracts was determined by using the DPPH radical scavenging method and results are presented as DPPH% radical scavenging activity of different extracts (ng/mL) according to inhibition curves and IC50 values, the best free radical scavenging activity was obtained by methanolic leaves extract of C. arvense with 366 ng/mL IC50 value (). IC50 values for ascorbic acid, gallic acid, and quercetin solutions which were used as a positive control were 5.144, 0.264, and 1.685 μg/mL, respectively ().
Figure 1. Percent free radical scavenging activity of acetone, ethanol, and methanol extracts of O. acanthium flowers (ONA-C), methanol extract of C. arvense leaves (CIA-Y), and acetone extract of C. solstitialis flowers (CES-C) with IC50 values of 842, 1120, 723, 366, and 334 ng/mL, respectively.
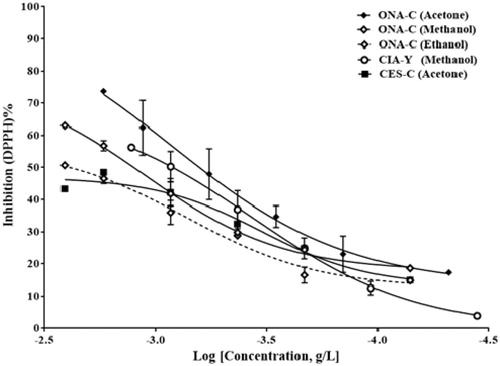
Figure 2. Percent free radical scavenging activity of ascorbic acid, gallic acid, and quercetin solutions with IC50 values of 5.144, 0.264, and 1.685 μg/mL, respectively.
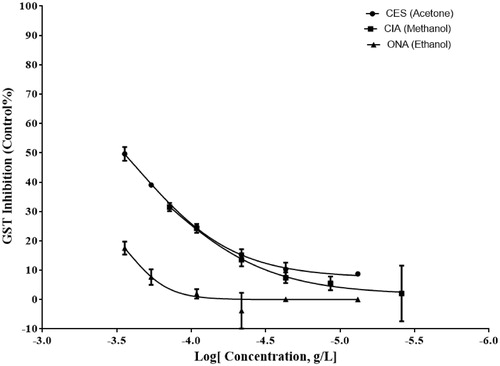
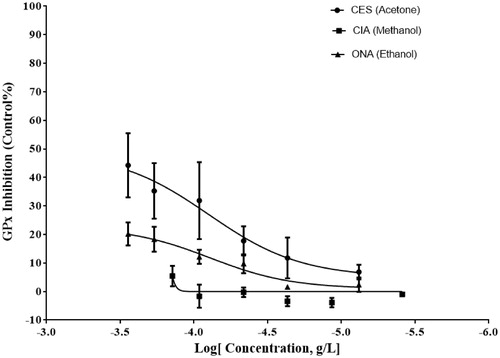
In order to calculate the percent inhibition of GST activity and IC50 values, the final concentration of plant extracts used in the assay was within the range of 2–285 ng/mL. The best inhibition was exhibited in the crude acetone extract of flowers of C. solstitialis with an IC50 value of 232 ng/mL (). The crude extracts were used within the final concentration of 2–285 ng/mL to calculate the percent inhibition of GPx activity and IC50 values. The acetone extract of C. solstitialis flowers showed the best GPx inhibition activity with IC50 values of 79 ng/mL (). In this study, none of the extracts evaluated showed any reasonable CAT inhibition.
Discussion
In this study, we evaluated the effects of the acetone, ethanol, and methanol extracts which were prepared from flowers and leaves of O. acanthium, C. acanthoides, C. Arvense, and C. solstitialis to determine the free radical scavenging potential and the effects on GST, GPx and catalase activities.
Cirsium arvense is a native plant of Europe and Asia. Phytochemical investigation shows that C. arvense is a good source of phytochemical compounds, such as polyphenols, tannins, flavonoids, and their derivatives (Nazaruk et al., Citation2008; Norton, Citation2000). Also, C. arvense juice of the leaves is used in some parts of the world for wound healing. Nazaruk (Citation2008) studied the antioxidant activity and total phenolic contents of extract from leaves of C. arvense and chloroform (CHCl3), diethyl ether (Et2O), ethyl acetate (EtOAc), and n-butanol (n-BuOH) fractions which were prepared from the methanolic extract. The results obtained from the experiments showed antioxidant activities of Et2O and EtOAc; n-BuOH fractions were more effective radical scavengers compared with MeOH extracts. Also, Nazaruk et al. (Citation2008) determined that the aqueous extract prepared from the leaves of C. arvense showed moderately significant antioxidant effect and total phenolic contents.
Some species of Onopordum are used in traditional medicine. The fruit of O. tauricum is sold as a spice and used to treat liver diseases (Ugur et al., Citation2011). The flowers of O. acanthium are used as diuretic and antipyretic and the roots are used as a diuretic and for stomachache (Baytop, Citation1999). Angelov et al. (Citation2012) reported that ethanolic extracts of O. acanthium flowers and leaves contained a large amount of phenolic contents and antioxidant capacity. Sharifi et al. (Citation2013) isolated a new activity compound [(E)-1-oxo-3,4-dihydro-1-h-isochromen-7-yl-3 -(3,4-dihydroxyphenyl) acrylate] from O. acanthium methanolic seed extracts. They demonstrated that this compound produced a high inhibition activity on the angiotensin-converting enzyme (ACE) and antioxidant activity.
Centaurea is a medicinal genus herb from Asteraceae family with 168 species available in Turkey. The aerial parts of Centaurea species are known for their antidiabetic, antidiarrhetic, antirhematic, anti-inflammatory, colagog, digestive stomachic, hypotensive, cytotoxic, and antibacterial effects (Arif et al., Citation2004; Kaij-A-Kamb et al., Citation1992). Centaurea species also contain secondary metabolites such as flavonoids, terpenoids, and sesquiterpene lactones (Gurbuz & Yesilada, Citation2007; Koca et al., Citation2009). Centaurea solstitialis which grows all over Turkey is used for stomach problems, common colds, herpes infections around lips, and abdominal pain (Ozcelik et al., Citation2009). Şen et al. (Citation2013) found that the methanolic extracts of capitulums and aerial parts of the C. solstitialis contained a small amount of phenolic contents but had good effects on the scavenging free radicals. Species of the genus Carduus are traditionally used as diuretic, cardiotonic, and antihemorroidal remedies in folk medicine (Zheleva-Dimitrova et al., Citation2011).
In this study, we found that flower extracts of C. acanthoides which were prepared with acetone contained significant amounts of phenolic and flavonoid contents. Also, the methanolic extracts of C. arvense leaves showed high potential of free radical scavenging. Flower extracts of C. solstitialis which were obtained from acetone exhibited a good inhibition effect on GST and GPx enzymes.
Conclusions
In this research, for the first time, we considered the biological potential of O. acanthium, C. acanthoides, C. arvense, and C. solstitialis on the antioxidant defense system such as GST, GPx, and CAT enzymes. We showed that the acetone extract of C. solstitialis flowers has good potential to inhibit GPx and GST enzymes. Also, we found that the methanolic extract of C. arvense leaves has high free radical scavenging potential and antioxidant activity.
Declaration of interest
The authors report no declarations of interest. This study was supported by the grant from The Coordination of Scientific Research Projects of Ankara University awarded to Professor Dr. Ozlem Yildirim (Grant no. 13L4240003).
References
- Aebi H. (1984). Catalase in vitro. Meth Enzymol 105:121–6
- Angelov G, Georgieva S, Petkova-Parlapanska K. (2012). Antioxidant activity of extracts from cotton thistle (Onopordum acanthium L.). Nat Math Sci 2:19–23
- Arif R, Küpeli E, Ergun F. (2004). The biological activity of Centaurea L. species. G Ü Fen Bilim Der 17:149–64
- Ata A, Van den Bosch SA, Harwanik DJ, Pidwinski GE. (2007). Glutathione-S-transferase- and acetylcholinesterase-inhibiting natural products from medicinally important plants. Pure Appl Chem 79:2269–76
- Bai JZ, Saafi EL, Zhang SP, Cooper GJS. (1999). Role of Ca2+ in apoptosis evoked by human amylin in pancreatic islet beta-cells. Biochem J 343:53–61
- Baytop T, ed. (1999). Therapy with Medicinal Plants in Turkey Past and Present. İstanbul: Nobel Tip Kitabevleri, 118–19
- Chang CC, Yang MH, Wen HM, Chern JC. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–82
- Coruh N, Sagdicoglu Celep AG, Ozgokce F, Iscan M. (2007). Antioxidant capacities of Gundelia tourneforii L. extracts and inhibition on glutathione-S-transferase activity. Food Chem 100:1249–53
- Ertürk Ö, Demirbağ Z. (2003). Scorzonare mollis Bieb (Compositae) bitkisinin antimikrobiyal aktivitesi. Çevre Koruma 12:27–31
- Fan MY, Chambers TC. (2001). Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Drug Resist Update 4:253–67
- Fossati P, Romon M, Sagnier MC. (1980). Nutritional investigations and dietetic note-book – An instrument to establish the diet of hyperlipidemic patients. Nouv Presse Med 9:2955–63
- Gurbuz I, Yesilada E. (2007). Evaluation of the anti-ulcerogenic effect of sesquiterpene lactones from Centaurea solstitialis L. ssp solstitialis by using various in vivo and biochemical techniques. J Ethnopharmacol 112:284–91
- Habig WH, Pabst MJ, Jakoby WB. (1974). Glutathione-S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–9
- Isgor BS, Isgor YG. (2012). Effect of alpha-1-adrenoceptor blocker on cytosolic enzyme targets for potential use in cancer chemotherapy. Int J Pharmacol 8:333–43
- Isgor YG, Iscan M, Ozturk HS, Durak I. (2008). Effects of water soluble garlic extract on human leukemia HL60 cell lines. Turk J Biochem 33:78–84
- Kaıj-A-Kamb M, Amoros M, Girre L. (1992). Chemical and biological activity of the genus Centaurea. Pharm Acta Helv 67:178–88
- Koca U, Peşin Süntar I, Keles H, et al. (2009). In vivo anti-inflammatory and wound healing activities of Centaurea iberica Trevex Spreng. J Ethnopharmacol 126:551–6
- Koehn FE, Carter GT. (2005). The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4:206–20
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75
- Nabekura T. (2010). Overcoming multidrug resistance in human cancer cells by natural compounds. Toxins 2:1207–24
- Nazaruk J. (2008). Antioxidant activity and total phenolic content in Cirsium five species from north-east region of Poland. Fitoterapie 79:194–6
- Nazaruk J, Czechowska SK, Markiewicz R, Borawska MH. (2008). Polyphenolic compounds and in vitro antimicrobial and antioxidant activity of aqueous extracts from leaves of some Cirsium species. Nat Prod Res 22:1583–8
- Norton SA. (2000). Botanical heritage of dermatology. In: Dermatologic Botany. CRC Press
- Ozcelik B, Gurbuz I, Karaoglu T, Yesilada E. (2009). Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp solstitialis. Microbiol Res 164:545–52
- Paglia DE, Valentine WN. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–69
- Potapova O, Haghighi A, Bost F, et al. (1997). The Jun kinase stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatin. J Biol Chem 272:14041–4
- Prasad KN, Cole WC, Kumar B, Prasad KC. (2002). Pros and cons of antioxidant use during radiation therapy. Cancer Treat Rev 28:79–91
- Şen A, Bitiş L, Birteksöz-Tan S, Bulut G. (2013). In vitro evaluation of antioxidant and antimicrobial activities of some Centaurea L. species. Marmara Pharmaceut J 17:42–5
- Sharifi N, Souri E, Ziai SA, et al. (2013). Isolation, identification and molecular docking studies of a new isolated compound, from Onopordon acanthium: A novel angiotensin converting enzyme (ACE) inhibitor. J Ethnopharmacol 148:934–9
- Sharma OP, Bhat TK. (2009). DPPH antioxidant assay revisited. Food Chem 113:1202–5
- Sheehan D, Meade G, Foley VM, Dowd CA. (2001). Structure, function and evolution of glutathione-S-transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360:1–16
- Shing B, Sahu PM, Sharma MK. (2002). Anti-inflammatory and antimicrobial activities of triterpenoids from strobilanthes callosus Ness. Phytomedicine 9:355–9
- Simone CB, Simone NL, Simone V, Simone CB. (2007). Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part 1. Alternat Ther Health Med 13:22–8
- Slinkard K, Singleton VL. (1977). Total phenol analyses: Automation and comparison with manual methods. J Enol Viticul 28:49–55
- Tan AC, Konczak I, Sze DMY, Ramzan I. (2011). Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr Cancer Int J 63:495–505
- Townsend DM, Tew KD. (2003). The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogenesis 22:7369–75
- Udenigwe CC, Ata A, Samarasekera R. (2007). Glutathione-S-transferase inhibiting chemical constituents of Caesalpinia bonduc. Chem Pharm Bull 55:442–5
- Ugur A, Sarac N, Duru ME. (2011). Chemical composition and antimicrobial activity of endemic Onopordum caricum. Middle East J Sci Res 8:594–8
- Zheleva-Dimitrova D, Zhelev I, Dimitrova-Dyulgerova I. (2011). Antioxidant activity of some Carduus species growing in Bulgaria. Free Rad Antiox 1:15–20