Abstract
Context: Indian mustard [Brassica juncea (L.) Czern. & Coss. (Brassicaceae)] is reported to possess diverse pharmacological properties. However, limited information is available concerning its hepatoprotective activity and mechanism of action.
Objective: To study the protective mechanism of mustard seed extract against acetaminophen (APAP) toxicity in a hepatocellular carcinoma (HepG2) cell line.
Materials and methods: Hepatotoxicity models were established using APAP (2.5–22.5 mM) based on the cytotoxicity profile. An antioxidant-rich fraction from mustard seeds was extracted and evaluated for its hepatoprotective potential. The mechanism of action was elucidated using various in vitro antioxidant assays, the detection of intracellular generation of reactive oxygen species (ROS), and cell cycle analysis. The phytoconstituents isolated via HPLC-DAD were also evaluated for hepatoprotective activity.
Results: Hydromethanolic seed extract exhibited hepatoprotective activity in post- and pre-treatment models of 20 mM APAP toxicity and restored the elevated levels of liver indices to normal values (p < 0.05). Post-treatment suppressed the generation of ROS by 58.37% and pre-treatment effectively prevented the generation of ROS by 90.5%. The mechanism of ROS suppression was further supported by antioxidant activity (IC50) data from DPPH (103.37 ± 4.2 µg AAE/mg), FRAP (83.26 ± 1.1 µg AAE/mg), ORAC (1115 µM GAE/ml), ABTS (83.05 µg GAE/ml), and superoxide (345.22 ± 5.15 µg AAE/mg) scavenging assays and by the restoration of cell cycle alterations. HPLC-DAD analysis revealed the presence quercetin, vitamin E, and catechin, which exhibited hepatoprotective activity.
Discussion and conclusions: A phytoextract of mustard seeds acts by suppressing the generation of ROS in response to APAP toxicity.
Introduction
Acetaminophen (APAP) is considered a safe and effective analgesic and antipyretic drug (Temple et al., Citation2007). APAP is widely available as a single-component medication and as a component of several combination products of over-the-counter and prescription medications. In 2009, the American Association of Poison Control Centers’ National Poison Data System reported 401 deaths caused by APAP or its combination products in the U.S. alone (Hodgman & Garrard, Citation2012). APAP overdose is the most common cause of acute liver failure in western countries with an increasing tendency (Fontana, Citation2008). APAP undergoes extensive hepatic metabolism. Up to 10% of APAP undergoes phase I oxidation by CYP450 to generate the reactive intermediate, N-acetyl-para-benzoquinone imine (NAPQI), which is normally conjugated with glutathione (GSH) to non-toxic cysteine and mercapturate metabolites (Gelotte et al., Citation2007). At very high doses of APAP, the continued production of NAPQI eventually results in the depletion of GSH. Once GSH stores have been depleted by approximately 70%, NAPQI binds to cellular proteins and leads to cell injury (McGill et al., Citation2012).
GSH depletion is a cascade of intracellular events that include mitochondrial oxidative stress, the generation of reactive oxygen (ROS) and nitrogen species, the activation of stress proteins and gene transcription mediators, and the mobilization of the liver’s innate immune system. The balance between these pathways ultimately determines whether recovery or cell death occurs. As a consequence, antioxidants have been proposed as an adjunct therapy for various liver diseases (Muriel, Citation2009). Mitochondrial oxidative stress also triggers the formation of mitochondrial membrane permeability transition pore, the loss of the mitochondrial membrane potential, the depletion of ATP, and the release of intermembrane proteins that are responsible for the typical nuclear DNA fragmentation that is associated with APAP-induced cell death (Jaeschke & Bajt, Citation2006). Antioxidants, such as silymarin (Pascual et al., Citation1993), exert liver protection against several toxins including APAP overdoses (Muriel et al., Citation1992). Reduced GSH can effectively protect the liver both by scavenging NAPQI and by detoxifying ROS and RNS, such as peroxynitrite. This mechanism is the basis for the rational clinical use of N-acetylcysteine, which is a GSH precursor, as an antidote against APAP toxicity (Polson & Lee, Citation2005).
Brassica juncea (L.) Czern. & Coss. (Brassicaceae), popularly known as Indian mustard, has both edible and medicinal values. The seeds of this plant are widely used as a spice. It has been reported that the mixture of crushed mustard seed with a decoction of Moringa oleifera Lam (Moringaceae) root is useful in liver and spleen diseases (Reddy et al., Citation2010). Mustard preparations are widely known for mildly laxative, diuretic, and their liver bile stimulatory effect (Desai, Citation2005). As documented in classical Ayurvedic texts, mustard seeds are used to purge the toxins out of the body (Manohar & Pushpan, Citation2009). The leaf extracts of B. juncea have been reported to exhibit antioxidant, anti-nociceptive, and anti-hyperglycemic activities both in vitro and in vivo (Kumar et al., Citation2011). The leaf extracts of B. juncea have also been reported to significantly prevent the development of insulin resistance in rats (Walia et al., Citation2011). However, the mechanism responsible of the protective effect of B. juncea seed extract in the context of APAP-induced liver toxicity in HepG2 cells still remains unknown. Thus, the purpose of the study was to elucidate the mechanism of action of this extract at the cellular level by monitoring several enzymatic systems, the generation of ROS, and cell cycle analysis.
Materials and methods
Chemicals and reagents
The reference standards were purchased from Sigma-Aldrich Corporation, St. Louis, MO; tissue culture media and chemicals from Genetix Biotech Asia Pvt. Ltd., New Delhi, India. The other chemicals and reagents used were of highest purity and purchased from, Fisher Scientific, Waltham, MA.
Preparation of seed extracts
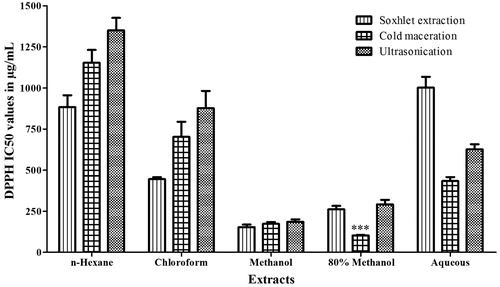
Brassica juncea seeds were purchased (2011) from a local grocery shop in vacuum packaged bags. The seeds were identified and authenticated at Agharkar Research Institute in Pune, India (Voucher specimen number: S-158). For extraction, three different methods were employed. Crushed seeds (15 g) of Brassica juncea were extracted using a Soxhlet apparatus (6 h), a rotary shaker (150 rpm at room temperature), and an ultrasonicator. About 90 ml of solvents, i.e., n-hexane, chloroform, methanol, hydromethanol (methanol:water in an 80:20 ratio), and water, were used in each method. The extracts were screened for antioxidant potential using DPPH radical scavenging assay according to previously described methodology (Silva et al., Citation2011). Based on a comparison of IC50 values, the extract obtained using a rotary shaker and hydromethanol, which was referred to as B. juncea hydromethanolic extract (BJHME), was used for further study.
Maintenance of human hepatocellular carcinoma cells – HepG2 cell line
HepG2 cell line derived from the liver tissue of one volunteer with differentiated hepatocellular carcinoma was obtained as a gift from Regenerative Medicine Lab, Reliance Life Sciences, Mumbai. Culture was grown in 10% FBS in DMEM containing 100 U/ml penicillin and 100 μ/ml streptomycin at 37 °C in a humidified CO2 incubator. The cells were frozen (1 − 1.5 × 106 cells/vial) from passage (p) 25–28, which were thawed as per the requirement of the experiments. HepG2 cell line used for experimentation was within the range of p25–p29, to ensure consistency and to avoid time-dependent genotype variation.
Determination of APAP toxicity
The toxicity of APAP was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay. Briefly, HepG2 cells (5 × 104 cells/well) were seeded in a 96-well plate in medium containing APAP at various concentrations ranging from 2.5 to 22.5 mM for 24 h. After the treatment, 20 µl of MTT solution was added and the cells were incubated for 4 h. Following the incubation, the supernatant was discarded and formazan crystals were dissolved in 100 µl of DMSO. The absorbance was measured at 570 and 655 nm to determine IC50 values to establish the toxicity models.
Efficacy testing of BJHME against APAP-induced hepatotoxicity on HepG2 cells
Post-treatment model
Briefly, HepG2 cells were seeded for 24 h and incubated with 20 mM APAP. The cells were post-treated with medium containing BJHME at several concentrations ranging from 50 to 1600 µg/ml for 24, 48, and 72 h, and the viability was assessed using the MTT assay. EC50 values were determined by plotting the dose–response curve and the treatment time was selected. Based on these results, the supernatant was monitored for alanine aminotransferase (ALT), aspartate aminotransferase (AST) alkaline phosphatase (ALP), and γ-glutamyl transferase (GGT). The damage control was maintained for the experiment. 10% (v/v) Liv-52 and 40 µg/ml silymarin were used as positive controls for the study.
Pre-treatment model
HepG2 cells were pre-treated with medium containing BJHME at several concentrations ranging from 50 to 1600 µg/ml for 24, 48, and 72 h. Next, the cells were exposed to 20 mM APAP for 24 h and the cell viability was assessed to plot the dose–response curve and EC50 values were determined and the treatment time was selected. Based on these results, the supernatant was monitored for AST, ALT, ALP, and GGT levels. The damage control was maintained for the experiment. About 10% (v/v) Liv-52 and 40 µg/ml silymarin were used as positive controls for the study.
Detection of morphological changes related to APAP-induced hepatotoxicity using acridine orange/ethidium bromide (AO/EB) fluorescent staining
HepG2 cells that were grown in a 35 mm dish treated with BJHME and APAP (as described above) were washed with Dulbecco’s phosphate buffered saline (DPBS). Ethidium bromide (100 μg/ml) and acridine orange (100 μg/ml) were mixed 1:1, added to cells and observed under a fluorescent microscope to evaluate morphological characteristics.
Mechanism of action
In vitro antioxidant activity
The free radical scavenging activity of BJHME was measured using a FRAP assay (Prasad et al., Citation2009) and a superoxide scavenging assay (Silva et al., Citation2011). BJHME was analyzed for ABTS radical scavenging assay and ORAC assay at Natural Remedies Pvt. Ltd., Bangalore, India.
Detection of intracellular ROS
Intracellular ROS was detected by means of the oxidation-sensitive fluorescent probe 2,7-dichlorodihydrofluorescein diacetate (DCFDA). For analysis, the treated cells were harvested via trypsinization and washed with DPBS. Next, the cells were incubated with 20 µM DCFDA at room temperature for 1 h and fluorescent intensity was measured with a FACScan flow cytometer (BD Biosciences, San Jose, CA).
Cell-cycle analysis
Cell-cycle analysis was performed using a propidium iodide (PI) staining solution and a FACScan flow cytometer (BD Biosciences, San Jose, CA). For the experiment, the treated cells were washed with DPBS, harvested, and fixed in ice-cold 70% ethanol for 1 h at 4 °C. Next, the cells were washed, suspended in a PI staining solution, incubated at room temperature for 1 h, and analyzed.
HPLC-DAD analysis of BJHME for the detection of characteristic phytoconstituents
The standard solutions and BJHME were prepared in methanol and filtered through a 0.2-µm filter for analysis. The detection of polyphenols in the extract was performed using RP-HPLC (UFLC Prominence Gradient: Shimadzu Corporation, Kyoto, Japan) in an Enable C18 G: 5 µm 250 × 4.6 mm column (Spinotech Pvt. Ltd, Chennai, India) using a diode array UV detector (SPD-M20A). A binary gradient (pump: LC 20AD) of 10 mM KH2PO4 (pH: 7) in deionized water (solvent A), and methanol (solvent B) was used as follows: from 10% to 50% solvent B in 5 min, from 50% to 80% solvent B in 5 min, from 80% to 70% solvent B in 5 min, from 70% to 60% solvent B in 5 min, from 60% to 50% solvent B in 5 min, and then isocratic conditions of 10% solvent B for a further 5 min. The flow rate was 1 ml/min. The calibration curves were plotted and the phytoconstituents were quantified by comparing the retention time, peak areas, and spectra of BJHME with those of standards.
Efficacy testing of phytoconstituents detected via HPLC-DAD in BJHME in an APAP-induced toxicity model
The EC50 values of the phytoconstituents were determined using a MTT assay on APAP toxicity model to analyze the supernatants for the release of AST, ALT, GGT, and ALP in the culture medium.
Statistical analysis
All experiments were performed in triplicate and the data were analyzed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). The dose–response curves were plotted to obtain the EC50 values and Dunnette’s multiple test was used to compare values among the treatment groups. Two-way ANOVA followed by Bonferroni’s post-test was applied to assess the significance of difference observed among the enzyme levels at different doses.
Results
Brassica juncea hydromethanolic extract (BJHME) showed maximum antioxidant activity
Indian mustard seeds were extracted using solvents that ranged from non-polar to polar using three different methods. The IC50 values of the DPPH scavenging activity of the extracts are depicted in . The antioxidant effect of the hydromethanolic extract resulted in 50% inhibition at the lowest concentration of 103.37 ± 4.2 µg/mg when compared with other extracts. Hence, BJHME was used for further studies. The yield of BJHME was calculated to be 7.24 ± 0.45% (w/w).
20 mM APAP-induced hepatotoxicity on HepG2 cells
The APAP exhibited toxicity in HepG2 cell line at concentrations from 0.25 mM to 22.5 mM. The IC50 value of APAP was 18.81 mM; thus, 20 mM APAP was used to establish the toxicity models. The toxicity of 20 mM APAP was reflected as a multiple fold increase in the release of AST (15-fold), ALT (6-fold), GGT (6-fold), and ALP (7-fold) in the post-treatment model () and AST (23-fold), ALT (12-fold), GGT (8-fold), and ALP (7-fold) in the pre-treatment model () resulting in a significant damage to the cells. Elevated levels of these enzymes indicate cellular leakage and a loss of functional integrity of the hepatocyte cell membrane.
Table 1. Effect of BJHME post-treatment on AST, ALT, GGT and ALP levels (U/L).
Table 2. Effect of BJHME pre-treatment on AST, ALT, GGT, and ALP levels (U/L).
BJHME treatment significantly reversed 20 mM APAP-induced hepatotoxicity in HepG2 cells
The efficiency of BJHME post-treatment against 20 mM APAP-induced toxicity was assessed in the HepG2 cell line for 24 h, 48 h, and 72 h, the dose–response curve was plotted and the EC50 values were found to be 442 ± 13.1, 502 ± 23, and 1086 ± 167 µg/ml for 24, 48, and 72 h, respectively. The values were compared and the viability of the cells was evident after treatment with BJHME for 24 h; thus, the enzyme levels of this treatment were monitored. As depicted in , treatment with 300, 500, and 700 µg/ml of BJHME caused a significant reversal of enzyme leakage compared with damaged cells. The positive controls 10% Liv-52 and 40 µg silymarin which are known hepatoprotective drugs (Del Prete et al., Citation2012; Shaker et al., Citation2010), resulted in complete reversal of damage as expected (p < 0.05). Treatments with 700 µg/ml of BJHME resulted in a maximum reversal (p < 0.001), effectively restoring the control levels.
Pre-treatment of BJHME was also performed for 24, 48, and 72 h to evaluate the efficacy of the extract in preventing the damage caused by 20 mM APAP. The dose–response curve was plotted and the EC50 value for the 24 h treatment was found to be 886 ± 37 µg/ml. Although 48 and 72 h pre-treatments with BJHME were administered to the cells, the later time points were not reproducible; thus, the enzyme levels of the 24 h treatment were assessed. As shown in , 10% liv-52 and silymarin resulted in the prevention of APAP-induced damage on hepatocytes. Further, 24 h pretreatment with BJHME at concentrations of 600, 800, and 1000 µg/ml resulted in a significant control of elevated enzyme levels compared with damaged cells. In addition, pre-treatment with 1000 µg/ml of BJHME resulted in maximum prevention of damage (p < 0.001).
Fluorescence microscopy reveals a remarkable reduction of APAP-induced damage after BJHME treatment
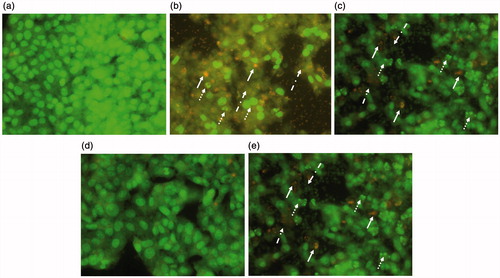
APAP caused extensive cellular damage in both models as evidenced by DNA fragmentation, membrane damage, and leakage of cellular contents. Apoptotic bodies were also observed. Post-treatment of cells with BJHME (700 µg) triggered the regeneration of cells with a reduction in damage (). In contrast, pre-treatment of cells with BJHME (1000 µg) largely prevented the observed cellular damage ().
Figure 2. AO/EB revealed the cellular damage of APAP toxicity and BJHME treatments: (a) control HepG2 cells, (b) Cells damaged with 20 mM APAP in post-treatment model showing extensive cellular damage (dotted arrow: DNA fragmentation, plain arrow: apoptosis, dotted dashed arrow: cellular leakage), (c) BJHME 700μg treatment against 20 mM APAP in post-treatment model showing reversal of damage, (d) pre-treatment of 1000 μg BJHME on HepG2 cells preventing the toxicity of 20 mM APAP, (e) absence of pretreatment causes cellular damage by virtue of 20 mM APAP toxicity.
BJHME acts via the suppression of ROS due to its antioxidant potential
BJHME exhibited antioxidant potential when assessed using different antioxidant assays. As observed, the IC50 of BJHME in the DPPH, ferric reducing power, superoxide anion scavenging, and ABTS radical scavenging assay were 103.37 ± 4.2 µg AAE/mg, 83.26 ± 1.11 µg AAE/mg, 345.22 ± 5.15 µg AAE/mg, and 83.05 µg GAE/ml, respectively. The oxygen radical-scavenging potential, which was assessed using ORAC assay, was 1115 µmoles TE/g of extract. Thus, BJHME showed various radical scavenging capacities in vitro, depicting its antioxidant potential in vivo.
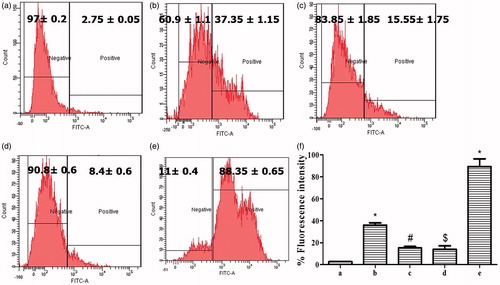
Further, the ROS generated in HepG2 cells during APAP toxicity was determined using the DCFDA dye. Intracellular acetases cleave DCFDA to DCF, which becomes excited upon combining with ROS, this effect can be quantified using flow cytometry. Treatment with 20 mM APAP leads to excessive generation of ROS in HepG2 cell line (), with values increasing from 2.75 ± 0.05% in control cells to 37.35 ± 1.15% in damaged cells, corresponding to an increase of more than 30-fold. Post-treatment of these damaged cells with BJHME (700 µg) resulted in a reduction of ROS generation by 58.37 to 15.55 ± 1.75% (). In contrast, pre-treatment of cells with BJHME (1000 µg) effectively prevented the generation of ROS under APAP-induced stress conditions (). The generation of ROS in pre-treated cells was 8.4 ± 0.6% versus 88.35 ± 0.65% in untreated damaged cells; thus, treatment prevented the generation of ROS by 90.5%.
Figure 3. Detection of ROS from HepG2 cells on APAP toxicity and BJHME treatment: (a) control cells show endogenous production of ROS (gated negative); (b) % ROS generation in cells damaged with 20 mM APAP; (c) cells post-treated with 700 μg BJHME show reduction in generation of ROS; (d) 1000 μg BJHME pre-treatment prevents the generation of ROS as opposed to (e) cells not given the BJHME pre-treatment; and (f) analysis of detection of ROS generation in (a–e) treatments data expressed as mean ± SEM (n = 3); *p < 0.05 when compared with a #p < 0.05 when compared with b, $p < 0.05 when compared with e.
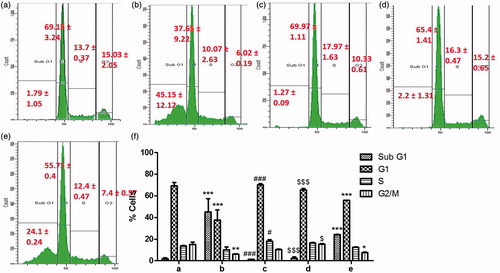
It was also observed that the toxicity caused by APAP results in an alteration of the cell cycle. As depicted in , 20 mM APAP-induced 45.15 ± 12.12 and 24.1 ± 0.24% apoptosis in post-treatment and pre-treatment models, respectively. APAP toxicity resulted in a loss of healthy cells from-G1 phase. BJHME (700 µg/ml for 24 h) post-treatment () resulted in a reduction of apoptosis to 1.27 ± 0.09% and a restoration of the cell cycle with 69.97 ± 1.11% of the cells in G1 phase, 17.97 ± 1.63% of the cells in S phase, and 10.33 ± 0.61% of the cells in G2/M phase. Pre-treatment of the cells with BJHME (1000 µg/ml for 24 h) resulted in protection of the cells, and reduced toxicity was observed after treatment with 20 mM APAP (). The percentage of cells undergoing apoptosis was reduced to 2.2 ± 1.31% compared with 24.1 ± 0.24% in post-treatment damage. Further, the normal cell cycle, with 65.4 ± 1.41% of the cells in G1 phase, 16.3 ± 0.47% of the cells in S phase, and 15.2 ± 0.65% of the cells in G2 phase was also maintained.
Figure 4. Cell-cycle analysis of HepG2 cells: (a) control: normal cell cycle (sub-G1, -G1, -S & -G2/M phases from left to right); (b) cells damaged with 20 mM APAP increase cell death (sub-G1 population); (c) cells post-treated with 700 μg BJHME show reduction in apoptosis; (d) 1000 μg BJHME pre-treatment prevents apoptosis as opposed to (e) cells not given the BJHME pre-treatment; (f) cell-cycle analysis of (a–e) treatments, data expressed as mean ± SEM (n = 3); *p < 0.05, **p < 0.01, ***p < 0.001 when compared to a; #p < 0.05, ###p < 0.001 when compared to b; $p < 0.05, $$$p < 0.001 when compared to e.
Thus, in both BJHME pre-treatment and post-treatment models, BJHME efficiently restored the normal cell cycle.
Quercetin, vitamin E, vanillin, catechin, and sinigrin were detected in the BJHME
An HPLC-DAD method was developed for the identification and quantification of characteristic compounds in BJHME. Phenolic compounds quercetin, vitamin E, vanillin, catechin, and sinigrin – which is a glucosinolate present in the Brassicaceae family, were detected () and quantified by plotting calibration curves ().
Figure 5. Optimized HPLC chromatogram at 273 nm: (a) chromatogram of mixture of standards with their respective retention times sinigrin (5.716 min), catechin (20.743 min), vanillin (23.528 min), quercetin (25.387 min), and vitamin E (26.097 min); (b) chromatogram of BJHAE showing peaks of sinigrin (5.669 min), catechin (20.92 min), vanillin (23.538 min), quercetin (26.011 min), and vitamin E (26.984 min).
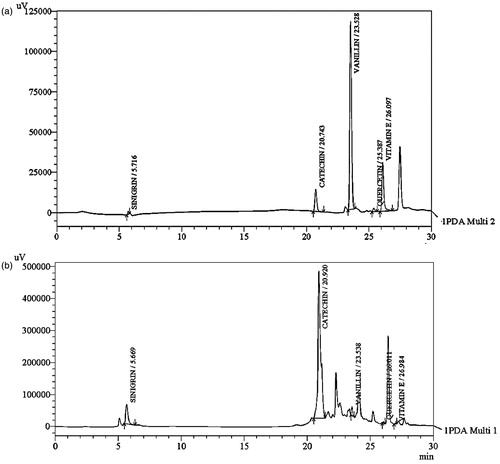
Table 3. Detection and quantification of compounds from BJHME using HPLC-DAD analysis.
Vitamin E, quercetin, and catechin present in BJHME contributes to hepatoprotection
The effects of the phytoconstituents detected in the BJHME on APAP-induced toxicity in HepG2 cells were evaluated and the EC50 values of vitamin E, quercetin, and catechin were 86.14 µg/ml (0.2 mM), 90.67 µg/ml (0.3 mM), and 29.02 µg/ml (0.1 mM), respectively, based on the MTT assay (data not shown). Vanillin (1 mM) and sinigrin (1 mM) failed to exhibit activity. However, vitamin E, quercetin, and catechin mediated a remarkable reversal of the elevated levels of AST, ALT, GGT, and ALP ().
Table 4. Effect of isolated compounds from BJHME on AST, ALT, GGT and ALP levels (U/L).
Discussion
Mitochondrial dysfunction is an important mechanism in APAP-induced hepatotoxicity (Jaeschke et al., Citation2002; Kucera et al., Citation2012). The mitochondrial permeability transition (MPT) occurs with the formation of superoxide, leading to the formation of peroxynitrite and tyrosine nitration (Ben-Shachar et al., Citation2012; Jaeschke et al., Citation2011). Increased superoxide production is anticipated to be a critical event because it leads to increased hydrogen peroxide and peroxynitrite formation in the cell. Thus, the superoxide scavenging activity of BJHME is one of the mechanisms of protection against APAP-induced cytotoxicity. This role is further supported by the detection of low intensity fluorescence when using the DCF dye which becomes excited in the presence of ROS. The protective effect of BJHME can also be attributed to the capacity of this extract to act as a free radical scavenger during the cellular state of GSH scarcity. GSH is one of the most important natural antioxidants of hepatocytes; the absence of this renders the cell susceptible to oxidative stress (Bessems & Vermeulen, Citation2001; James et al., Citation2003; Jaeschke et al., Citation2002). The GSH depleted during APAP-induced hepatotoxicity is also a cofactor for glutathione peroxidase. Thus, a major mechanism of peroxide detoxification is compromised, leading to increased intracellular peroxide levels and increased oxidative stress via the Fenton mechanism (Hinson et al., Citation2010). BJHME possesses an iron-chelating capacity as indicated by the FRAP assay. Thus, BJHME inhibits the Fenton reaction and thereby exhibits potential as a hepatoprotective agent. This effect was also observed when HepG2 cells were pretreated with BJHME, along with the possibility of rapid replenishment of GSH, as a consequence of the antioxidant property of BJHME.
Liv-52 is a polyherbal drug with Achillea millefolium, Capparis spinosa, Cassia occidentalis, Cichorium intybus, Solanum nigrum, Tamarix gallica, and Terminalia arjuna manufactured by Himalaya Drug Company, Bangalore, India, as a liver tonic (Girish et al., Citation2009), extensively reported to be antioxidant and hepatoprotective against various hepatotoxicants. Hence, Liv-52 was used as a positive control for comparative evaluation of BJHME along with silymarin. Silymarin is a mixture of flavonolignans from the fruit of Silybum marianum, widely known for its hepatoprotective potential against liver toxics (Pradeep et al., Citation2007). BJHME showed a hepatoprotective effect similar to the previous products (p < 0.05), further extending the applicability of mustard seeds for future applications.
Phytochemical analysis of BJHME revealed the presence of phenolics, flavonoids, tannins, and vitamins; the observable antioxidant activity exhibited a linear correlation with phenolic content. These compounds act by scavenging free radicals, chelating transitional metals, as reducing agents and activators of antioxidative defense enzyme systems to suppress radical damage. The vitamin E, quercetin, and catechin, detected in BJHME (), also exhibited hepatoprotective activity in the HepG2 cell line (). Moreover, the antioxidant activity of these phytoconstituents has been well documented (Abdel-Azeem et al., Citation2013; Chen, Citation2010; Effi et al., Citation2012; Oyinbo et al., Citation2006; Pavanato et al., Citation2003; Tsuchiya, Citation1999). Vitamin E which was detected in BJHME at 800 µg/g is known to compete for scavenging deleterious peroxyl radicals much faster than polyunsaturated fatty acids and nearly 200-times faster than the commercial antioxidant butylated hydroxytoluene (Burton & Traber, Citation1990) protecting cellular membranes. Thus, only a small amount of vitamin E is able to protect a large amount of polyunsaturated fat in the membranes, this contributing to the therapeutic value to the extract. The quercetin in BJHME detected at 100 µg/g, prevents the propagation of lipid peroxidation, increases GSH levels, increases antioxidant enzyme function, and prevents Ca2+-dependent cell death (Ansari et al., Citation2010; Bentz, Citation2009). Catechin, which is known to reduce lipid peroxidation and increase GSH production (Mehra et al., Citation2013), was also found in BJHME at 4.2 mg/g concentration, protects the cells from APAP damage. Mustard seeds are reported to contain a high content of cysteine residues (Wanasundara, Citation2008), which may even bind with oxygen electrophiles, further augmenting antioxidant potency. Altogether, these phyconstituents contribute to the protective efficacy of BJHME for the suppression of increased ROS generation.
Although extensive studies have been performed to evaluate the potential of phytoextracts to act as protective agents in the HepG2 cell line, most of these studies focused on evaluating the toxicity profiles of the extract. However, considering the multiplicity and complexity of liver functions, no single test can exhaustively establish disturbances in liver function. In the current study, a series of liver function tests was employed to accurately assess the severity of the damage. The magnitude of liver dysfunction that is triggered by hepatotoxins is measured using the levels of AST, ALT, GGT, and ALP. When the hepatic cell membrane is damaged, these enzymes, which are normally located in the cytosol, leak into the circulation from hepatocytes. We established an enzyme-based damage model to monitor the levels of these enzymes. APAP-induced liver injury resulted in elevated levels of ALT, AST, GGT, and ALP, which are suggestive of cellular leakage and the loss of functional integrity of the cell membrane. BJHME treatment resulted in the restoration of elevated levels of AST, ALT, ALP, and GGT due to the stabilization of the plasma membrane, which re-established the structural integrity of the hepatocytes. An increase in the viability of liver cells is attributable to the inhibitory effect of BJHME on ROS production which is primarily responsible for hepatocyte injury and inflammation.
APAP and other non-steroidal anti-inflammatory drugs have been evaluated extensively for their hepatotoxicity, and herbal extracts have been assessed for their protective nature against APAP toxicity. However, the mechanism of action of these extracts has been explored less thoroughly. The hepatoprotective activity of seeds of Brassica juncea against CCl4 toxicity on rats was reported (John, Citation2011). However, there was limited characterization of the extract. In addition, the mechanism of hepatoprotection was not established at the cellular level. The focus of the current investigation was to evaluate the mechanism of protection of BJHME against APAP-induced toxicity in HepG2 cell line. Our data demonstrate that BJHME suppresses the extensive generation of ROS that is caused by APAP, thus preventing the oxidative stress which seems to be responsible of APAP toxicity.
It is well established that plant extracts with antioxidant potential can protect against the oxidative damage caused by APAP hepatotoxicity (Parmar et al., Citation2010). We confirmed this observation and demonstrated that BJHME substantially reduced the oxidative stress in HepG2 cell line and protected the membrane integrity as indicated by the enzyme release profile.
Conclusion
This study successfully establishes an in vitro model of hepatotoxicity using HepG2 cells that can be used as a reliable model for drug toxicity screening. Further, BJHME successfully reversed APAP-induced hepatotoxicity, restoring the elevated levels of AST, ALT, GGT, and ALP. The ability to reduce these enzyme levels could be associated with the ability of BJHME to prevent the peroxidative degradation of membrane lipids by inhibiting the binding of activated radicals to the macromolecules. This effect could be achieved via various antioxidant mechanisms of BJHME. To summarize, the study indicates that the hepatoprotective activity of BJHME involve, the synergistic action and antioxidant potential of its phytoconstituents, and establishes the potential medicinal value of BJHME for future pre-clinical studies.
Acknowledgements
The authors express sincere thanks to SAIF, IIT Bombay for allowing us to use flow cytometer and Dr. P. S. Ramanathan advanced instrumentation center, Ramnarain Ruia College, Mumbai, for allowing the use of the best available HPLC facility and kind support for this work.
Declaration of interest
The authors report that they have no conflict of interest.
References
- Abdel-Azeem AS, Hegazy AM, Ibrahim KS, et al. (2013). Hepatoprotective, antioxidant, and ameliorative effects of ginger (Zingiber officinale Roscoe) and vitamin E in acetaminophen treated rats. J Diet Suppl 10:195–209
- Ansari M, Abdul H, Joshi G, et al. (2010). Protective effect of quercetin in primary neurons against Aβ (1-42): Relevance to Alzheimer’s disease. J Nutr Biochem 20:269–75
- Ben-Shachar R, Chen Y, Luo S, et al. (2012). The biochemistry of acetaminophen hepatotoxicity and rescue: A mathematical model. Theor Biol Med Model 9:55–77
- Bentz AB. (2009). A review of quercetin: Chemistry, antioxidant properties, and bioavailability. J Young Investig 19:1–8
- Bessems JG, Vermeulen NP. (2001). Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 31:55–138
- Burton GW, Traber MG. (1990). Vitamin E: Antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr 10:357–82
- Chen X. (2010). Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag 6:135–41
- Del Prete A, Scalera A, Iadevaia MD, et al. (2012). Herbal products: Benefits, limits, and applications in chronic liver disease. Evid Based Complement Alternat Med 2012:837939–58
- Desai U. (2005). The Ayurvedic Cookbook: A Personalized Guide to Good Nutrition and Health. Delhi, India: Motilal Banarsidass Publisher
- Effi F, Ebong PE, Akpan HD, Usoh IF. (2012). Hepatoprotective effect of vitamins C and E against gasoline vapor-induced liver injury in male rats. Turk J Biol 36:217–23
- Fontana RJ. (2008). Acute liver failure including acetaminophen overdose. Med Clin North Am 92:761–94
- Gelotte CK, Auiler JF, Lynch JM, et al. (2007). Disposition of acetaminophen at 4, 6, and 8 g/day for 3 days in healthy young adults. Int J Clin Pharmacol Ther 81:840–8
- Girish C, Koner BC, Jayanthi S, et al. (2009). Hepatoprotective activity of six polyherbal formulations in paracetamol induced liver toxicity in mice. Indian J Med Res 129:569–78
- Hinson JA, Roberts DW, James LP. (2010). Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 196:369–405
- Hodgman MJ, Garrard AR. (2012). A review of acetaminophen poisoning. Crit Care Clin 28:499–516
- Jaeschke H, Bajt ML. (2006). Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89:31–41
- Jaeschke H, Gores GJ, Cederbaum AI, et al. (2002). Mechanisms of hepatotoxicity. Toxicol Sci 65:166–76
- Jaeschke H, Williams CD, Farhood A. (2011). No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology 53:748–9
- James LP, Mayeux PR, Hinson JA. (2003). Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 31:1499–506
- John AA. (2011). Hepatoprotective activity of Brassica juncea (L) Czern against carbon tetrachloride induced hepatotoxicity in albino rats. Pharmacologyonline 3:609–21
- Kucera O, Al-dury S, Lotková H, et al. (2012). Steatotic rat hepatocytes in primary culture are more susceptible to the acute toxic effect of acetaminophen. Physiol Res 61:93–101
- Kumar V, Thakur AK, Barothia ND, Chatterjee SS. (2011). Therapeutic potentials of Brassica juncea: An overview. TANG 1:8–24
- Manohar P, Pushpan R SR. (2009). Mustard and its uses in Ayurveda. Indian J Tradit Know 8:400–4
- McGill MR, Sharpe MR, Williams CD, et al. (2012). The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 122:1574–83
- Mehra P, Garg M, Koul A, Bansal DD. (2013). Effect of (+)-catechin hydrate on oxidative stress induced by high sucrose and high fat diet in male Wistar rats. Indian J Exp Biol 51:823–7
- Muriel P. (2009). Role of free radicals in liver diseases. Hepatol Int 3:526–36
- Muriel P, Garciapiña T, Perez-Alvarez V, Mourelle M. (1992). Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol 12:439–42
- Oyinbo C, Dare W, Okogun G, et al. (2006). The hepatoprotective effect of vitamin C and E on hepatotoxicity induced by ethanol in Sprague–Dawley rats. Pak J Nutr 5:507–11
- Parmar SR, Vashrambhai PH, Kalia K. (2010). Hepatoprotective activity of some plants extract against paracetamol induced hepatotoxicity in rats. J Herb Med Toxicol 4:101–6
- Pascual C, Gonz R, Armesto J, Muriel P. (1993). Effect of silymarin and silybinin on oxygen radicals. Drug Dev Res 29:73–7
- Pavanato A, Tuñón MJ, Sánchez-Campos S, et al. (2003). Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci 48:824–9
- Polson J, Lee WM. (2005). AASLD position paper: The management of acute liver failure. Hepatology 41:1179–97
- Pradeep K, Mohan CVR, Gobianand K, Karthikeyan S. (2007). Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol 560:110–16
- Prasad N, Hao J, Yi C, et al. (2009). Antioxidant and anticancer activities of wampee (Clausena lansium (Lour.) Skeels) peel. J Biomed Biotechnol 2009:612805–11
- Reddy J, Gnanasekaran D, Vijay D, Ranganathan TV. (2010). Studies on hepatoprotective activity of traditional Ayurvedic formulation “Vidakana Choornam” against carbon tetrachloride induced hepatotoxicity in albino rat. Int J Pharm Anal 2:5–16
- Shaker E, Mahmoud H, Mnaa S. (2010). Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol 48:803–6
- Silva CHTPD, Sobrinho TJDSP, Lima DDCA, Amorim ELCD. (2011). Antioxidant capacity and phenolic content of Caesalpinia pyramidalis Tul. and Sapium glandulosum (L.) Morong from Northeastern Brazil. Molecules 16:4728–39
- Temple AR, Lynch JM, Vena J, et al. (2007). Aminotransferase activities in healthy subjects receiving three-day dosing of 4, 6, or 8 grams per day of acetaminophen. Clin Toxicol (Philadelphia, Pa.) 45:36–44
- Tsuchiya H. (1999). Effects of green tea catechins on membrane fluidity. Pharmacology 59:34–44
- Walia A, Malan R, Saini S, et al. (2011). Hepatoprotective effects from the leaf extracts of Brassica juncea in CCl4 induced rat model. Der Pharm Sinica 2:288–99
- Wanasundara J. (2008). Potential of mustard as a protein Crop. Agric Agric-Food Canada Available from: http://www.cropweek.com/presentations/2008/2008-jan09-mustard-wanasundara.pdf [last accessed 5 May 2014]