Abstract
Context: Syzygium cumini (L.) Skeels (Myrtaceae), commonly known as jamun, is an Indian plant, traditionally well known for its medicinal properties including antidiabetic activity.
Objective: To isolate the antidiabetic compounds from Syzygium cumini seeds and evaluate their activity using aldose reductase (AR) and protein-tyrosine phosphatase 1B (PTP1B) inhibition assays.
Materials and methods: The dried seeds were extracted with methanol and partitioned with ethyl acetate, butanol, and water. The extracts were screened for antidiabetic activity at a concentration of 100 µg/mL using in vitro AR and PTP 1B inhibition assays.
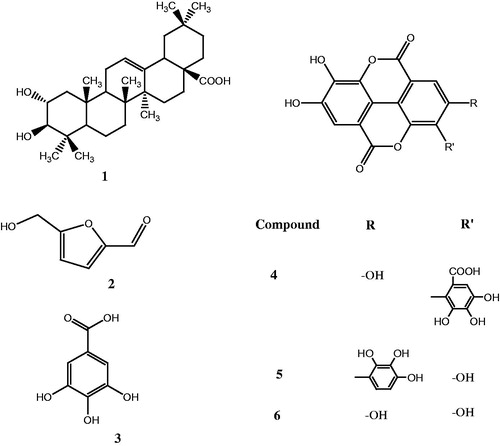
Results and discussion: The highly enriched fractions obtained from broad ethyl acetate fraction yielded maslinic acid (1), 5-(hydroxymethyl) furfural (2), gallic acid (3), valoneic acid dilactone (4), rubuphenol (5), and ellagic acid (6). Structures were elucidated by 1H-NMR and 13C-NMR. The initial ethyl acetate fraction showed AR inhibitory activity with the IC50 value of 2.50 μg/mL and PTP1B enzyme inhibition with the IC50 value of 26.36 μg/mL. Compounds 3, 4, 5, and 6 were found to inhibit AR with IC50 values of 0.77, 0.075, 0.165, and 0.12 μg/mL while the compounds 4, 5, and 6 inhibited PTP1B with IC50 values of 9.37, 28.14, and 25.96 μg/mL, respectively.
Conclusion: The results of this study demonstrate that the isolated constituents show promising in vitro antidiabetic activity and, therefore, can be candidates for in vivo biological screening using relevant models to ascertain their antidiabetic activity.
Introduction
Syzygium cumini (L.) Skeels (Myrtaceae), commonly known as jamun, is known for its beneficial effects in diabetes and is used as one of the alternative natural healing systems in the Ayurveda, Unani, and Chinese medicines (Baliga et al., Citation2013; Chaudhary & Mukhopadhyay, Citation2012). It is a widely distributed forest tree in India including Andaman Islands, Bangladesh, Burma, and Sri Lanka and other tropical and sub-tropical regions of the world (Kumar et al., Citation2009). The plant has been reported to contain anthocyanins, glucoside, ellagic acid, isoquercetin, kaemferol, and myrecetin (Aqil et al., Citation2012; Kumar et al., Citation2009; Sagrawat et al., Citation2006; Simoes-Pires et al., Citation2009). The seeds are claimed to contain alkaloids, jambosine, and glycoside, jamboline or antimellin, which halt the diastatic conversion of starch into sugar (Ayyanar & Babu, Citation2012; Gowri & Vasantha, Citation2010; Srivastava & Chandra, Citation2013).
All parts of S. cumini tree, namely seeds, fruits, leaves, flower, and bark, are used in folklore medicine (Ayyanar et al., Citation2013; Saravanan & Pari, Citation2008). Pharmacological activities like chemoprotective, hypoglycaemic, hyperglycemic, analgesic, anti-inflammatory, anti-allergic, antihyperlipidemic, antiplaque, antimicrobial, antidiarrheal, anti-oxidant, gastro-protective, astringent to bowels, and antibacterial (Arun et al., Citation2011; Banerjee & Narendhirakannan, Citation2011; Bona et al., Citation2014; Brito et al., Citation2007; Helmstadter, Citation2007; Jadhav et al., Citation2009; Khan et al., Citation2011; Muruganandan et al., Citation2001; Rekha et al., Citation2010; Sharma et al., Citation2006) have been reported in the literature. In modern literature, S. cumini seed powder and its ethanolic extract have been proved to possess antidiabetic properties in type 2 diabetic rats (Bhuyan et al., Citation2010). Hydrolyzable tannins of jamun have recently been reported for α-glucosidase inhibitory activity (Omar et al., Citation2012).
Although S. cumini seeds are known to have antidiabetic activity (Ponnusamy et al., Citation2011; Oliveira et al., Citation2005; Rodrigues et al., Citation2012; Sharma et al., Citation2008), no systematic study has been carried out to identify the constituents responsible for inhibition of aldose reductase (AR) and protein-tyrosine phosphatase 1B (PTP-1B) enzymes. Preliminary studies performed in our lab indicated that the methanolic extract of jamun seeds inhibits AR and PTP-1B. As an extension of this study, we have performed bioactivity-guided fractionation of methanolic extract of S. cumini seeds to identify the active constituents responsible for the inhibition of these enzymes using in vitro models. For the first time, we report rubuphenol and valoneic acid dilactone as antidiabetic bioactive compounds of jamun seeds using in vitro AR and PTP-1B inhibition assays.
Materials and methods
Chemicals
Potassium dihydrogen orthophosphate, dipotassium hydrogen orthophosphate, 2-marceptoethanol, sodium chloride, EDTA disodium salt dihydrate, and lithium sulfate were purchased from Himedia, Bombay, India. dl-Glyceraldehyde, nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), and quercetin hydrate were procured from Sigma, New Delhi, India. Ethanol was purchased from Changshu Yangyuan Chemicals, Jiangsu, China, while dimethyl sulfoxide and sodium hydroxide were purchased from Ranbaxy, Gurgaon, India. Bovine serum albumin (BSA) solution was purchased from New England Biolabs, Ipswich, MA. PTP-1B was purchased from Millipore, Mumbai, India. p-Nitrophenyl phosphate (p-NPP), 4 -(2-hydroxyethyl)piperazine-1-ethanesulonic acid (HEPES), and dl-dithiothreitol (DTT) were purchased from Fluka, Newport News, VA.
Plant material
Fruits of S. cumini were collected from Bangalore, India, in December 2010. The plant material was identified by National Institute of Science Communication and Information Resources (NISCAR), New Delhi and Dr. P. Santhan, in-house taxonomist, Pharmacognosy Department, R&D Centre, Natural Remedies Pvt. Ltd, Bangalore, India. The seeds were separated from the juicy pericarp, sun dried, and stored. A voucher specimen (NRPL-299) was deposited at the herbarium of Botanical Survey of India, southern circle, Coimbatore, Tamil Nadu, as well as in the in-house herbarium.
Instrumentation
Melting point was determined with a Thermonik melting point apparatus manufactured by Campbell Electronics, Mumbai, India. An IR spectrum (KBr) was recorded using a Shimadzu system IR Prestige-21 spectrometer and UV spectrum with a Shimadzu-2401 PC spectrometer (Shimadzu Corporation, Kyoto, Japan). 1H- and 13C-NMR spectra were recorded with a Bruker AVANCE II 400 spectrometer (Bruker Corporation, Chennai, India). Chemical shifts (parts per million) relative to internal tetramethylsilane were used as a reference. 1H–1H COSY, HMQC, and HMBC NMR experiments were performed on the same spectrometer. MS spectra were measured with a LCQ Fleet-Thermo Fisher Scientific instrument (Thermo Fisher Scientific, Inc., Waltham, MA).
Analytical HPLC system composed of Shimadzu 2010CHT HPLC (Shimadzu Corporation, Kyoto, Japan) consisting of a quaternary pump with a vacuum degasser, thermostatic column compartment, autosampler, UV detector, and reversed-phase column Phenomenex Luna C18 (5 μ particle size, 250 × 4.6 mm) (Phenomenex Inc., Torrance, CA). Preparative HPLC consisted of the Shimadzu model VP LC-8 A HPLC system with a SPD-M10A detector and Kromasil C-18 column (7 μm, 21.2 × 250 mm2) (Sigma-Aldrich, St. Gallen, Switzerland). Silica gel (60–120 mesh, ASTM; Merck Co., White House Station, NJ), Sephadex™ LH-20 (18–111 μm, GE Healthcare, El Paso, TX), and Diaion resin HP-20 (Labion, Kitten) were used for column chromatography. TLC was performed on silica gel 60 F254 plates purchased from Merck (White House Station, NJ).
Extraction and isolation
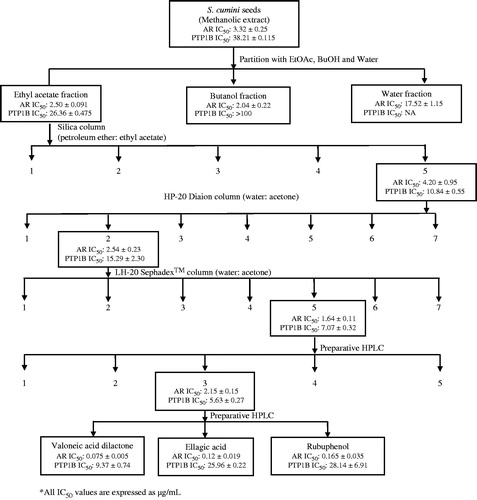
The dried seeds of S. cumini (20 kg) were refluxed three-times with methanol to obtain methanolic extract (4 kg). The methanolic extract was suspended in H2O and partitioned successively with ethyl acetate (EtOAc) and n-butanol (n-BuOH). The EtOAc fraction (500 g) was subjected to column chromatography (CC) over silica gel and eluted with a petroleum ether:EtOAc step gradient to give five fractions (Fr. 1–Fr. 5). All the fractions obtained were tested for biological activity (AR and PTP-1B inhibition assays) and also monitored by TLC and HPLC to select the fraction to be subjected to the next rounds of bioactivity-guided fractionation. Fraction 5 was selected and subjected to CC over Diaion HP-20 eluted with a H2O:CH3COCH3 step gradient to give seven fractions (Fr. 5-1–Fr. 5-7). Active fraction 5-2 was applied to CC over Sephadex™ LH-20 eluted with a H2O:CH3COCH3 step gradient to give seven fractions (Fr. 5-2-1–Fr. 5-2-7). Compounds 1 (140 mg) and 2 (150 mg) were isolated from Fr. 5-2-3 by repeated silica column chromatography. Compound 3 (230 mg) was isolated from Fr. 5-2-1 by repeated silica column chromatography. Compounds 4 (160 mg), 5 (137 mg), and 6 (142 mg) were isolated from Fr. 5-2-5 by repeated preparative HPLC [Kromasil C-18 column (7 μm, 21.2 × 250 mm) acetonitrile:H2O {gradient elution, at a flow rate of 20 mL/min}, λmax 205 and 270 nm] (). The purity of isolated compounds was checked by HPLC, and its spectroscopic data were recorded with IR, mass, and NMR spectrometers.
HPLC analysis of isolated compounds
Standard compounds were prepared at a concentration of 0.1 mg/mL in methanol. Dried and finely milled plant tissues (∼3.0 g) of S. cumini were extracted with the aid of boiling in a water bath and sonication in 50 mL methanol. The supernatant was transferred to a flask. The procedure was repeated three-times. The pooled extracts were concentrated under vacuum and re-dissolved in 100 mL methanol for HPLC analysis.
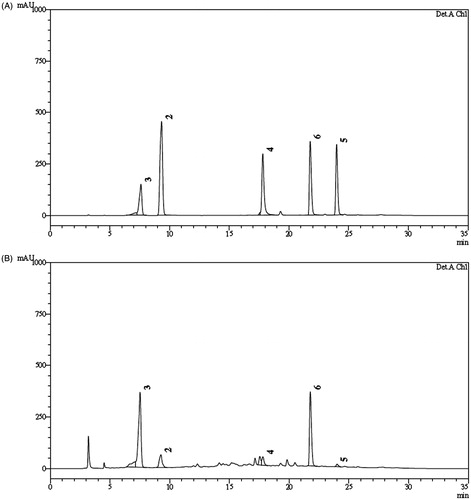
The HPLC method used a mobile phase consisting of mobile phase (A) and acetonitrile (B). The mobile phase (A) was prepared by dissolving 0.136 g potassium dihydrogen orthophosphate and 0.5 mL of orthophosphoric acid in 900 mL HPLC grade water and made up the solution to 1000 mL. The solution was filtered using a 0.45-µ membrane filter and degassed just before use. The following gradient elution was used: 0 min, 5% B; in next 20 min to 25% B; and maintained at 25% B for 5 min, decreased the solvent B to 5% in next 5 min followed by an equilibration period of 5 min. The flow rate was 1.0 mL/min, and the injection volume was 20 μL. The eluents were detected at 270 nm. The column temperature was maintained at 30 °C. The content of compounds 2–6 in methanol extract of seeds was quantified by HPLC and found to be 0.23, 2.15, 0.12, 0.04, and 0.92% ().
In vitro method using rat lens homogenate
Preparation of lens homogenate
Crude AR was isolated from rat lens according to the method described by Lee (Citation2002) with few modifications. Male Wistar albino rats weighing 150 g were selected and sacrificed by cervical dislocation. An incision was made on the eye balls and the lenses were enucleated through the posterior approach. The lenses were immediately placed in a small amount of 135 mM potassium phosphate buffer (pH 7.0) containing 10 mM 2-mercaptoethanol (1:12 volumes) to avoid drying of lens. All the above procedures were carried out on ice (4 °C). The lenses were homogenized and the homogenate was centrifuged for 30 min at 15 000 rpm, and the supernatant was collected and stored at −80 °C in the Forma™ 900 series −86 °C upright ultra-low temperature freezer, model 902 and used as the enzyme. The protein content was determined by the Biuret method (Weichselbaum, Citation1946).
AR inhibitory activity
AR inhibitory activity was assayed spectrophotometrically according to the method described by Hayman and Kinoshita (Citation1965) with few modifications. In the reaction mixture (250 µL), 670 mM potassium phosphate buffer (pH 6.2) was diluted to a final concentration of 67 mM by the addition of 0.4 M lithium sulfate, 150 µM NADPH, 220 µM dl-glyceraldehyde, 50 µL of crude enzyme isolated from rat lens, and different concentrations of quercetin test sample. Appropriate blanks were prepared for control and each of the test samples, without dl-glyceraldehyde. The reaction was initiated by adding NADPH and incubated at 37 °C. The decrease in absorbance at 340 nm was recorded for 10 min at 1 min interval using a FLUOstar multimode plate reader from BMG Labtech, Offenburg, Germany.
The raw data obtained from the above kinetic assay was exported from Fluostar Optima software (BMG Labtech, Offenburg, Germany) to an excel sheet. A change in absorbance for 10 min was calculated for blank, control, reference standard, and sample wells. Activity and percent inhibition were calculated as follows:
Activity of control = ΔAcontrol−ΔAblank
Activity in the presence of ref. standard = ΔAref. standard−ΔAref. standard blank
Activity in the presence of sample = ΔAsample−ΔAsample blank
% Activity = (activity sample × 100)/activity control
% Inhibition = 100−(% activity)
where ΔA is the change in absorbance in 10 min.
The IC50 values were calculated by using the Finney software (BMC Software, Houston, TX).
Protein tyrosine phosphatase 1B inhibitory activity
PTP-1B inhibition assay was carried out according to the method described by Burke et al. (Citation1996) with few modifications. The enzyme activity was performed using p-nitrophenyl phosphate (p-NPP) as a substrate. Each 384-well in a final volume of 50 μL contained BSA (45 μg/mL), PTP-1B enzyme (0.817 U) in a buffer containing 25 mM HEPES (pH 7.2), 50 mM NaCl, 2.5 mM EDTA, and 5 mM dithiothreitol (DTT) with or without test compounds. The reaction mixture was pre-incubated at 37 °C for 10 min. The reaction was initiated by adding 5 μL of 12.75 mM p-NPP to the pre-incubation mixture. The amount of p-nitrophenol produced was estimated by measuring the absorbance at 405 nm. The non-enzymatic hydrolysis of p-NPP was corrected by measuring the increase in absorbance at 405 nm obtained in the absence of PTP-1B enzyme. The product formation was monitored using a multiplate reader (PHERAstar, BMG Labtech, Offenburg, Germany). The percent inhibition was calculated as follows:
The IC50 values were calculated by using the Finney software (BMC Software, Houston, TX).
Results and discussion
In the course of screening for AR and PTP1B inhibitors from natural resources, an EtOAc-fraction of methanolic extract of S. cumini seeds was found to inhibit AR and PTP-1B showing IC50 values of 2.50 ± 0.091 and 26.36 ± 0.475 µg/mL, respectively, and was selected for further investigation. To identify the active principles, bioassay-guided isolation was performed, resulting in isolation of compounds 1–6, i.e., maslinic acid, 5-(hydroxymethyl)furfural, gallic acid, valoneic acid dilactone, rubuphenol, and ellagic acid ().
Figure 3. Representative chromatograms of (A) standard mixture and (B) methanolic extract of S. cumini seeds.
Compounds 5-(hydroxymethyl)furfural, valoneic acid dilactone, and rubuphenol had undergone detailed spectral analysis of 1D and 2D NMR (1H–1H COSY, HMQC, HMBC, and NOESY) which was in agreement with previously reported literature (Barakat et al., Citation1997; Cui et al., Citation2002; Yamada et al., Citation2011). In the present study, rubuphenol was reported for the first time from the seeds of S. cumini.
Other known compounds such as maslinic acid, gallic acid, and ellagic acid were also isolated and confirmed by direct comparison of their physiochemical and spectroscopic data with previously reported literature (Aher et al., Citation2010; Gohar et al., Citation2003; Tanaka et al., Citation2003). All the isolates were assayed for their inhibitory activity against AR and PTP-1B, and the results are presented in .
Table 1. The inhibitory activity of extracts and isolated compounds 1–6 from S. cumini seeds against AR and PTP1B.
Valoneic acid dilactone, rubuphenol, and ellagic acid exhibited the strong inhibitory activities against AR with IC50 values of 0.075 ± 0.005, 0.165 ± 0.035, and 0.12 ± 0.019 µg/mL and against PTP-1B with IC50 values of 9.37 ± 0.74, 28.14 ± 6.91, and 25.96 ± 0.22 µg/mL, respectively. In this communication, we for the first time report the AR and the PTP1B inhibitory activity of the isolated compounds 1–6 from the seeds of S. cumini. Based on this study, it can be concluded that ellagic acid moiety is responsible for the inhibition of AR and PTP1B enzymes.
Conclusion
The present work provided initial evidence that the methanolic extract of S. cumini seeds, its EtOAc fraction, and its major constituent ellagic acid derivatives possess strong AR and PTP-1B inhibitory activities with IC50 values <1 μg/mL for AR and <30 μg/mL for PTP-1B, respectively. Such IC50 values substantiate its potential for use as an adjunct herbal supplement to manage diabetes mellitus in patients to minimize the secondary complications associated with diabetes. Further, valoneic acid dilactone and rubuphenol can be used as biomarkers for biological standardization to ensure consistent quality.
Declaration of interest
The authors report no conflict of interest. The authors are grateful to National Medicinal Plants Board (NMPB), Department of AYUSH, New Delhi, India, for providing the financial assistance to carry out this work.
References
- Aher AN, Pal SC, Yadav SK, et al. (2010). Isolation and characterization of phytoconstituents from Casuarina equisetifolia (Casuarinaceae). Asian J Chem 22:3429–34
- Aqil F, Gupta A, Munagala R, et al. (2012). Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr Cancer 64:428–38
- Arun R, Prakash MVD, Abraham SK, Premkumar K. (2011). Role of Syzygium cumini seed extract in the chemoprevention of in vivo genomic damage and oxidative stress. J Ethnopharmacol 134:329–33
- Ayyanar M, Babu PS, Ignacimuthu S. (2013). Syzygium cumini (L.) Skeels, a novel therapeutic agent for diabetes: Folk medicinal and pharmacological evidences. Complement Ther Med 2:232–43
- Ayyanar M, Babu PS. (2012). Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed 2:240–6
- Baliga MS, Fernandes S, Thilakchand KR, et al. (2013). Scientific validation of the antidiabetic effects of Syzygium jambolanum DC (black plum), a traditional medicinal plant of India. J Altern Complement Med 19:191–7
- Banerjee J, Narendhirakannan RT. (2011). Phytochemical analyses, antibacterial, in vitro antioxidant and cytotoxic activities of ethanolic extract of Syzygium cumini (L.) seed extract. Int J Pharm Sci Res 2:1799–806
- Barakat HH, Hussein SAM, Marzouk MS, et al. (1997). Polyphenolic metabolites of Epilobium hirsutum. Phytochemistry 46:935–41
- Bhuyan ZA, Rokeya B, Masum N, et al. (2010). Antidiabetic effect of Syzygium cumini L. seed on Type 2 diabetic rats. Dhaka Univ J Biol Sci 19:157–64
- Bona DKS, Bonfanti G, Bitencourt PE, et al. (2014). Syzygium cumini is more effective in preventing the increase of erythrocytic ADA activity than phenolic compounds under hyperglycemic conditions in vitro. J Physiol Biochem 70:321–30
- Brito FA, Lima LA, Ramos MFS, et al. (2007). Pharmacological study of anti-allergic activity of Syzygium cumini (L.) Skeels. Braz J Med Biol Res 40:105–15
- Burke TR Jr, Ye B, Yan X, et al. (1996). Small molecule interactions with protein-tyrosine phosphatase PTP1B and their use in inhibitor design. Biochemistry 35:15989–96
- Chaudhary B, Mukhopadhyay K. (2012). Syzygium cumini (L.) Skeels: A potential source of nutraceuticals. Int J Pharm Bio Sci 2:46–53
- Cui CB, Zhao QC, Cai B, et al. (2002). Two new and four known polyphenolics obtained as new cell-cycle inhibitors from Rubus aleaefolius Poir. J Asian Nat Prod Res 4:243–52
- Gohar AA, Lahloub MF, Niwa M. (2003). Antibacterial polyphenol from Erodium glaucophyllum. Z Naturforsch 58:670–4
- Gowri SS, Vasantha K. (2010). Phytochemical screening and antibacterial activity of Syzygium cumini (L.) (Myrtaceae) leaves extracts. Int J Pharm Tech Res 2:1569–73
- Hayman S, Kinoshita JH. (1965). Isolation and properties of lens aldose reductase. J Biol Chem 240:877–82
- Helmstadter A. (2007). Antidiabetic drugs used in Europe prior to the discovery of insulin. Pharmazie 62:717–20
- Jadhav VM, Kamble SS, Kadam VJ. (2009). Herbal medicine: Syzygium cumini: A review. J Pharm Res 2:1212–19
- Khan N, Pathan JK, Harsoliya MS, et al. (2011). Evaluation of analgesic activity of Syzygium cumini (L.) Skeels leaves extract per se & its interactions with diclofenac sodium in thermal and chemical induced pain models. J Pharm Res 4:4129–31
- Kumar A, Jayachandran T, Aravindhan P, et al. (2009). Neutral components in the leaves and seeds of Syzygium cumini. Afr J Pharm Pharacol 3:560–1
- Lee HS. (2002). Inhibitory activity of Cinnamomum cassia bark-derived component against rat lens aldose reductase. J Pharm Pharm Sci 5:226–30
- Muruganandan S, Srinivasan K, Chandra S, et al. (2001). Anti-inflammatory activity of Syzygium cumini bark. Fitoterapia 72:369–75
- Oliveira AC, Endringer DC, Amorim LA, et al. (2005). Effect of the extracts and fractions of Baccharis trimera and Syzygium cumini on glycaemia of diabetic and non-diabetic mice. J Ethnopharmacol 102:465–9
- Omar R, Li L, Yuan T, Seeram NP. (2012). α-Glucosidase inhibitory hydrolyzable tannins from Eugenia jambolana seeds. J Nat Prod 75:1505–9
- Ponnusamy S, Ravindran R, Zinjarde S, et al. (2011). Evaluation of traditional Indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evid Based Complement Alternat Med. 2011:515647
- Rekha N, Balaji R, Deecaraman M. (2010). Antihyperglycemic and antihyperlipidemic effects of extracts of the pulp of Syzygium cumini and bark of Cinnamon zeylanicum in streptozotocin-induced diabetic rats. J Appl Biosci 28:1718–30
- Rodrigues MT, Alves TL, Soares GL, Ritter MR. (2012). Plants used as antidiabetics in popular medicine in Rio Grande do Sul, southern Brazil. J Ethnopharmacol 139:155–63
- Sagrawat H, Mann A, Kharya MD. (2006). Pharmacological potential of Eugenia jambolana: A review. Pharmaco-genesis Mag 2:96–105
- Saravanan G, Pari L. (2008). Hypoglycaemic and antihyperglycaemic effect of Syzygium cumini bark in streptozotocin-induced diabetic rats. J Pharmacol Toxicol 3:1–10
- Sharma B, Viswanath G, Salunke R, Roy P. (2008). Effects of flavonoid-rich extract from seeds of Eugenia jambolana (L.) on carbohydrate and lipid metabolism in diabetic mice. Food Chem 110:697–705
- Sharma SB, Nasir A, Prabhu KM, Murthy PS. (2006). Antihyperglycemic effect of the fruit-pulp of Eugenia jambolana in experimental diabetes mellitus. J Ethnopharmacol 104:367–73
- Simoes-Pires CA, Vargas S, Marston A, et al. (2009). Ellagic acid derivatives from Syzygium cumini stem bark: Investigation of their antiplasmodial activity. Nat Prod Commun 4:1371–6
- Srivastava S, Chandra D. (2013). Pharmacological potentials of Syzygium cumini: A review. J Sci Food Agric 93:2084–93
- Tanaka JCA, Vidotti GJ, Silva CCD. (2003). A new tormentic acid derivative from Luehea divaricata Mart. (Tiliaceae). J Braz Chem Soc 14:475–8
- Weichselbaum TE. (1946). An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. AJCP 10:40–9
- Yamada P, Nemoto M, Shigemori H, et al. (2011). Isolation of 5-(hydroxymethyl)furfural from Lycium chinense and its inhibitory effect on the chemical mediator release by basophilic cells. Planta Med 77:434–40