Abstract
Context: Eupatorium triplinerve Vahl (Asteraceae), popularly known as Japana, is widely used in folk medicine, due its analgesic, anticoagulant, antianorexic, antiparasitic, anthelmintic, sedative, antifungal, and antibacterial properties.
Objective: The present study evaluated the chemical composition and antimicrobial activity of E. triplinerve extracts from different parts of the plant and identified the extract with the highest antimicrobial potential.
Materials and methods: Extracts were obtained by maceration of all parts of plant, and subjected to bioassay-guided fractionation of methanol extract by partition column chromatography. The major chemical groups, saponins, reducing sugars, alkaloids, steroids, triterpenoids, phenols, tannins, flavonoids, and others were screened by standard techniques. The antimicrobial activity of the different extracts was performed by microdilution assay and the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values were reported.
Results: Phytochemical screening of hydroalcoholic extract from all parts of E. triplinerve identified mainly steroids, coumarins, alkaloids, saponins, tannins, depsides and absence of polysaccharides and flavonoids. The methanol extract of leaves presented the highest content of coumarins and lower MIC values of 62 and 75 µg/mL against Enterococcus faecalis and Staphylococcus aureus, respectively. In addition, its non-polar fractions showed antimicrobial activity with MIC ranging from 16 to 125 µg/mL against Gram-negative bacteria, mainly Escherichia coli.
Discussion and conclusion: Data showed that non-polar fractions of E. triplinerve methanolic extract has better antimicrobial activity and most likely depends on the presence of several compounds, such as depsidones, coumarins, saponins, and triterpenes on crude extract. The results can be exploited largely in research of new antibacterial agents.
Introduction
Amazonian biodiversity is relevant to research of new plant-based treatments that are based on medicinal plants used by indigenous communities either directly as folk remedies or on indigenous information systems (Baptista, Citation2007). Eupatorium triplinerve Vahl (Asteraceae), also referred as Ayapana triplinervis (Vahl) R.M. King & H. Rob. and Eupatorium triplinerve Vahl by other authors (www.tropics.org), and it is native to South America, particularly the Amazon region of Brazil (Trang et al., Citation1993). It has also been found in Hawaii, India, Vietnam, and the Mascarene Islands (Gauvin-Bialecki & Marodon, Citation2009). This plant grows up to 1 m high and is an ornamental erect perennial herb that is semi-woody at the base. The leaves (4.5–10.5 cm long and 0.8–1.7 cm wide) are aromatic, smooth, simple, opposite, sub-sessile, 3-nerved, acuminate, glabrous, and lanceolate. The stems are reddish brown. The many flowering heads are each 6–13 mm long and bear approximately 40 pink flowers (Gauvin-Bialecki & Marodon, Citation2009).
Eupatorium triplinerve is widely used in folk medicine and its analgesic, anticoagulant, antianorexic, antiparasitic, anthelmintic, sedative, antifungal, and antibacterial properties have been reported (Bose et al., Citation2007; Chaurasia & Kher, Citation1978; Garg & Nakhare, Citation1993; Gupta et al., Citation2002; Jelager et al., Citation1998; Kokate et al., Citation1971; Verpoorte & Dihal, Citation1987; Yadava & Saini, Citation1990). In addition, the plant extract is used as an antiseptic to treat ulcers, hemorrhages (Ghani, 1998), and also as sedative, anxiolytic, and antidepressive (Melo et al., Citation2013).
Although several studies have shown antibacterial and antifungal activities in various extracts from different plant parts of E. triplinerve, the data are still quite controversial, and little is known about the likely components associated with these activities (Gupta et al., Citation2002; Sharma & Singh, Citation1979; Verpoorte & Dihal, Citation1987). The oil of the plant has been found to possess antimicrobial activity (Yadava & Saini, Citation1990). Among different plant derivatives, secondary metabolites have been proven to be the most important group of compounds, with a wide range of antibacterial and antifungal activities (Ahmad et al., Citation2002; Rahman et al., 1999). Thus, this study aimed to evaluate the chemical composition and antimicrobial activity of E. triplinerve extracts from different plant parts and to identify the most active fraction.
Materials and methods
Collection and identification of E. triplinerve
Eupatorium triplinerve was collected from São Raimundo, a village in Acará city (Pará state, Amazon region) in March, 2010. The region is situated at latitude 01132.684′ and longitude 048123.984′ (geographic coordinates obtained using global positioning system [GPS] equipment). The botanical identification was performed by Dr. Mário Jardim, a specialist from Emilio Goeldi Museum (Pará, Brazil), where a sample was deposited as a voucher specimen under number MG123913.
Preparation of hydroalcoholic extract of different parts of E. triplinerve
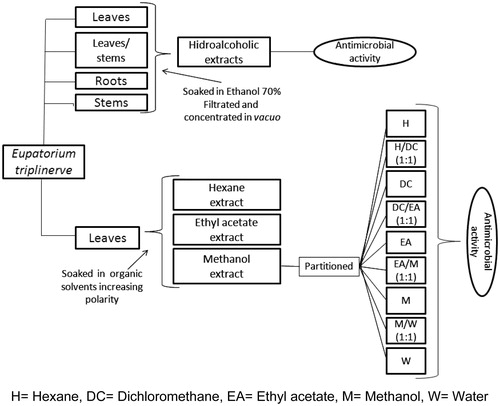
The whole plant was washed with water and 10% ethanol solution and separated into leaves, stems, leaves/stems, and roots. The plant parts were dried at room temperature for 2 d, dried in an oven with forced air circulation at an average temperature of 40 °C for 6 d and then crushed in a knife mill to obtain the drug spray. After grinding, the dust was macerated for 5 d in 70% ethanol to obtain the hydroalcoholic extract of E. triplinerve. The mash was dried in a rotator evaporator (Laborata 4000 efficient; Heidolph Instruments GmbH & Co. KG, Schwabach, Germany) at 45 °C, 1 atm pressure and 120 rpm. For total removal of the solvent, the mash was kept in the oven and water bath at 40 °C. Leaves and stem extracts (∼231 g, corresponding to 820 g of the dried plant yield of the extract was 28.16%) were obtained and used in animal treatments. All hydroalcoholic extracts were analyzed to identify the plant parts with antibacterial properties.
Different extracts of the leaf and bioguided fractionation of the methanol extract of E. triplinerve leaves
Eupatorium triplinerve leaves (100 g) were extracted with 500 mL of increasing polarity solvents, including hexane, ethyl acetate, and methanol, at room temperature for a period of 7 d each. The solvent was evaporated under reduced pressure. The methanol extracts were subjected to partition column chromatography using silica gel 60 (Macherey-Nagel, Sigma-Aldrich, St. Louis, MO); were partitioned with hexane, dichloromethane, ethyl acetate, and methanol; and were concentrated as described. The extracts were investigated for their phytochemical characteristics; then, the extracts and fractions obtained from the methanol extract were evaluated for antibacterial activity ().
Phytochemical screening
The crude extracts were analyzed for the presence of major chemical groups (i.e., saponins, reducing sugars, alkaloids, steroids and triterpenoids, phenols, tannins, flavonoids, polysaccharides, coumarin, depsides, and depsidones) by standard phytochemical techniques (Barbosa et al., Citation2001).
In vitro antimicrobial activity
Microorganisms
Antibacterial activity was evaluated on the following standard strains: (i) Gram-positive bacteria: Staphylococcus aureus (ATCC 6538) and Enterococcus faecalis (ATCC 29212) and (ii) Gram-negative bacteria: Pseudomonas aeruginosa (ATCC 25853), Escherichia coli (ATCC 8739), Klebsiella pneumonie (ATCC 4352), and Proteus mirabilis (ATCC 15290). All strains were obtained from the INCQS/FIOCRUZ (National Institute for Health Quality Control, Rio de Janeiro, Brazil). In addition, one clinical isolate of E. coli was used as a test organism. The clinic isolate was obtained of a culture from patient samples collected in the Microbiology Laboratory, Pará, Brazil.
The microorganisms used in the study were maintained in the Laboratory of Microbiology in the Pharmacy Faculty, Federal University of Pará/UFPA. The standard strains were kept in nutrient agar at room temperature. For the tests, all strains were grown in Petri dishes containing media specific to each bacterium. For S. aureus, mannitol salt agar medium was used; for E. faecalis, nutrient agar was used; for P. aeruginosa, cetrimide agar was used; and for E. coli, K. pneumonia, and P. mirabilis, MacConkey agar was used. All plates were incubated at 37 °C for 24 h to induce exponential growth.
Determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
To prepare the bacterial inoculum, strains were grown in the exponential phase in Mueller–Hinton broth (Merck, Darmstadt, Germany) at 37 °C for 18 h. The turbidity was adjusted to 0.5 on the McFarland scale (approximately of 2 × 108 CFU/mL) by diluting fresh cultures and then diluted to 1 × 103 CFU/mL as described by the Clinical and Laboratory Standards Institute (CLSI, 2012).
MIC and MBC assays were performed using the broth microdilution method in the Mueller–Hinton broth as described by the Clinical and Laboratory Standards Institute (CLSI, 2012). The MIC is defined as the lowest concentration of an extract or fraction that inhibits the growth of microorganism. The E. triplinerve extract or fraction was dissolved in a solution of 50% dimethylsulfoxide (DMSO), and serial two-fold dilutions were made until a final concentration of 16 μg/mL in 1 mL sterile test tubes containing the Mueller–Hinton broth (MHA).
For the microdilution test, the inoculums (100 μL) containing 5 × 108 CFU/mL were added to each well, and 100 μL of the serial dilutions was transferred into consecutive wells. After 24 h of incubation, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenytetrazolium bromide (MTT), a tetrazolium salt that is reduced by metabolically active cells to a colored water-soluble formazan derivative, was added to the wells to allow visual identification of metabolic activity (Mosmann, Citation1983). The final concentration of MTT after inoculation was 0.005% (v/v). After incubation, growth was indicated by the development of a blue color. The MIC was read as the lowest concentration of the extracts at which a change in color occurred. To determine the MBC, 10 μL of broth was taken from each well and incubated in Mueller–Hinton agar at 37 °C for 24 h. The MBC was defined as the lowest concentration of extracts or fractions that resulted in either no growth or fewer than three colonies (99.9% killing) as described by Quadros et al. (Citation2011). Each test was performed in three replicates and repeated twice. The negative control consisted of 100 μL of the bacterial inoculum in 100 μL of DMSO. Chloramphenicol (50 µg/mL) and gentamicin (10 µg/mL) were used as positive controls for Gram-positive and Gram-negative bacteria, respectively.
Results
There were no qualitative differences in the chemical composition of the hydroalcoholic extracts from different parts of E. triplinerve. shows the presence of saponin, reducing sugars, alkaloids, phenols, tannins, steroids/triterpenoids, depsides/depsidones, coumarin derivatives, and sesquiterpene lactones and the absence of polysaccharides and flavonoids in all the hydroalcoholic plant extracts.
Table 1. Phytochemical screening of the hydroalcoholic extracts of leaves and stalks, stems, and roots of E. triplinerve.
shows that all extracts from the different parts of E. triplinerve were effective in inhibiting the growth of Gram-negative bacteria, particularly the leaves extract. The data showed that the MIC values of the leaves plus stems, stems, or roots extracts were 187 μg/mL against P. aeruginosa and E. coli, while the MIC value of the leaves extract was 94 μg/mL against both bacteria. The MBC value for all extracts was 375 μg/mL. Among Gram-positive bacteria, the E. faecalis strain was sensitive to the extract of leaves plus stems, with an MIC value of 37 μg/mL and an MBC value of 75 μg/mL, whereas S. aureus was more sensitive to the leaves extract, with an MIC value of 75 μg/mL.
Table 2. The minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the hydroalcoholic extracts of leaves and stalks, stems, leaves, and root E. triplinerve against the different microorganisms.
The phytochemical screening of different E. triplinerve leaf extracts of increasing polarity showed the presence of alkaloids in all extracts; furthermore, saponins, reducing sugars, and high concentration of coumarins were detected only in the methanol extract. Low concentrations of depsides, depsidones, and coumarins and high concentrations of triterpenoids and steroids were found in the ethyl acetate extract, whereas in the hexane extract, only depsides, depsidones, and alkaloids were found ().
Table 3. Phytochemical screening of different extracts of increasing polarity of leaves of E. triplinerve.
The results demonstrate that the methanol extract of E. triplinerve presented the best antimicrobial activity with MIC values of 62 and 75 µg/mL against Gram-positive bacteria E. faecalis and S. aureus, respectively. In addition, the MIC values against Gram-negative bacteria were at least 125 µg/mL. Other extracts showed antimicrobial activity with MIC values at least 125 µg/mL against Gram-negative bacteria and 259 µg/mL against Gram-positive bacteria ().
Table 4. The minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of different extracts of increasing polarity of leaves of E. triplinerve against different bacteria.
The bioactivity-guided fractionation of the methanol extract of E. triplinerve showed that the active antimicrobial components were mainly found in the hexane, dichloromethane, and dichloromethane/ethyl acetate fractions. The hexane and dichloromethane/ethyl acetate fractions exhibited the best MIC and CBM values against E. coli ATCC at 16 and 31 µg/mL, respectively. The dichloromethane fraction was more effective against the E. coli clinic isolate and P. aeruginosa, with MIC values of 31 and 62 µg/mL, respectively, and the CBM value was at least 125 µg/mL ().
Table 5. The minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) in μg/mL of fractions from methanolic extract of the leaves of E. triplinerve against the different microorganisms.
Discussion
The purpose of this study was to evaluate the chemical composition and antimicrobial activity of different extracts of E. triplinerve, a medicinal plant used by indigenous communities from Amazon region as hemostatic, cardiotonic, diaphoretic, emetic, antiseptic, antimicrobial, hepatoprotective, antidepressive, and anxiolytic (Melo et al., Citation2013). Even though the chemical composition of E. triplinerve has already been studied by other authors, here we also investigated the presence of the major secondary metabolites, because climatic factors such as seasonality, rainfall (affecting the availability of nutrients and water), and changes in the circadian cycle may influence the biochemical and physiological processes of plants and thus changing its composition (Czelusniak et al., Citation2012; Gobbo Neto et al., Citation2007).
In this study, the phytochemical screening of the leaves extract of E. triplinerve showed the presence of steroids, coumarins, alkaloids, and saponins. The methanol extract of the leaves presented better antimicrobial activity compared with other extracts with increasing polarity, and the non-polar leaf fractions showed good antimicrobial activity against Gram-negative bacteria.
The initial phytochemical screening of the hydroalcoholic extract of E. triplinerve showed the presence of saponins, reducing sugars, alkaloids, coumarins, phenols, steroids, and depsides. However, there was no qualitative difference in the metabolites present in different plant parts (). The presence of these metabolites in the Eupatorium genus has been reported previously (Bose & Roy, Citation1936; Inya-agha et al., Citation1987; Jukic et al., Citation2007; Maas & Hensel, Citation2008; Maas et al., Citation2009; Zhang et al., Citation2008).
The hydroalcoholic leaf extract showed better antimicrobial activity with lower MIC values (94 μg/mL) against Gram-negative bacteria. Therefore, the leaf extract was selected for fractionation into daughter fractions of different polarities. Gupta et al. (Citation2002) reported the broad-spectrum antimicrobial activity of the petroleum ether and methanol extracts from leaves of E. triplinerve against Gram-positive and Gram-negative bacteria at concentrations of 250–1000 µg/mL. A similar antibacterial activity was also reported by Rahman and Junaid (Citation2008), who showed that the crude leaf extract of E. triplinerve has antibacterial and antifungal properties and can be used as a novel antimicrobial agent.
Taking into account the different leave extract fractions, the methanol extract exhibited the highest antimicrobial activity against Gram-positive and Gram-negative bacteria and contained traces of coumarins ( and , respectively). In addition, coumarins have shown higher activity against Gram-negative bacteria than Gram-positive bacteria when isolated from other plants (Basile et al., Citation1997, Citation1999, Citation2009; Souza et al., Citation2005). The polarity of the solvent can influence the interaction between bacterial cell wall components and polar constituents present in methanol extracts, such as coumarin derivatives, saponins, reducing sugars, sesquiterpene lactones, and depsides as revealed by this phytochemical screening (). The bioactivity-guided assay led to the identification of the dichloromethane fraction of the methanolic extract as a bioactive fraction with good antibacterial activity. More studies are necessary to identify the component(s) responsible for this activity to facilitate its possible use as a new phytomedicine.
According to previous phytochemical studies, the most characteristic secondary metabolites of this plant are coumarins. Ayapanin (or herniarin), ayapin, daphnetin, daphnetin dimethyl ether, daphnetin-7-methyl ether, hydrangetin, and umbelliferone have been identified in the plant (Gauvin-Bialecki & Marodon, Citation2009). This plant has an odor that is slightly coumarin like, and the taste is mildly astringent and aromatic. The leaves are recommended to treat indigestion, pectoral complaints, and cholera, and they are employed externally and internally during the treatment. Several studies of E. triplinerve leaves have identified the presence of 7-ethoxycoumarin (ayapanin), 6,7-dimethoxycoumarin (ayapin), carotene, vitamin C, and stigmasterol (Bose & Roy, Citation1936; Chaturvedi & Mulchandani, Citation1989; Natarajan & Natarajan, Citation1979; Trang, Citation1992; Trang et al., Citation1993). Other important compounds include thymohydroquinone and terpenoids, which are also present in high percentages (Jukic et al., Citation2007). Coumarins are components involved in defense response of plants to abiotic and biotic stresses, providing antimicrobial or anti-inflammatory activity, acting as inhibitors of numerous enzyme systems (Murray et al., Citation1982). Some studies reported that coumarins are responsible for many biological activities such as antithrombotic, anti-inflammatory, vasodilator, and antimicrobial activities (Bose & Roy, Citation1936; Chaturvedi & Mulchandani, Citation1989; Murray et al., Citation1982; Späth et al., Citation1937; Trang, Citation1992; Trang et al., Citation1993). Recently, our group showed that leaf and stem extracts of Ayapana triplinervis exert mild sedative, anxiolytic, and antidepressive behavioral effects in the central nervous system (CNS) of rat and antinociceptive and antioxidant activities in animal models (Melo et al., Citation2013).
Kayser and Kolodziej (Citation1999) suggested that the fairly high antibacterial activity of coumarin is due to both its lipophilic character and planar molecular structure, which contribute to its penetration through the bacterial cell membrane or cell walls. These authors also suggested that the antibacterial activity of oxygenated coumarins depends on the position of polar (OH) and less polar (OMe, Me) functions at the aromatic nucleus of the coumarin structure. In addition, Tegos et al. (Citation2002) reported that scopoletin, a coumarin, displays better antibacterial activity against Gram-positive bacteria than Gram-negative bacteria, perhaps because of an efficient efflux pumps on Gram-negative bacteria. Efflux pumps extrude the drug from the cell before they attain an adequate concentration at the site of action (Tenover, Citation2006).
Another major component of the majority of extracts is the saponins, which are widely distributed in the plant kingdom and have several biological properties such as antimicrobial action (Avato et al., Citation2006; Oleszek, Citation1996). Studies have shown that leaf extracts of Eupatorium odoratum suppress the growth of Propionibacterium acne (Chomnaweng et al., Citation2005). Triterpenes and steroids were found in the phytochemical screening, and these substances are found in essential oils, also called the fifth essence and are responsible for the fragrance of plants (Cavalcanti et al., Citation2004). Certain representatives of this group, such as capsaicin, have an antimicrobial effect through disruption of the plasma membrane (Cowan, Citation1999). Coumarins, saponins, and triterpenes were found in the methanol extract and might be involved in the antibacterial activity of this fraction due to a synergistic interaction between them.
Depsides have been shown to reduce bacterial and fungal growth (Manojlovic et al., Citation2012; Schmeda-Hirschmann et al., Citation2008; Vartia, Citation1973) and have other properties such as antioxidant (Hidalgo et al., Citation1994), antiviral (Neamati et al., Citation1997), antitumor (Yamamoto et al., Citation1995), analgesic, and antipyretic (Okuyama et al., Citation1995). These compounds were only found in the hexane and ethyl acetate extracts.
Some criteria should be considered to avoid incorrect analysis of the antimicrobial data and false positive results such as the use of high concentrations of crude extracts or isolated compounds. Concentrations of 100 µg/mL and 25 mM are considered optimal but the type of microorganism being tested must be taken into consideration (Cos et al., Citation2006). The antimicrobial activity of E. triplinerve is most likely due to the presence of depsidones, coumarins, saponins, and triterpenes, which are important antimicrobial compounds present in the methanol extracts, as reported in this study.
Our previous and current studies indicated that E. triplinerve contains steroids, coumarins, alkaloids, and saponins. The leaf methanol extract showed the best antimicrobial activity, and its non-polar fractions showed good antimicrobial activity against Gram-negative bacteria. Studies to isolate and identify these compounds should be performed.
Acknowledgements
We are grateful to Dr. Mario Jardim and the Emilio Goeldi Museum for identifying the plant and production of voucher specimen and to FAPESPA and the Federal University of Pará for financial support.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. The authors thank the Federal University of Pará for financial support.
References
- Ahmad FI, Hafiz A, Asi AA, et al. (2002). Mango varietal susceptibility to malformation and its control. Asian J Pl Sci 1:158–9
- Avato P, Bucci R, Tava A, et al. (2006). Antimicrobial activity of saponins from Medicago sp.: Structure-activity relationship. Phytother Res 20:454–7
- Baptista ER. (2007). Knowledge and healing practices in rural Amazonian communities: Therapeutic plant resources [Thesis]. Federal University of Pará
- Barbosa WLR, Quignard E, Tavares ICC, et al. (2001). Manual para análise fitoquímica e cromatográfica de extratos vegetais. Rev Científica da UFPA 1:4
- Basile A, Giordano S, Lopez-Saez JÁ, Castaldo Cobianchi R. (1999). Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry 52:1479–82
- Basile A, Sorbo S, Spadaro V, et al. (2009). Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules 14:939–52
- Basile A, Vuotto ML, Violante U, et al. (1997). Antibacterial activity in Actinidia chinensis, Feijoa sellowiana and Aberia caffra. Int J Antimicrob Agents 8:199–203
- Bose P, Gupta M, Mazumder UK, et al. (2007). Hepatoprotective and antioxidant effects of Eupatorium ayapana against carbon tetrachloride induced hepatotoxicity in rats. Int J Pharm Technol 6:127–33
- Bose PK, Roy AC. (1936). The constitution of ayapanin. Indian Chem Soc 13:586–7
- Cavalcanti ES, Morais SM, Lima MA, Santana EW. (2004). Larvicidal activity of essential oils from brazilian plants against Aedes aegypti L. Mem Inst Oswaldo Cruz 99:541–4
- Chaturvedi R, Mulchandani NB. (1989). Coumarins from Eupatorium ayapana. J Ind Chem Soc 66:286–7
- Chaurasia SC, Kher A. (1978). Activity of essential oils of three medicinal plants against various pathogenic and nonpathogenic fungi. Indian J Hosp Pharm 15:139–41
- CLSI. (2012). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard–Ninth Edition. Clinical and Laboratory Standards Institute (CLSI) document M07-A9 [ISBN 1-56238-783-9] USA
- Chomnaweng MT, Suvimol S, Venna SN, Gritsanapan W. (2005). Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J Ethnopharmacol 101:330–3
- Cos P, Vlietinck AJ, Berghe DV, Maes L. (2006). Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol 106:290–302
- Cowan MM. (1999). Plants products as antimicrobial agents. Clin Microbiol Rev 12:564–82
- Czelusniak KE, Brocco A, Pereira DF, Freitas GBL. (2012). Farmacobotânica, fitoquímica e farmacologia do Guaco: Revisão considerando Mikania glomerata Sprengel e Mikania laevigata Schulyz Bip. ex Baker. Rev Bras Plantas Med 14:400–9
- Garg SC, Nakhare S. (1993). Studies on the essential oil from the flowers of Eupatorium triplinerve. Índian Perfumer 37:318–23
- Gauvin-Bialecki A, Marodon C. (2009). Essential oil of Ayapana triplinervis from Reunion Island: A good natural source of thymohydroquinone dimethyl ether. Biochem Syst Ecol 36:853–8
- Ghani A. (1998) Medicinal plants of Bangladesh: Chemical constituents and uses. Asiatic Society of Bangladesh
- Gobbo-Neto L, Lopes NP. (2007). Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim Nova 30:374–81
- Gupta M, Mazumder UK, Chaudhuri I, et al. (2002). Antimicrobial activity of Eupatorium ayapana. Fitoterapia 73:168–70
- Hidalgo ME, Fernandez E, Quilhot W, Lissi E. (1994). Antioxidant activity of depsides and depsidones. Phytochemistry 37:1585–7
- Inya-agha SI, Oguntimein BO, Sofowora A, Benjamin TV. (1987). Phytochemical and antibacterial studies on the essential oil of Eupatorium odoratum. Pharm Biol 25:49–52
- Jelager L, Gurib-Fakim A, Andersen A. (1998). Antibacterial and antifungal activity of medicinal plants of Mauritius. Pharm Biol 36:153–61
- Jukic M, Politeo O, Maksimovic M, et al. (2007). In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother Res 21:259–61
- Kayser O, Kolodziej H. (1999). Antibacterial activity of simple coumarins: Structural requirements for biological activity. Z Naturforsch 54:169–74
- Kokate CK, Rao RE, Varma KC. (1971). Pharmacological studies on the essential oil of Eupatorium triplinerve Vahl I. The effects on the central nervous system and antimicrobial activity. Flavour Industry 2:177–80
- Maas M, Hensel A. (2008). Eupatorium perfoliatum L. – Alte Arzneipflanzeneuentdeckt. Z Phytother 5:249–54
- Maas M, Petereit F, Hensel A. (2009). Caffeic acid derivatives from Eupatorium perfoliatum L. Molecules 14:36–45
- Manojlovic NT, Vasiljevic PJ, Maskovic PZ, et al. (2012). Chemical composition, antioxidant, and antimicrobial activities of lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid-Based Complement Alternat Med 2012:1–8
- Melo AS, Monteiro MC, Silva JB, et al. (2013). Antinociceptive, neurobehavioral and antioxidant effects of Eupatorium triplinerve Vahl on rats. J Ethnopharmacol 147:293–301
- Mosmann T. (1983). Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J Immunol Methods 65:55–63
- Murray R, Mendez DH, Brown SA. (1982). The Natural Coumarins: Occurrence, Chemistry and Biochemistry. New York: Wiley & Sons, 702 p
- Natarajan RK, Natarajan M. (1979). Phytochemical investigation of Eupatorium ayapana. J Res Indian Med Yoga Homeopathy 14:155–6
- Neamati N, Hong H, Mazumder A, et al. (1997). Depsides and depsidones as inhibitors of HIV-1 integrase: Discovery of novel inhibitors through 3D database searching. J Med Chem 40:942–51
- Okuyama E, Umeyama K, Yamazaki M, et al. (1995). Usnic acid and diffractic acid ass analgesic and antipyretic components of Usnea diffracta. Planta Med 61:113–5
- Oleszek W. (1996). Alfalfa saponins: Structure, biological activity and chemotaxonomy. Adv Exp Med Biol 405:155–70
- Quadros AU, Bini D, Pereira PAT, et al. (2011). Antifungal activity of some cyclooxygenase inhibitors on Candida albicans: PGE2-dependent mechanism. Folia Microbiol (Praha) 56:349–52
- Rahman MDS, Junaid M. (2008). Antimicrobial activity of leaf extracts of Eupatorium triplinerve Vehl. Against some human pathogenic bacteria and phytopathogenic fungi. Bangl J Bot 37:89–92
- Rahman MS, Anwar MN, Chowdhury AZMS. (1999). Antibacterial activity of secondary metabolites from Holarrhena antidysenterica stem bark. Bangl J Microbiol 16:101–5
- Schmeda-Hirschmann G, Tapia A, Lima B, et al. (2008). A new antifungal and antiprotozoal depside from the Andean lichen Protousnea poeppigii. Phytother Res 22:349–55
- Sharma SK, Singh VP. (1979). The antifungal activity of some essential oils. Indian Drugs Pharm Ind 14:3–6
- Souza SM, Delle Monache F, Smânia Jr A. (2005). Antibacterial activity of coumarins. Z Naturforsch C 60:693–700
- Späth E, Bose PK, Schläger J. (1937). Konstitution und synthese von Ayapin (XXVI. Mitteil.übernaturliche Cumarine). Ber Dtsch Chem Ges A/B 70:702–4
- Tegos G, Stermitz FR, Lomovskayd O, Lewis K. (2002). Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob Agents Chemother 46:3133–41
- Tenover FC. (2006). Mechanisms of antimicrobial resistance in bacteria. Am J Med 119:S3–10
- Trang NTD. (1992). The 13 C-NMR spectroscopy of ayapin isolated from Eupatorium ayapana Vent. from Vietnam. Tap Chi Hó a Hoc J Chem 30:62–3
- Trang NTD, Wanner MJ, Phuong LVN, et al. (1993). Thymoquinone from Eupatorium ayapana. Planta Med 59:99–100
- Vartia KO. (1973). Antibiotics in lichens. In: Ahmadjian V, Hale ME, eds. The Lichens. New York: Academic Press, 547–61
- Verpoorte R, Dihal PP. (1987). Medicinal plants of Surinam IV. Antimicrobial activity of some medicinal plants. J Ethnopharmacol 21:315–8
- Yadava RN, Saini VK. (1990). In vitro antimicrobial efficacy of the essential oil of Eupatorium triplinerve leaves. Índian Perfumer 34:61–3
- Yamamoto Y, Miura Y, Kinoshita Y, et al. (1995). Screening of tissue culture and thalli of lichens and some of their active constituents for inhibition of tumor promoter-induced Epstein-Barr virus activation. Chem Pharm Bull 43:1388–90
- Zhang ML, Wu M, Zhang JJ, et al. (2008). Chemical constituents of plants from the genus Eupatorium. Chem Biodivers 5:40–55