Abstract
Context: Psoralen, an active ingredient from Fructus Psoraleae (FP), is used in Traditional Chinese Medicine (TCM) to treat bone diseases. However, the effect of psoralen on cartilage is unknown.
Objective: To investigate the effects of psoralen on chondrocytes isolated from rats.
Materials and methods: Chondrocytes were treated with different concentrations of psoralen (1, 10, and 100 μM) in vitro at 3-d and 9-d intervals. MTS assay, Alcian blue colorimetry, western blotting, and qRT-PCR, respectively, were used to evaluate the effects of psoralen on cell viability, glycosaminoglycan (GAG) synthesis, collagen synthesis, and cartilage-specific gene expression.
Results: Psoralen dosages of 1–10 μM exhibited low cytotoxicity toward chondrocytes. However, a dosage of 100 μM suppressed the proliferation of chondrocytes. Different concentrations of psoralen treatments on chondrocytes revealed that GAG and Type II collagen synthesis increased, especially at 100 μM, by 0.39-fold and 0.48-fold, respectively, on day 3, and by 0.51-fold and 0.56-fold, respectively, on day 9. Similarly, gene expression of Type II collagen, aggrecan, and SOX-9 were all up-regulated on days 3 and 9, particularly aggrecan which increased significantly by 9.37-fold and 7.32-fold at 100 μM. Additionally, Type I collagen was inhibited both in gene expression and in protein synthesis.
Conclusion: The results showed that psoralen promotes cartilaginous extracellular matrix (ECM) synthesis, as well as increased cartilaginous gene expression, and it may be a useful bioactive component for activating the cartilaginous cellular functions of chondrocytes.
Introduction
Cartilage is the dense, connective tissue located in the joints between bones, in the ears, intervertebral discs, as well as other areas in the body (Jasin, Citation1995; Thomas et al., Citation2013). It is not as hard and rigid as bone, but it is stiffer and less flexible than muscle. Cartilage structural components include chondrocytes and ECM. Hyaline cartilage is located on the articular surfaces of bone. It is composed of an ECM with sparse distribution of chondrocytes (highly specialized cells). Articular cartilage provides a smooth, lubricated surface for movement and reduces friction during load transmission. Due to insufficient blood supply, lack of lymphatics, nerves and low cell density, articular cartilage carries a limited capacity for intrinsic healing and repair. The harsh biochemical environment that articular cartilage is subjected to also makes them susceptible to injuries, trauma, and age-related diseases such as osteoporosis (OP) (Lauder et al., Citation2001) and osteoarthritis (OA) (Bobinac et al., Citation2013; Calvo et al., Citation2007).
Chondrocytes are responsible for the production of ECM. OP and OA occur when the dynamic equilibrium between normal cartilage ECM synthesis and degradation is broken. Several strategies for treating articular cartilage injuries have been designed including the use of grafts and growth hormones, such as transforming growth factor (TGF-β) and insulin-like growth factor (IGF) (Bosnakovski et al., Citation2006; Kawamura et al., Citation2005). However, these techniques are very expensive and carry a low success rates. Therefore, the need for cost-effective and highly successful techniques is necessary.
Fructus Psoraleae (FP), the seeds of Psoralea corylifolia L. (Leguminosae), has been widely used in TCM to treat osteoporosis, bone fractures, and osteomalacia. The extracts from FP include coumarins, flavonoids, monoterpene phenols, saponins, polysaccharides, and lipoids (Qiao et al., Citation2007; Xu et al., Citation2012; Zhao et al., Citation2005).
Psoralen is the main ingredient in FP. A previous report demonstrated that psoralen can trigger osteogenesis (Tang et al., Citation2011). Another report stated that psoralen had a stimulatory effect on local new bone formation in vivo (Wong & Rabie, Citation2011). Recently, it has been reported that prenyl compounds of FP, including psoralen, may be essential for osteoblast proliferation and differentiation (Li et al., Citation2013). Some research has indicated that the phenomenon of chondrogenesis resembled the osteogenesis process and that they have similar molecular mechanisms concerning Bone Morphogenetic Protein (BMP) signaling (Ichinose et al., Citation2013; Nepal et al., Citation2013; Tang et al., Citation2011). Although the psoralen stimulatory effect on osteogenesis is confirmed, its effect on cartilage is unknown. Therefore, we designed the experiment on chondrocytes treated with different concentrations of psoralen (1, 10, and 100 μM) in vitro. In this study, we hypothesize that psoralen can have a stimulatory effect on the cartilaginous cellular functions of chondrocytes resulting in the production of cartilaginous ECM.
This in vitro study was designed to investigate the activating effects of psoralen on chondrocytes. We examined the immunophenotype, morphology, and proliferation of cells after treating them with different concentrations of psoralen. We then investigated the effect of psoralen on glycosaminoglycan (GAG) synthesis, collagen synthesis, and gene expression of cartilage-specific genes. Post-treatment gene expression levels of Type II collagen, aggrecan, and SOX-9 were all increased. Additionally, synthesis of cartilaginous ECM Type II collagen and GAG was also increased. These results suggest that there is an in vitro activation of chondrocytes after psoralen treatment and offer some therapeutic prospects for OP, OA, and other diseases affecting the cartilage.
Materials and methods
Isolation and identification of articular chondrocytes
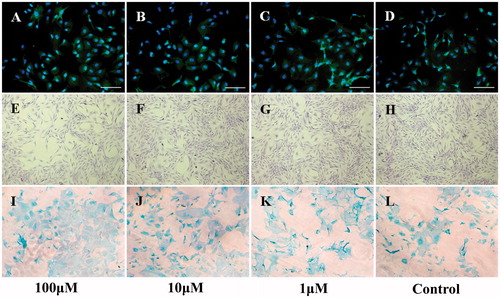
Articular cartilage from the knee was isolated from healthy Sprague–Dawley rats (Wang et al., Citation2013). The cartilage was then minced, and the pieces were digested with 2 mg/ml of collagenase type II (Gibco) under 5% CO2 at 37 °C for 4 h. After digestion, any undigested tissue was removed using a 70-mm nylon sieve. The cell suspensions were centrifuged at 300 g for 5 min. The supernatant was discarded, and the cell pellets were re-suspended using low-glucose DMEM (Gibco, Carlsbad, CA) medium supplemented with 10% FBS, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. The cultured cells were recorded as passage P0. The second passage (P2) was used for the study, and the culture medium was changed every 2 d. To observe the cell morphology, psoralen-treated cells and the controls were fixed in 4% PFA for 30 min. Afterwards, they were stained with Alcian blue and crystal violet solution. The cells were also stained with rabbit anti-Type II collagen (1:100, bs-8859R, Bioss, Woburn, MA) antibody for immunofluorescent identification. Alexa Fluor 488 dye-labeled goat anti-rabbit antibody was used as a secondary identifying antibody.
Preparation of psoralen-conditioned culture medium
Psoralen (NIFDC, batch number 110739-201115, 99.3% pure) was first dissolved in DMSO (Sigma, St. Louis, MO) to prepare a psoralen-conditioned medium stock solution. Low-glucose DMEM (Gibco, Carlsbad, CA) plus 10% FBS (Gibco, Carlsbad, CA) were used to prepare psoralen concentrations of 100, 10, and 1 μM (DMSO concentration was 0.1% in the conditioned culture medium). All the preparations were replicated three times.
Cell proliferation assay
Cell proliferation was determined by the MTS assay (Promega, Madison, WI, G358C) according to the manufacturer’s instructions. Briefly, a standard curve was first established to test the linear relationship between the cell number and the OD value. Then, chondrocytes were seeded at a density of 2 × 104 cells/well in a 24-well plate. The cells were then treated with low-glucose DMEM (Gibco, Carlsbad, CA) culture medium supplemented with 10% FBS containing different psoralen concentrations. The cells were then cultured for 1–9 d. At the end of each time interval, cell samples were washed with PBS and incubated with serum-free medium containing 10% MTS reagent. After 3 h of incubation at 37 °C under 5% CO2, aliquots were pipetted into a 96-well plate and measured at 490 nm using an enzyme-labeling instrument (Bio-Rad, Berkeley, CA).
GAG determination
Approximately 5 × 105 chondrocytes were seeded into 6 cm dishes. The cells were cultured in psoralen-conditioned culture medium (for the treatment group) or in normal culture medium (for the control group) for 3 and 9 d. After the treatment, cells were washed with PBS and lysed using cell lysis buffer (Beyotime, Shanghai, China). Cell numbers were counted for data normalization. GAG was measured by Alcian blue colorimetry. The samples were briefly incubated in Alcian blue acid dyes at 37 °C for 30 min. The plates were then measured at 490 nm. The assays were replicated three times.
Western blotting assay
Approximately 5 × 105 chondrocytes were seeded into 6 cm dishes. The cells were then treated with normal culture medium or psoralen-conditioned medium and cultured for 3 and 9 d. The chondrocytes were lysed in lysis buffer (Beyotime, Shanghai, China) containing 1% PMSF protease inhibitors. They were then centrifuged at 12 000 rpm for 10 min to remove cell debris. Protein concentration was determined by BCA Protein Assay Kit (Beyotime, Shanghai, China). All the samples were separated by electrophoresis in 8% SDS-PAGE gel (Bio-Rad, Berkeley, CA) at 110 V for 60 min. The proteins were then transferred to a PVDF membrane (Millipore, Billerica, MA) using the wet-transfer method at 200 mA for 3 h. After transfer, the membrane was washed with TBST solution and then blocked with 5% skim milk for 1 h at 37 °C. It was then incubated with rabbit anti-Type I collagen (1:500, Bioss, Woburn, MA, bs-0578R), Type II collagen (1:500, Bioss, Woburn, MA, bs-8859R), and β-actin primary antibodies overnight at 4 °C. Afterwards, the membrane was incubated with HRP-conjugated goat anti-rabbit secondary antibody for 1 h at 37 °C. Finally, ECL (Beyotime, Shanghai, China) was used to detect targeted proteins which were imaged using the VersaDoc imaging system (Bio-Rad, Berkeley, CA).
Quantitative RT-PCR assay
Chondrocytes were seeded at a density of 1 × 105 per well into 6-well plates. The cells were then cultured in normal culture medium or psoralen-conditioned medium. Gene expression was evaluated by qRT-PCR after 3 and 9 d. Total RNA was extracted using the total RNA extraction kit (BioTeke Corporation, Mainland, China) according to the manufacturer’s instructions. First strand cDNA synthesis was followed with the RT-PCR protocol (Thermo Scientific Rt-First Strand cDNA Synthesis Kit, K1622, Thermo Scientific, Waltham, MA). PCR conditions were 65 °C for 5 min, then 42 °C for 60 min, and the termination at 70 °C for 5 min. SD rat-specific primers were designed using the NCBI Primer-BLAST () and synthesized by Invitrogen (Carlsbad, CA). The SsoAdvanced Universal SYBR Green Supermix (No. 1725264, Bio-Rad, Berkeley, CA) and CFX96 Real-Time PCR Detection System (Bio-Rad, Berkeley, CA) were used to perform qRT-PCR. The real-time PCR condition was as follows: 95 °C for 30 s followed by 39 cycles of a two-temperature program for 5 s at 95 °C and 30 s at 60 °C. The gene expression levels were evaluated by the 2−ΔΔct method.
Table 1. List of primer sequences for real-time PCR.
Statistical analysis
All analyses were conducted using SPSS 17.0 (SPSS Inc., Chicago, IL), expressed as mean ± standard deviation (SD). Statistical comparisons were performed using one-way ANOVA. A p value of <0.05 was accepted as statistically significant.
Results
Influence of psoralen on cell immunophenotype and morphology
Type II collagen immunofluorescent () and Alcian blue staining () were used to study the immunophenotype of cells after treatment with different psoralen concentrations. As shown in , there was no significant effect on immunophenotype in neither the treatment nor the control group. Morphologically, the cells grew well and were polygonal, elliptical, and spindle-shaped, shown by crystal violet staining (). This test confirms that the cells we isolated from the knee articular cartilage were indeed chondrocytes. However, the cell density in the psoralen-treated group was lower than the control group, especially for the high-dosage treatment. These results indicate that psoralen treatment did not influence the cell immunophenotype and cell morphology. Nonetheless, slow growth was observed in the 100 μM concentration group.
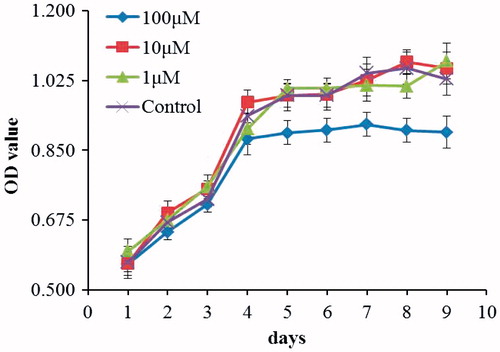
Psoralen effect on cell viability
The effect of psoralen on cell viability was examined using the MTS assay. The results of the MTS assay at different psoralen concentrations are shown in . In this study, the different psoralen dosages of 1–100 μM exhibited no cytotoxic effect on chondrocytes. Moreover, on day 4, the 100-μM treatment group showed stagnated proliferation compared with the control group. These results are in agreement with the cell morphology results ().
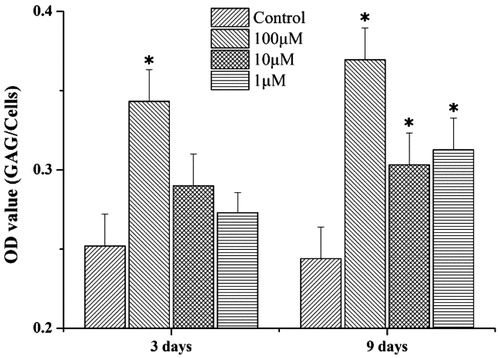
Glycosaminoglycan content
According to the spectrophotometry reading, the psoralen-treated group showed an increase in GAG synthesis compared with the control group (). On day 3, GAG content in the cell was dose dependently increased by 0.39-fold (p < 0.05), 0.15-fold, and 0.08-fold. On day 9, GAG content revealed a 0.51-fold (p < 0.05), 0.25-fold (p < 0.05), and 0.28-fold (p < 0.05) increase. The results are in a dose-decreasing order.
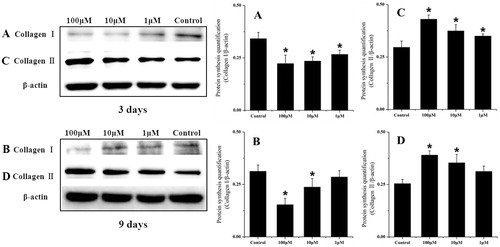
Collagen synthesis
To confirm the collagen synthesis of chondrocytes treated with psoralen, western blotting was performed () on days 3 and 9 after treatment. After 3 d, the expression of Type I collagen was down-regulated by 0.35-fold (p < 0.05), 0.31-fold (p < 0.05), and 0.21-fold (p < 0.05) in a dose-dependent manner. By contrast, the expression of Type II collagen showed a 0.48-fold (p < 0.05), 0.3-fold (p < 0.05), and 0.21-fold (p < 0.05) increase. After 9 d, Type I collagen synthesis was still suppressed, showing a 0.5-fold (p < 0.05), 0.24-fold (p < 0.05), and 0.18-fold decrease, while the expression of Type II collagen increased by 0.56-fold (p < 0.05), 0.41-fold (p < 0.05), and 0.21-fold. The results indicate that psoralen treatment could stimulate Type II collagen expression while inhibiting the Type I collagen synthesis in rat chondrocytes in vitro.
Figure 4. Western blotting analysis of Collagen I and Collagen II protein synthesis in chondrocytes treated with different psoralen concentrations at day 3 (A: collagen I and C: collagen II) and day 9 (B: collagen I and D: collagen II). The graphs represent band intensity (target/β-actin), which were analyzed with the software Quantity one (SPSS Inc., Chicago, IL) (mean ± sd, n = 3). *p < 0.05 was accepted as statistically significant.
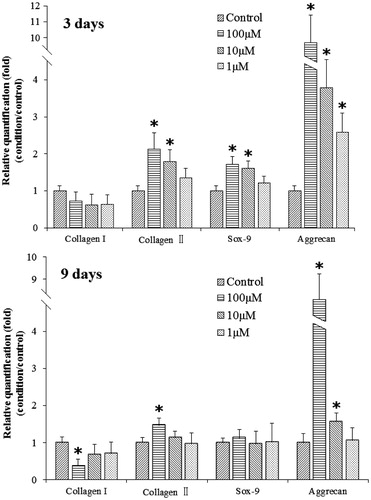
Effect of psoralen on gene expression
As shown in , the gene expression level of Type II collagen, aggrecan, and SOX-9 was dose dependently increased after treatment with different concentrations of psoralen on day 3 (, 3d results). Among them, Type II collagen was increased by 1.13-fold (p < 0.05) with the 100 μM dosage, 0.59-fold (p < 0.05) with the 10 μM dosage, and 0.31-fold with the 1 μM dosage. Aggrecan expression was increased by 9.37-fold (p < 0.05), 2.85-fold (p < 0.05), and 1.58-fold (p < 0.05). SOX-9 was increased by 0.72-fold (p < 0.05), 0.61-fold (p < 0.05), and 0.22-fold. Type I collagen gene expression was not affected. After 9 d, SOX-9 gene expression was not affected by psoralen treatments, and only high dosages of psoralen enhanced Type II collagen and aggrecan expression. Type II collagen was increased by 0.46-fold (p < 0.05) with the 100 μM dosage while aggrecan expression was increased by 7.32-fold (p < 0.05) with the 100 μM dosage and 0.58-fold (p < 0.05) with the 10 μM dosage. On the other hand, Type I collagen was down-regulated 9 d post-treatment. A significant effect was observed with the 100 μM dosage.
Discussion and conclusion
In our experiment, we investigated the activating effects of psoralen on chondrocytes in vitro, providing a reference for potential research on cartilage protection and treatment of cartilage diseases. We found that psoralen minimally influences the cell immunophenotype and morphology and has no apparent cytotoxic effects at concentrations of 1, 10, and 100 μM. However, the cell density of the 100 μM psoralen concentration treatment group was slightly lower than the control group. Moreover, according to MTS assay, we found that the proliferation of chondrocytes reached stagnation at a psoralen concentration of 100 μM compared with the control group on day 4. This cell proliferation result was in agreement with previous cell density findings.
It has been reported that psoralen stimulates osteogenesis and could promote new bone formation (Tang et al., Citation2011; Wong & Rabie, Citation2011). In the present study, we found that psoralen treatments on chondrocytes not only increased the expression of cartilage related genes, such as Type II collagen, aggrecan, and SOX-9, but also promoted GAG and Type II collagen synthesis. These results suggest the possibility of chondrocytes producing cartilage with psoralen treatments. In addition, the decreased Type I collagen expression might prove that psoralen would not induce the differentiation of chondrocytes or change their immunophenotype. Interestingly, the trend of gene expression and protein synthesis is related to previous findings of cell proliferation. These results showed that the chondrocytes had increased ECM gene expression instead of cell proliferation at psoralen concentrations of 100 μM on day 3. It indicates that cells start to synthesize ECM after a period of time with psoralen treatment.
Furthermore, in the early stages of psoralen treatments, cartilage-related genes were up-regulated, especially aggrecan, which is known as the cartilage-specific proteoglycan core protein (CSPCP) in an integral part of the ECM in cartilaginous tissue. It has been reported that proteoglycan core protein enables the cartilage to withstand compression (Ingavle et al., Citation2013; Papagiannopoulos et al., Citation2006; Roughley, Citation2006). Aggrecan expression was significantly increased to almost 10-fold compared with the control group. These promising effects of psoralen provide hope for the treatment of cartilage diseases (Rizkalla et al., Citation1992). However, as time progressed, cartilage-related gene expression weakened; some genes showed no significant changes in expression. We suppose that this was due to cell regulation or cell senescence, resulting in a decrease of gene transcription.
In conclusion, our research demonstrates that psoralen was shown to increase gene expression of Type II collagen, aggrecan, and SOX-9, while promoting GAG and Type II collagen protein synthesis. The results indicate that psoralen can activate chondrocytes in vitro and provide potential therapies for OP, OA, and other cartilage diseases.
Although we showed that psoralen can activate chondrocytes from articular cartilage of the rat knee, there are some limitations to this study. We did not investigate other types of cartilage, such as elastic cartilage and fibrocartilage. It is unknown whether there are any differences in the effect of psoralen on these other types. Second, there is a lack of human studies. The source of our specimens was rats, since it is difficult to obtain pure chondrocytes from humans. Nevertheless, we propose that psoralen could be a key bioactive component, at least in rat chondrocytes in vitro.
Declaration of interest
The authors have declared that no conflict of interest exists. This work was supported by National Innovation and Attracting Talents Project (“111” Project) (B06023), College of Biomedical Engineering, Chongqing University, and National Natural Science Foundation of China (81274085).
References
- Bobinac D, Marinovic M, Bazdulj E, et al. (2013). Microstructural alterations of femoral head articular cartilage and subchondral bone in osteoarthritis and osteoporosis. Osteoarthr Cartil 21:1724–30
- Bosnakovski D, Mizuno M, Kim G, et al. (2006). Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 93:1152–63
- Calvo E, Castaneda S, Largo R, et al. (2007). Osteoporosis increases the severity of cartilage damage in an experimental model of osteoarthritis in rabbits. Osteoarthr Cartil 15:69–77
- Ichinose S, Tagami M, Muneta T, et al. (2013). Comparative sequential morphological analyses during in vitro chondrogenesis and osteogenesis of mesenchymal stem cells embedded in collagen gels. Med Mol Morphol 46:24–33
- Ingavle GC, Frei AW, Gehrke SH, Detamore MS. (2013). Incorporation of aggrecan in interpenetrating network hydrogels to improve cellular performance for cartilage tissue engineering. Tissue Eng Part A 19:1349–59
- Jasin HE. (1995). Structure and function of the articular cartilage surface. Scand J Rheumatol Suppl 101:51–5
- Kawamura K, Chu CR, Sobajima S, et al. (2005). Adenoviral-mediated transfer of TGF-beta1 but not IGF-1 induces chondrogenic differentiation of human mesenchymal stem cells in pellet cultures. Exp Hematol 33:865–72
- Lauder RM, Huckerby TN, Brown GM, et al. (2001). Age-related changes in the sulphation of the chondroitin sulphate linkage region from human articular cartilage aggrecan. Biochem J 358:523–8
- Li WD, Yan CP, Wu Y, et al. (2013). Osteoblasts proliferation and differentiation stimulating activities of the main components of Fructus Psoraleae corylifoliae. Phytomedicine 21:400–5
- Nepal M, Li L, Cho HK, et al. (2013). Kaempferol induces chondrogenesis in ATDC5 cells through activation of ERK/BMP-2 signaling pathway. Food Chem Toxicol 62:238–45
- Papagiannopoulos A, Waigh TA, Hardingham T, Heinrich M. (2006). Solution structure and dynamics of cartilage aggrecan. Biomacromolecules 7:2162–72
- Qiao CF, Han QB, Song JZ, et al. (2007). Chemical fingerprint and quantitative analysis of Fructus psoraleae by high-performance liquid chromatography. J Sep Sci 30:813–18
- Rizkalla G, Reiner A, Bogoch E, Poole AR. (1992). Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest 90:2268–77
- Roughley PJ. (2006). The structure and function of cartilage proteoglycans. Eur Cell Mater 12:92–101
- Tang DZ, Yang F, Yang Z, et al. (2011). Psoralen stimulates osteoblast differentiation through activation of BMP signaling. Biochem Biophys Res Commun 405:256–61
- Thomas JT, Schneider BS, Frank EL, Krizan SJ. (2013). Cartilage repair and replacement in the knee: A regulatory perspective. Trends Biotechnol 31:665–7
- Wang J, Zhu X, Liu L, et al. (2013). Effects of strontium on collagen content and expression of related genes in rat chondrocytes cultured in vitro. Biol Trace Elem Res 153:212–19
- Wong RW, Rabie AB. (2011). Effect of psoralen on bone formation. J Orthop Res 29:158–64
- Xu MJ, Wu B, Ding T, et al. (2012). Simultaneous characterization of prenylated flavonoids and isoflavonoids in Psoralea corylifolia L. by liquid chromatography with diode-array detection and quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 26:2343–58
- Zhao L, Huang C, Shan Z, et al. (2005). Fingerprint analysis of Psoralea corylifolia L. by HPLC and LC-MS. J Chromatogr B Analyt Technol Biomed Life Sci 821:67–74