Abstract
Context: Salvia lavandulifolia has been employed in folk medicine for the treatment of memory and dementia problems. This specie contains numerous bioactive terpenes which may contribute to its effectiveness.
Objective: To analyze the composition of essential oil of S. lavandulifolia and to investigate the potential in vitro cytoprotective and antioxidant activities of its major compounds, α-pinene and 1,8-cineole, against H2O2-induced oxidative stress in the U373-MG cell line.
Materials and methods: Chemical composition was analyzed by gas chromatography; antioxidant capacity was measured using the ORAC assay, and cytoprotective activity was evaluated using the MTT assay (for cell viability) (range of concentrations: 10–400 μM), DCFH-DA assay (for intracellular ROS generation), thiobarbituric acid reactive substances (TBARS) method (for lipid peroxidation), and spectrofometric techniques and Western blot (for enzymatic activity and protein expression, respectively) at 10 and 25 µM.
Results: α-Pinene (18.39%) and 1,8-cineole (19.57%) were identified as major compounds in S. lavandulifolia essential oil. Pretreatments with these monoterpenes protected U373-MG cells against H2O2-induced oxidative injury by attenuating the loss of cell viability (IC50 : 79.70 µM to α-pinene and 66.23 µM to 1,8-cineole) and cell morphology, inhibiting ROS production (the most active compound was 1,8-cineole by decreasing the ROS production over 30–45% at 10 and 25 μM) and lipid peroxidation and increasing the endogenous antioxidant status (glutathione levels and CAT, SOD, GR, GPx, and HO-1 activity and protein expression).
Conclusions: These findings demonstrate for the first time the effects of the monoterpenes 1,8-cineole and α-pinene identified in S. lavandulifolia essential oil as regulators of cellular redox balance in astrocytes.
Introduction
An excessive production of ROS (i.e. hydroxyl radical, superoxide anion, and hydrogen peroxide) or a failure in the endogenous antioxidant defense system activity can lead to a perturbation of cellular redox balance, and ultimately resulting in an oxidative stress situation. As a consequence of oxidative stress, ROS oxidize indiscriminately biological molecules, inducing lipid peroxidation, mitochondrial dysfunction, protein carbonilation, and strand breaks and base damage of DNA (Halliwel, Citation1992). Strong evidence supports that oxidative stress is implicated in the early phase of several forms of dementia including Alzheimer's disease (Mao, Citation2013).
Over the last recent years, natural antioxidants have been considered as a promising approach to face the harmful effects of ROS, and consequently prevent further oxidative injury or attenuate the progression of dementia associated with oxidative stress (Jaydeokar et al., 2012). Essential oils contain different organic compounds, mainly terpene-type, some of which have been reported to exhibit potential antioxidant properties (González-Burgos & Gómez-Serranillos, Citation2012).
The genus Salvia (Lamiaceae) contains high quantities of essential oil which have been traditionally employed for its therapeutic properties (Sáez, Citation2010). The essential oil of S. lavandulifolia Vahl. (Spanish sage) is a popular plant remedy widely used to improve memory. Its useful and therapeutic effect for cognitive disorders associated with ageing is attributed to its anti-cholinesterase, estrogenic, and anti-inflammatory properties (Perry et al., Citation2003). Moreover, S. lavandulifolia essential oil has been previously reported as an important source of bioactive principles with potential natural antioxidant activity to prevent oxidative stress accompanying degenerative diseases (Tildesley et al., Citation2003). Monoterpenes contained in essential oil are the mainly responsible for the described activities (Chang et al., Citation2007; Perry et al., Citation2001; Savelev et al., Citation2004). Despite available research concerning the chemical characterization and activities of S. lavandulifolia essential oil (Herraiz-Peñalver et al., Citation2010), little is known about the role of its major monoterpenes as potential protective agents against oxidative damage at the central nervous system (CNS) level (Mitic-Culafic et al., 2009).
In view of the importance of therapy research against oxidative stress induced-dementia and based on the traditional use of S. lavandulifolia essential oil, the aim of this work was to evaluate the protective effect of the major monoterpenes present in S. lavandulifolia essential oil on astrocytes. The antioxidant and cytoprotective role of α-Pinene and 1,8-cineole against H2O2-induced oxidative stress in a human astrocytoma U373-MG cell line has been analyzed to examine their contribution as regulators of cellular redox balance. The essential oil chemical composition of S. lavandulifolia and the effect of the major monoterpenes on cell viability and cell morphology, endogenous antioxidant enzymes protein expression, and lipid peroxidation have been investigated.
Materials and methods
Plant material
The aerial parts of Salvia lavandulifolia were collected from a crop of Solsona, in the province of Lerida, Spain, in 2008. The plant materials were identified botanically by Dr. Mª Ángeles Cases Capdevila from the National Institute of Agricultural and Food Research and Technology (INIA, Spain).
Chemicals
α-Pinene was procured from Sigma-Aldrich (St Louis, MO) and 1,8-cineole and camphor were purchased from International Flavours & Fragances (Barcelona, Spain) (The chemical structures of these monoterpenes are presented in ). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate buffered saline (PBS), and trypsin–EDTA were obtained from Invitrogen (Carlsbarg, CA). Dimethylsulfoxide (DMSO) was purchased from Panreac (Barcelona, Spain). 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH), 2′,7′-dichlorofluorescein diacetate, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), hydrogen peroxide (H2O2), sodium dodecyl sulfate (SDS), Triton X-100, Trolox, and the antibodies CAT, SOD, and β-actine for the Western blot assay were purchased from Sigma-Aldrich (St Louis, MO). The antibodies GPx and GR were supplied from Abcam (Cambridge, UK) and HO-1 and Nrf2 were from Santa Cruz Biotechnology (Santa Cruz, CA).
Extraction of essential oil
The essential oil was isolated from the aerial parts of S. lavandulifolia by hydrodistillation (2 h) using a Clevenger-type apparatus, according to the procedure described in The Real Farmacopea Española (2002). The essential oil was dried over anhydrous sodium sulfate and stored in dark glass vials in a refrigerator.
Gas chromatography (GC) analysis
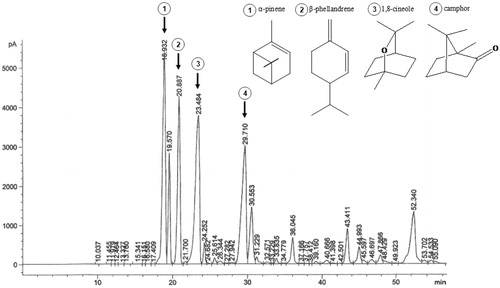
The qualitative and quantitative composition of the volatile constituents of S. lavandulifolia was carried out by GC on a Hewlett Packard 6890 chromatograph (Agilent Technologies, Santa Clara, CA), equipped with a flame ionization detector (FID) and a 5% phenylmethyl silicone capillary column (30 m × 0.25 mm). The column oven temperature was programmed from 70 °C to 240 °C at 3 °C/min. Injection of all samples of 2 µL was performed at 25 °C. The volatile constituents were identified on the basis of their retention index using authentic standards.
Peroxyl radical scavenging ability
Oxygen radical absorbance capacity (ORAC) was measured following the method described by Davalos et al (Citation2004). The monoterpenes α-pinene and 1,8-cineole were dissolved in methanol (1:1 v/v) and subsequently different dilutions were prepared in phosphate saline buffer (75 mM, pH 7.4). Trolox, a water-soluble vitamin E analog, was employed as a control standard; AAPH as a peroxyl radical generator, and fluorescein as the fluorescent probe. Fluorescence was monitored every minute for 104 cycles at an excitation wavelength of 485 nm and an emission wavelength of 520 nm using a FLUOstar OPTIMA (BMG Labtech, Durham, NC) fluorometer. The temperature was set at 37 ºC. The quantification of the antioxidant activity was determined using the area under the curve (AUC) and comparing the results with AUC of the Trolox. The results were expressed as μmol of Trolox Equivalents (TE)/mg of monoterpene.
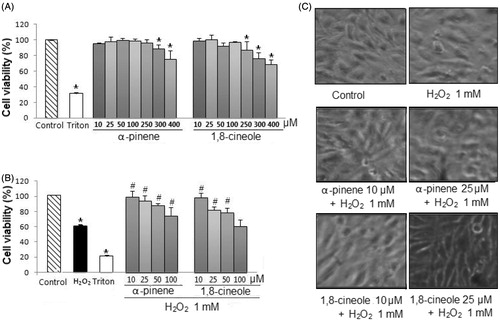
Figure 2. (A) Effect of monoterpenes on cell viability. U373-MG cells were treated with α-pinene and 1,8-cineole (a range of concentrations from 10 to 400 μM) for 24 h. Cell viability was measured using MTT assay. Experiments were performed in triplicate and results are expressed as mean ± S.D. *p < 0.05 versus control. (B) Cytoprotective effect of monoterpenes. U373-MG cells were pretreated with α-pinene and 1,8-cineole (a range of concentrations from 10 to 100 μM) for 24 h, previous to the treatment with H2O2 (1 mM) for 30 min. Cell viability was measured using the MTT assay. Experiments were performed in triplicate and results are expressed as mean ± S.D. *p < 0.05 versus control, # p < 0.05 versus H2O2-treated cells. (C) Effect of monoterpenes on cell morphology. U373-MG cells were pretreated with α-pinene and 1,8-cineole (10 and 25 μM) for 24 h, previous to the treatment with H2O2 (1 mM) for 30 min. Representative images are shown.
Cell culture and treatment
The human astrocytoma cell line U373-MG was obtained from the European Collection of Cell Cultures (ECACC, Salisbury, Wiltshire, England, UK). Cells were cultured in DMEM with 10% FBS and 0.5% gentamicin, and maintained in cell culture flasks (75 cm3; surface area) in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. For experiments, cells were plated in 96-well plates and in 100-mm Petri dishes.
Monoterpenes were dissolved in DMSO and then diluted in PBS to get the desired concentration (DMSO; 1% final concentration). Cells were pretreated with different concentrations of α-pinene and 1,8-cineole (a range of concentrations from 10 to 400 μM) for 24 h, prior to H2O2 exposition (1 mM, 30 min).
Cytotoxic and cytoprotective assay
The cytotoxic and cytoprotective effects of α-pinene and 1,8-cineole were determined by measuring cell viability using the MTT assay (Mosmann, Citation1983). Briefly, after treatments, cells were incubated with 100 μL of MTT (2 mg/mL) for 1 h at 37 °C. Once the incubation time finished, the MTT solution was carefully removed and the dark-blue formazan crystals were dissolved by adding 200 μL of DMSO. The absorbance was then measured at a wavelength of 550 nm using a microplate reader (Digiscan 340, Asys Hitech, Eugendorf, Austria). The percentage of cell viability (%) was calculated using the following expression: (mean absorbance of sample/mean absorbance of control) × 100].
Morphological study
The U373-MG cellular morphological changes were monitored by inverted phase-contrast microscopy. Photographs were taken using a Motic Moticam 2500 camera (Moticam, Decatur, GA).
Determination of intracellular ROS generation
Intracellular ROS formation was measured using the 2′7′-dichlorofluorescin diacetate (DCFH-DA) method (LeBel et al., Citation1992). Briefly, after cell treatments, cells were incubated with DCFH-DA (10 μM) in PBS-glucose for 30 min at 37 ºC in the dark. After removal of the medium, the cells were washed twice with PBS-glucose. The fluorescence intensity was monitored for 2 h at an excitation wavelength of 480 nm and an emission wavelength of 530 nm using a microplate fluorescence reader (FLx800, Bio-Tek Instrumentation, Inc., Winooski, VT). The fluorescence intensity is directly related to the intracellular ROS amount.
Protein concentration determination
The protein concentration was determined by the bicinchonic acid method (Smith et al., Citation1985), using bovine serum albumin as a protein standard.
Thiobarbituric acid reactive substances determination
Lipid peroxidation was evidenced by the formation of thiobarbituric acid reactive substances (TBARS; (Mihara & Uchiyama, Citation1978). Briefly, after treatments, 50 µL of cellular extracts were kept for incubation with 100 µL TBA–TCA–HCl in boiling water bath for 10 min. Afterwards, samples were centrifuged at 3000 rpm and a temperature of 4 ºC. Thiobarbituric acid-reactive substances were determined spectrophotometrically at a wavelength of 535 nm using a microplate reader Digiscan 340 (Asys Hitech GmbH, Eugendorf, Austria). TBARS values were expressed as pmol/mg protein.
Western blot analysis
Total cell extracts were prepared for studying the protein expression of several antioxidant enzymes and nuclear and cytoplasmic cell extracts for the Nrf2 factor. In brief, proteins of cell extracts were separated by electrophoresis on 10–15% SDS polyacrylamide gels and electrophoretically transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Berkeley, CA). Then, membranes were immunoblotted with the primary bodies CAT (1:1000), SOD (1:1000), GPx (1:2000), GR (1:3000), HO-1 (1:1000), and Nrf2 (1:500) overnight at 4 °C. Horseradish peroxidase-conjugated with anti-rabbit and anti-mouse IgG (1:5000) were employed as secondary antibodies. β-Actine was used as a loading control. The protein bands were detected using an ECL (advanced chemiluminescence) kit (GE Healthcare, Chalfont St. Giles, UK). Densitometric analysis of specific bands was performed using an image analyzer, Syngene Multigenius BioImaging System (Syngene, Frederick, MD) with Image Quants program.
Caspase-3 activity
The measurement of caspase-3 activity was determined fluorimetrically using the specific substrate (Alexis Biochemicals, San Diego, CA). Briefly, 20 μg of total cellular extracts were incubated with 20 mM Ac–DEVD–AMC for 1 h at 37 °C in darkness. The fluorescence intensity was measured at an excitation wavelength of 380 nm and an emission wavelength of 460 nm using the FLx800 Fluorescence Microplate Reader (MTX Lab Systems, Inc., Vienna, VI).
Statistical analysis
The data were analyzed statistically by one-way ANOVA and expressed as the mean ± standard deviations (SD), using Statgraphics Plus Version 5.1 (Statpoint Technologies, Inc., Warrenton, VA). The differences between the means were considered significant for values of p < 0.05.
Results
Phytochemical analysis
The relative yield (% w/w) of the essential oil obtained from the aerial parts of S. lavandulifolia was 2.67%. The chemical composition and the GC chromatogram of the essential oil are shown in and , respectively. Twenty-eight compounds of a whole of 47 peaks in the chromatogram, which account for the total of 98.64% detected constituents, were identified. The most common classes of compounds found were oxygenated and hydrocarbon monoterpenes (44.85% and 39.12%, respectively). Oxygenated and hydrocarbon sesquiterpenes were detected in lower concentrations (9.25% and 5.42%). The major volatile oil constituents were 1,8-cineole (19.57%), followed by α-pinene (18.39%), camphor (15.52%), and β-phellandrene (12.82%).
Table 1. Chemical composition of the essential oil of S. lavandulifolia.
Antiradical activity of monoterpenes
The antiradical activity of the major monoterpenes found in the essential oils of S. lavandulifolia has been estimated using the ORAC assay and the results are listed in . On one hand, the highest ORAC value was for 1,8-cineole (0.045 µmol Trolox/mg of monoterpene), following by α-pinene (0.030 µmol Trolox/mg of monoterpene). On the other hand, camphor exhibited a weak activity (0.012 µmol Trolox/mg of monoterpene) as a radical scavenger. Considering these results, we further evaluated the antioxidant and cytoprotective responses of α-pinene and 1,8-cineole to H2O2-induced oxidative stress in astrocytes (U373-MG cell line).
Table 2. (A) Antiradical activity measured using the oxygen radical absorbance (ORAC) method. (B) Effect of monoterpenes on lipid peroxidation.
Cytoprotective effect of monoterpenes
In the first part of this work, to evaluate the cytoprotective effect of the major monoterpenes of S. lavandulifolia, the range of concentrations which did not result cytotoxic for cells was established. U373-MG were treated with α-pinene and 1,8-cineole in the presence of the following concentrations 10, 25, 50, 100, 250, 300, and 400 µM for 24 h. As shown in , the range of concentrations from 10 to 250 µM for α-pinene and from 10 to 100 µM for 1,8-cineole did not decrease cell viability as compared with control cells.
In the second part, the protective effect of non-cytotoxic concentrations of the major monoterpenes of S. lavandulifolia on oxidative cell damage induced by H2O2 in U373-MG cells was investigated. Cells were treated with α-pinene and 1,8-cineole (from 10 to 100 µM) for 24 h, previous to H2O2 exposition (1 mM, 30 min). As shown in , H2O2 caused a significant decrease in cell viability over 60%. However, pretreatments with α-pinene (at the concentrations of 10, 25, 50, and 100 µM) and 1,8-cineole (at the concentrations of 10, 25, and 50 µM) increased significantly U373-MG cell viability in a concentration-dependent manner. The IC50 value of α-pinene was 79.70 µM and of 1,8-cineole was 66.23 µM.
To confirm the cytoprotective activity of 1,8-cineole and α-pinene, the effect of cell morphology was then determined. For this purpose, and for further studies, the concentrations of 10 and 25 µM were employed. As shown in H2O2 exposure modified cell morphology including loss of ramifications and round of the body and it also decreased the cell number. However, we found that both monoterpenes markedly attenuated these morphological changes.
Effects of monoterpenes on intracellular ROS production
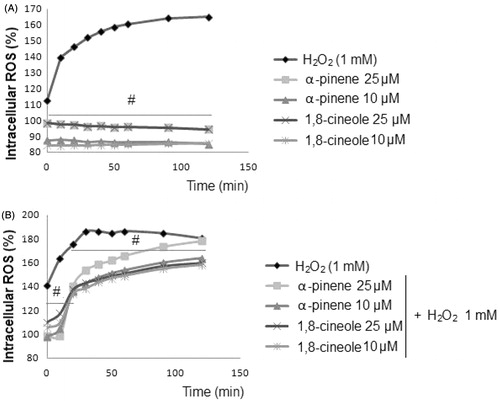
Next, we determine the effect of 1,8-cineole and α-pinene on intracellular ROS production using a dichlorofluorescein assay. In the presence of oxidant compounds such as H2O2, the non-fluorescence compound DCFH is oxidized to fluorescent DCF, being that the fluorescence intensity is directly proportional to ROS produced in cells (LeBel et al., Citation1992). As shown in , the levels of intracellular ROS after 1,8-cineole and α-pinene treatments were similar to that of the control. These results demonstrate that neither 1,8-cineole nor α-pinene is able to oxidize DCFH, and therefore, to produce ROS (IC50 value 7.13 µM α-pinene and 3.41 µM 1,8-cineole). However, on one hand, as shown in , H2O2 significantly increased the generation of ROS in astrocytes. On the other hand, the data in show that intracellular ROS production induced by H2O2 significantly declined when U373-MG cells were pretreated with both 1,8-cineole and α-pinene. The most active compound was 1,8-cineole by decreasing ROS production over 30–45% at 10 and 25 µM.
Figure 3. Effect of monoterpenes on intracellular ROS production. U373-MG cells were pretreated with α-pinene and 1,8-cineole (10 and 25 μM) for 24 h, previous to the treatment with H2O2 (1 mM) for 30 min. The intracellular ROS production was measured using the dichlorofluorescein assay. Data are expressed as percentage of ROS production, mean ± SD, #p < 0.05, versus H2O2-treated cells.
Effect of monoterpenes on lipid peroxidation
The levels of TBARS are an index of lipid peroxidation in biological materials. TBARS level () was significantly increased by 3-fold in H2O2-treated cells when compared with control cells. However, pretreatments with both monoterpenes tested at the concentrations of 10 and 25 µM inhibited markedly H2O2-induced lipid peroxidation. α-Pinene possessed the major ability to prevent lipid peroxidation.
Effect of monoterpenes on antioxidant enzymes expression
As shown in , pretreatments with 1,8-cineole and α-pinene enhanced the protein expression of the Nrf2-relating antioxidant and detoxifying enzymes CAT, SOD, GPx, GR, and HO-1, contributing to the protection of U373-MG against H2O2-induced oxidative stress. Particularly, pretreatment with α-pinene significantly elevated expression of GR and GPx at the highest concentration assayed (25 µM) and 1,8-cineole significantly increased the levels of CAT, SOD, and GPx protein expression compared with H2O2-treated cells.
Figure 4. (A) Effect of monoterpenes on protein expression of antioxidant enzymes. U373-MG cells were pretreated with α-pinene and 1,8-cineole (10 and 25 μM) for 24 h, previous to the treatment with H2O2 (1 mM) for 30 min. The levels of protein expression of CAT, SOD, GR, GPx, and HO-1 were measured by Western blots. (B) Effect of monoterpenes on Nrf2 protein expression. U373-MG cells were pretreated with α-pinene and 1,8-cineole (10 and 25 μM) for 24 h, previous to the treatment with H2O2 (1 mM) for 30 min. Nuclear and cytoplasmic Nrf2 protein expression were measured by Western blots. Data are expressed as mean ± SD. Results are representative of three independent experiments. *p < 0.05 versus control, # p < 0.05 versus H2O2.
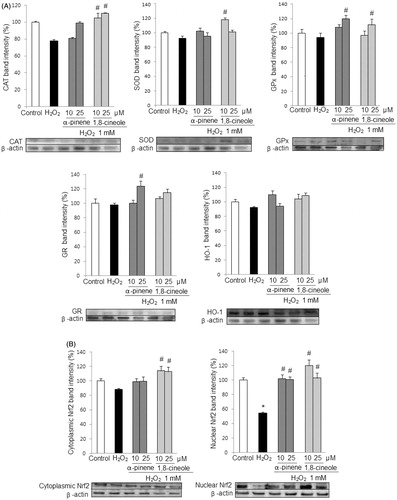
Since the expression of the studied enzymes is regulated by the transcription factor Nrf2, we further evaluated the effect of 1,8-cineole and α-pinene on the Nrf2 signaling pathway. As shown in , the pretreatments with 1,8-cineole and α-pinene, prior to H2O2 exposure, resulted in an increase of nuclear Nrf2 factor. These findings suggest that these monoterpenes induce up-regulation of antioxidant and detoxifying enzymes and activate the Nrf2 transcription factor.
Effect of monoterpenes on caspase-3 activity
Caspase-3 is a key mediator enzyme of oxidative stress-induced cell death by way of apoptosis. As shown in , when U373-MG cells were pretreated with monoterpenes, a significant decrease in caspase-3 activity was observed for α-pinene (10 and 25 µM) and 1,8-cineole (10 µM) compared with H2O2-treated cells.
Table 3. Effect of monoterpenes on caspase-3 activity.
Discussion
Recently, our research team has demonstrated the cytoprotective activity against oxidative stress of S. lavandulifolia essential oils at different phenological stages and plant densities (Porres-Martínez et al., Citation2013, Citation2014). The present study provides for the first time scientific evidence related to the potential role of the major monoterpenes, α-pinene and 1,8-cineole, found in the essential oil of S. lavandulifolia, as regulators of the cellular redox balance under conditions of oxidative stress.
The essential oil obtained from the aerial parts of S. lavandulifolia has been widely used in Mediterranean folk medicine for treating age-associated cognitive disorders such as loss of memory (Perry et al., Citation2003; Zrira et al., Citation2004). Data obtained from the compositional analysis of the essential oil of S. lavandulifolia by GC are in agreement with previous results of chemical composition evaluated in samples of S. lavandulifolia collected in other provinces of Spain. The monoterpenes 1,8-cineole and α-pinene were identified as major constituents (Guillén et al., Citation1996; Herraiz-Peñalver et al., Citation2010).
Monoterpenes have been demonstrated to possess antioxidant and radical scavenging activities against the DPPH, ORAC, and •OH free radicals in in vitro systems (Jin et al., Citation2012; Wang et al., Citation2008). In this study, the free radical scavenging potential of 1,8-cineole and α-pinene has been evaluated using the ORAC assay. This method employs a biological relevant radical source, peroxyl radical (AAPH)-induced, which makes it a common and valuable assay for measuring antioxidant capacities. Our results show that both monoterpenes are effective scavengers against AAPH-derived peroxyl radicals (found commonly in lipid peroxidation processes), suggesting they may prevent and reduce the oxidative stress that accompanies central nervous system diseases.
Oxidative stress has been implicated as a causative and consequential factor in the pathology of the memory decline and cognitive malfunction (Mao, Citation2013). Astrocytes, the major type of cells found in the central nervous system, provide physical, nutritional, and metabolic support and protection to neurons (Maragakis & Rothstein, Citation2006), constituting a potential therapeutic target for several diseases including oxidative stress-induced neurodegenerative diseases. Previous phytochemical and pharmacological studies have identified chemical constituents derived from medicinal plants, based on the traditional knowledge, as potential agents for the prevention and treatment of many types of cognitive disabilities (Kumar & Khanum, Citation2012). Our study shows that the major monoterpenes, α-pinene and 1,8-cineole, found in the essential oil of S. lavandulifolia, protect astrocytes against H2O2-induced oxidative stress cellular damage.
The H2O2-induced oxidative stress model to mimic cognitive decline in the elderly is widely employed in the evaluation of the antioxidant and cytoprotective action of isolated compounds, plant extracts, and essential oils. Hydrogen peroxide, which is the major ROS in the human body, has been physically characterized by its stability and its easy capacity to diffuse across cell membranes. Hydrogen peroxide is involved in different ROS-inducing reactions; these produced ROS cause the oxidative degradation of membrane lipids and alterations in antioxidant enzyme expression in astrocytes (Chen & Swanson, Citation2003; Röhrdanz et al., Citation2001). In the present work, α-pinene and 1,8-cineole exerted a protective action by maintaining cell viability and preserving morphological changes. Additionally, the studied major monoterpenes effectively prevented lipid peroxidation. The oxidative degradation of lipids in cell membranes, one of the major biochemical markers of oxidative stress, is involved in the pathophysiology of CNS injury (Halliwel, Citation1992). The antilipid peroxidative property has previously been demonstrated for other monoterpenes including camphene, geranyl acetate, and p-cymene (Quintans-Júnior et al., Citation2013). In this study, comparing both major monoterpenes from S. lavandulifolia essential oil, α-pinene had the major capacity for inhibiting lipid peroxidation. Its higher lipophilic character of this compound drives strong interactions with cell membranes (Elmann et al., Citation2009). Furthermore, it has been observed that after pre-treatments with α-pinene and 1,8-cineole, the activity and protein expression of the main antioxidant enzymes (CAT, SOD, GPx, GR, and HO-1) required for the defense against ROS were increased. The modulation of these enzymes constitutes a key target to counteract ROS-mediated damage (Zhang et al., 2007).
The Nrf2-signaling pathway regulates the studied enzymes, protecting against reactive oxygen species. Under normal conditions, Nrf2 is bound to Keap1 and retained in cytoplasm. In the presence of activating compounds including stimuli of oxidative stress, electrophilic compounds and chemopreventive agents, Nrf2 migrates to the nucleus and activate antioxidant response elements (ARE)-regulated genes, and subsequently enhances the expression of endogenous cytoprotective enzymes (Zhang et al., 2007). This work demonstrates for the first time that α-pinene and 1,8-cineole stimulate Nrf2-mediated antioxidant and cytoprotective enzymes in astrocytes. Consistent with other results that have identified several monoterpenes as activators of Nrf2 factor in the nervous system cells (Jinyoung et al., Citation2013), the present work provides evidence that pretreatments with 1,8-cineole and α-pinene, prior to H2O2 exposure, resulted in an increase of the nuclear Nrf2 factor. Thus, these findings suggest that these monoterpenes, at least in part, exert a protective effect against H2O2 in astrocytes by inducing the Nrf2 factor.
Finally, and based on previous work that has indicated that high concentrations of hydrogen peroxide have the ability to cause apoptosis (programmed cell death) in different cell types including neurons and astrocytes (Singh et al., Citation2011), the cytoprotective role of 1,8-cineole and α-pinene was evaluated against H2O2-triggered apoptotic cell death by measuring caspase-3 activity. Caspase-3 is a key mediator enzyme in both extrinsic and intrinsic apoptosis pathways. Under the experimental conditions established in our work, a significant increase in caspase-3 activity was observed in astrocyte cells treated with H2O2. However, pretreatments with 1,8-cineole and α-pinene protected astrocytes from apoptosis via inhibition of caspase-3, contributing to the prevention and amelioration of oxidative stress-induced cell damage. For 1,8-cineole, the lower concentration (10 µM) exerted higher protective activity against caspase-3 than the higher concentration (25 µM).This paradox is very common in natural products in which a low dose could stimulate whereas a high dose could inhibit (Calabrese et al., Citation2012).
Conclusion
The present work increases our knowledge about the role of α-pinene and 1,8-cineole, major monoterpenes found in Salvia lavandulifolia, as regulators of cellular redox balance. These monoterpenes exerted a protective effect under H2O2-induced oxidative stress conditions in astrocytes as evidenced by attenuation of loss in viability and morphological changes, inhibition of lipid peroxidation, and enhancement of expression of enzymatic antioxidant system. Furthermore, these compounds induce Nrf2 nuclear translocation and scavenge ROS. Acting as cytoprotective and antioxidants compounds, these monoterpenes may be beneficial in providing defense against cellular oxidative stress, and prevent ROS-induced damage that accompanies several neurodegenerative diseases such as Alzheimer's disease.
Acknowledgements
The authors thank Dr. Angelines Cases from the Spanish National Institute for Agricultural and Food Research and Technology (INIA) for her technical assistance with GC analysis.
Declaration of interest
The authors report no conflicts of interest.
References
- Calabrese V, Cornelius C, Dinkova-Kostova AT, et al. (2012). Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim Biophys Acta 1822:753–83
- Chang H, Kim HJ, Chun HS. (2007). Quantitative structure-activity relationship (QSAR) for neuroprotective activity of terpenoids. Life Sci 80:835–41
- Chen Y, Swanson RA. (2003). Astrocytes and brain injury. J Cerebr Blood Flow Metabol 23:137–49
- Davalos A, Gómez-Cordoves C, Bartolome B. (2004). Extending applicability of the oxygen radical absorbance capacity (ORAC Fluorescein) assay. J Agric Food Chem 52:48–54
- Elmann A, Mordechay S, Rindner M, et al. (2009). Protective effects of the essential oil of Salvia fructicosa and its constituents on astrocytic susceptibility to hydrogen peroxide-induced cell death. J Agric Food Chem 57:6636–41
- González-Burgos E, Gómez-Serranillos MP. (2012). Terpene compounds in the nature: A review of their potential antioxidant activity. Curr Med Chem 19:5319–41
- Guillén MD, Cabo N, Burillo J. (1996). Characterization of the essential oils of some cultivated aromatic plants of industrial interest. J Sci Food Agric 70:359–63
- Halliwell B. (1992). Reactive oxygen species and the central nervous system. J Neurochem 59:1609–23
- Herraiz-Peñalver D, Usano-Alemany J, Cuadrado J, et al. (2010). Essential oil composition of wild populations of Salvia lavandulifolia Vahl. from Castilla-La Mancha (Spain). Biochem Syst Ecol 38:1224–30
- Jaydeokar AV,Bandawane DD, Nipate SS, et al. (2012). Natural antioxidants: A review on therapeutic applications. Res J Pharmacol Phar 4:33–61
- Jin P, Wang SY, Gao H, et al. (2012). Effect of cultural system and essential oil treatment on antioxidant capacity in raspberries. Food Chem 132:399–405
- Jinyoung H, Cheon PS, Byung-Soo K, Songhee J. (2013). Borneol alleviates oxidative stress via upregulation of Nrf2 and Bcl-2 in SH-SY5Y cells. Pharm Biol 51:30–5
- Kumar GP, Khanum F. (2012). Neuroprotective potential of phytochemicals. Phcog Rev 6:81–90
- LeBel CP, Ischiropoulos H, Bondy SC. (1992). Evaluation of the probe 2',7-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–31
- Mao P. (2013). Oxidative stress and its clinical applications in dementia. J Neuro Dis 2013:1–15
- Maragakis NJ, Rothstein JD. (2006). Mechanisms of disease: Astrocytes in neurodegenerative disease. Nat Clin Pract Neurol 2:679–89
- Mihara M, Uchiyama M. (1978). Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–8
- Mitic-Culafic D, Zegura B, Nikolic B, et al. (2009). Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem Toxicol 47:260–6
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
- Perry NS, Houghton PJ, Sampson J, et al. (2001). In vitro activity of S.Lavandulaefolia (Spanish sage) relevant to treatment of Alzheimer's disease. J Pharm Pharmacol 53:1347–56
- Perry NS, Bollen C, Perry EK, Ballard C. (2003). Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial. Pharmacol Biochem Behav 75:651–9
- Porres-Martínez M, González-Burgos E, Carretero ME, Gómez-Serranillos MP. (2013). Phytochemical composition, antioxidant and cytoprotective activities of essential oil of Salvia lavandulifolia Vahl. Food Res Int 54:523–31
- Porres-Martínez M, González-Burgos E, Carretero ME, Gómez-Serranillos MP. (2014).Influence of phenological stage on chemical composition and antioxidant activity of Salvia lavandulifolia Vahl. essential oils. Ind Crop Prod 53:71–7
- Quintans-Júnior L, Moreira JC, Pasquali MA, et al. (2013). Antinociceptive activity and redox profile of the monoterpenes (+)-camphene, p-cymene, and geranyl acetate in experimental models. ISRN Toxicol 1–11
- Real Farmacopea Española. (2002). Ministerio de Sanidad y Consumo (2nd ed.). Madrid.
- Röhrdanz E, Schmuck G, Ohler S, et al. (2001). Changes in antioxidant enzyme expression in response to hydrogen peroxide in rat astroglial cells. Arch Toxicol 75:150–8
- Sáez L. (2010). Salvia L. Vol. XII.: Verbenaceae-Labiatae-Callitrichaceae. In: Morales R, Quintanar A, Cabezas F, Pujadas AJ, Cirujano S, eds. Flora ibérica. Madrid: Real Jardín Botánico de Madrid (CSIC), 298–326
- Savelev S, Okello E, Perry EK. (2004). Butyryl- and acetyl-cholinesterase inhibitory activities in the essential oils of Salvia species and their constituents. Phytother Res 18:315–24
- Singh S, Swarnkar S, Goswami P, Nath C. (2011). Astrocytes and microglia: Responses to neuropathological conditions. Int J Neurosci 121:589–97
- Smith PK, Krohn RI, Hermanson GT, et al. (1985). Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
- Tildesley NT, Kennedy DO, Perry EK, et al. (2003). Salvia lavandulaefolia (Spanish Sage) enhances memory in healthy young volunteers. Pharmacol Biochem Behav 75:669–74
- Wang CY, Wang SY, Chen C. (2008). Increasing antioxidant activity and reducing decay of blueberries by essential oils. J Agric Food Chem 56:3587–92
- Zhang L, Yu H, Sun Y, et al. (2007). Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol 564:18–25
- Zrira S, Menut C, Bessiere JM, et al. (2004). A study of the essential oil of Salvia lavandulifolia Vahl from Morocco. J Essent Oil Bear Pl 7:232–8