Abstract
Context: Alzheimer's disease (AD) is a neurodegenerative disorder characterized by the abnormal accumulation of β-amyloid (Aβ). Multiple Aβ-aggregated species have been identified, and neurotoxicity appears to be correlated with the amount of non-fibrillar oligomers. Potent inhibitors of Aβ oligomer formation or Aβ-induced cell toxicity have emerged as attractive means of therapeutic intervention. Eremochloa ophiuroide Hack. (Poaceae), also known as centipedegrass (CG), originates from China and South America and is reported to contain several C-glycosyl flavones and phenolic constituents.
Objective: We investigated whether CG could suppress Aβ aggregation, BACE1 activity, and toxicity at neuronal cell.
Materials and methods: The inhibitory effect of CG extracts toward aggregation of Aβ42 was investigated in the absence and presence of 50 µg/mL CG. We investigated the inhibitory effects of CG (0–5 µg/mL) on BACE1 using fluorescence resonance energy transfer (FRET)-based assay. The effects of CG (0–75 µg/mL) on Aβ42-induced neurotoxicity were examined in PC12 cells in the presence or absence of maysin and its derivatives of CG.
Results: We isolated EA-CG fraction (70% MeOH fraction from EtOAc extracts) from methanol extracts of CG, which contained approximately 60% maysin and its derivatives. In the present studies, we found that several Aβ oligomeric forms such as the monomer, dimer, trimer, and highly aggregated oligomeric forms were remarkably inhibited in the presence of 50 µg/mL of EA-CG. EA-CG also inhibited BACE1 enzyme activity in a dose-dependent manner. EA-CG treatment generated approximately 50% or 85% inhibition to the control at the tested concentrations of 1 or 5 µg/mL, respectively. Moreover, the neurotoxicity induced by Aβ42 was significantly reduced by treatment of EA-CG, and the 75 µg/mL EA-CG treatment significantly increased cell viability up to 82.5%.
Discussion and conclusion: These results suggested that the anti-Alzheimer’s effects of CG occurred through inhibition of neuronal cell death by intervening with oligomeric Aβ formation and reducing BACE1 activity. Maysin in CG could be an excellent therapeutic candidate for the prevention of AD.
Introduction
Alzheimer's disease (AD) is the most common form of dementia in the worldwide elderly population. The pathological hallmarks of AD are associated with profound neuronal loss in the hippocampus, entorhinal, and temporoparietal cortex; the presence of intraneuronal neurofibrillary tangles, astrogliosis, and the deposition of amyloid β-protein (Aβ); and memory impairment (Chishti et al., Citation2001). A small-sized Aβ is produced from sequential proteolytic cleavage of the amyloid precursor protein (APP) by β-secretase and γ-secretase (Opazo et al., Citation2002). The β-site APP-cleaving enzyme 1 (BACE1, also known as β-secretase) digests APP into the soluble APPβ (sAPPβ) and C99 peptide, and the latter is further hydrolyzed by γ-secretase to predominantly produce Aβ40 and Aβ42 (Vassar et al., Citation1999). BACE1 as a rate-limiting enzyme thus plays important roles in Aβ production; however, BACE1 knockout mice do not produce Aβ and seem to be phenotypically healthy, viable, and fertile (Luo et al., Citation2001). The deposition of Aβ into senile plaques in the extracellular space of the brain causes neurotoxicity, oxidative damage, and inflammation (Campbell & Gowran, Citation2007). Therefore, it seems increasingly likely that early soluble oligomers of Aβ are actually the toxic species responsible for neurodegeration and neuronal cell death; therefore, the inhibition of Aβ oligomerization is an attractive therapeutic target for AD.
One strategy for AD therapy is the use of natural products. Natural compounds usually tend to be safer and have fewer adverse effects than chemically synthesized drugs. Resveratrol (derived from red grape), (−)-epigallocatechin-3-gallate (EGCG; derived from green tea), and curcumin (derived from spice turmeric) have been shown to reduce the Aβ burden in the cerebral cortex (Karuppagounder et al., Citation2009; Ma et al., Citation2009; Rezai-Zadeh et al., Citation2005). It was also reported that cinnamon extract significantly inhibited the formation of toxic Aβ oligomers and prevented the toxicity of Aβ on neuronal PC12 cells as well as reduced senile plaques and improved the cognitive behavior of an AD mouse model (Chami & Checler, 2012). Caesar et al. (Citation2012) reported that the natural compound curcumin enhanced the clearance of toxic Aβ and alleviated Aβ toxicity in transgenic Drosophila. In this model, curcumin promoted amyloid fibril conversion by reducing the pre-fibrillar/oligomeric species of Aβ, which resulted in reduced neurotoxicity (Caesar et al., Citation2012; Caltagirone et al., Citation2012). Although it remains uncertain whether these effects are due to their antioxidant activities or by specific interaction with Aβs, the roles of natural compounds in AD therapy are being investigated widely.
Centipedegrass (CG) [Eremochloa ophiuroide Hack.] belongs to the genus Eremochloa (Poaceae), which includes eight species that are native to China and Southeast Asia, and is one of the most popular lawn grasses in South America (Barampuram et al., Citation2009). Maysin, a flavone C-glucoside that is found in CG, has been identified as an antibiotic that is resistant to the fall armyworm (FAW; Spodoptera frugiperda) (Byrne et al., Citation1996; Wiseman et al., Citation1990). A recent study has reported that the methanolic extracts from the leaves of CG exhibited an inhibitory effect against pancreatic lipase (Lee et al., Citation2010). CG also contains biologically active chemical components that could exert functional benefits such as an anti-inflammation effect, an antimicrobial effect, and an antiadipogenic activity (Lee et al., Citation2012; Park et al., Citation2012).
In the present study, we isolated maysin and its derivatives from CG. We then investigated the use of this CG-based, natural substance as an efficacious, therapeutic agent that inhibits Aβ oligomer formation, β-secretase activity, and Aβ-induced toxicity. We propose that maysin and its CG derivatives play a potential role in preventing neuronal cell death by intervening with oligomeric Aβ formation and reducing BACE1 activity.
Materials and methods
Preparation of CGs extracts
Eremochloa ophiuroides Hack. (Poaceae), CG, was supplied by Korea Atomic Energy Institute (KAERI). In brief, the leaves of CG were harvested in October 2012 and stored at −80 °C until use. The extraction method was selected based on an optimization study that was conducted by Lee et al. (Citation2012). The leaves were ground in an 80% (v/v) methanol solution by a mixer, followed by extraction of the samples for 3 d with vigorous shaking at room temperature. The methanol extracts were concentrated using rotary-vacuum evaporation at 50 °C and then freeze-dried (CGE). Furthermore, the MeOH extracts were fractionated with n-hexane and ethyl acetate (EA). The EA extracts were concentrated in vacuo and the dried compounds were dissolved in MeOH. Maysin and maysin derivatives were purified by a combination of column chromatographic techniques (Toyopearl, HW-40, YMC-GEL ODS-AQ 120-S50 and Sephadex LH-20 columns, YMC Co., Ltd, Kyoto, Japan) that were modified by Kasajima et al. (Citation2008). The dissolved MeOH extracts were diluted in 20% MeOH and chromatographed on TSK Toyopearl gel HW −40 °C resin (Tosoh, Tokyo, Japan) column using 70% MeOH. The final fraction was concentrated using rotary-vacuum evaporation at 50 °C and freeze-dried (EA-CG). The dried, final extracts from CG, EA-CG were reconstituted in 100% methanol for high-performance liquid chromatography (HPLC) analysis. In addition, CGE or EA-CG was dissolved in dimethylsulfoxide (DMSO) for cell treatments.
HPLC analysis of maysin and maysin-derivative compounds
CG extracts (EA-CG) were analyzed to identify maysin and its derivatives by using an Agilent Technologies 1200 series HPLC apparatus (Agilent Technologies Inc., Santa Clara, CA). Separation was performed using a YMC-Pack ODS A-302 column (4.6 mm i.d. × 150 mm; YMC Co., Ltd., Kyoto, Japan) as described by Lee et al. (Citation2012) at a flow rate of 1 mL/min. The mobile phase consisted of 1% formic acid (solution A) and 100% methanol (solution B), and the elution was performed by a combined step and linear gradient from 100:0 (solutions A: B) to 50:50 in 30 min, followed by 50:50–0:100 in 60 min. The eluted compounds were detected at a wavelength of 360 nm, and a sample volume of 20 µL was injected. The authentic standards, which included luteolin, isoorientin, rhamnosylisoorientin, derhamnosylmaysin, maysin, and luteoin-6-C-boivinopyranose, were used.
In vitro Aβ oligomerization assay
The procedure that was used has been described elsewhere (Dahlgren et al., Citation2002). Western blot analysis was performed to evaluate the immunoreactivity of oligomeric and fibrillar Aβ. Synthetic Aβ42 was purchased from US peptide, and synthetic Aβ1-42 was dissolved in 1 mM hexafluoroisopropanol (Sigma, St. Louis, MO), which was then removed under vacuum in a Speed Vac (Savant, Holbrook, NY). The residual peptide was re-suspended in DMSO (Sigma-Aldrich, St. Louis, MO) to a concentration of 5 mM. The concentration of the solution was diluted to 100 µM by adding phenol red-free Ham’s F-12 medium (Mediatech, Herndon, VA) to the re-suspended peptide, and the peptide solution was stored at 4 °C for 24 h. To measure the effect of EA-CG on the formation of monomer or oligomers, Aβ oligomers (100 µM) were incubated with 50 µg/mL of EA-CG for 2 h at 37 °C. The samples that were incubated with or without EA-CG were loaded onto a 12% Tris-Tricine SDS-PAGE gel, and Aβ oligomeric forms were isolated following electrophoresis. After transfer to a PVDF membrane, each oligomeric form on the membrane was reacted with 6E10 (Signet Pathology; Dedham, MA) following the Western blot procedure. The oligomeric signal in the presence or absence of EA-CG was detected using an enhanced chemiluminescence system (Amersham, Arlington Heights, IL).
Assessment of BACE1 inhibitory activity
The inhibitory activities of isolated EA-CG on BACE1 were evaluated using a fluorescence resonance energy transfer (FRET) assay kit (Panvera Corporation, Madison, WI) using purified baculovirus-expressed BACE1 and a specific substrate (Rh-EVNLDAEFK-Quencher) that is based on a Swedish mutation of the amyloid precursor protein (APP). Resveratrol was purchased from Sigma (St. Louis, MO). For the FRET assays, aliquots of the BACE1 enzyme (0.3 U/mL), 250 nM of substrate, and appropriate concentrations of the test compounds were added to 30 µL of 50 mM sodium acetate buffer (pH 4.5). After 1 h incubation at room temperature, 10 µL of the BACE1 stop solution was added to the reaction mixture to stop the reaction, and rhodamine fluorescence was monitored using a fluorometric plate reader, Victor 1 420 (Ex 545 nm/Em 585 nm, Victor Technologies Inc., Scotts Valley, CA). The inhibition ratio was calculated as a percentage of the control.
Aβ42 cytotoxicity assay
To evaluate the abilities of the CGE or EA-CG to protect or treat Aβ-induced neurotoxicity, we performed a bioassay according to a previously published method (Kang et al., Citation2011). PC12 cells were maintained at 37 °C/5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Gaithersburg, MD) supplemented with 5% horse serum (HS) v/v, 10% fetal bovine serum v/v, 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate. The CGE or EA-CG was scored as being able to protect PC12 cells from Aβ42 insult if it enhanced the conversion of MTT to MTT formazan within the cells, which is a process that directly reflects cell viability. For the assay, we seeded 500 µL of exponentially growing PC12 cells (1 × 105 cells per well) in 24-well tissue culture plates. After the PC12 cells were attached to the plate for 24 h, cells were pretreated with various concentrations (0, 25, 50, and 75 µg/mL) of CGE or EA-CG, or DMSO as a negative control (vehicle). One hour later, oligomeric Aβ42 (10 µM, prepared from a stock solution (100 µM in DMSO) was added to the pretreated cells and incubated for 24 h. MTT was added to each well, and the plates were incubated for 4 h at 37 °C. The liquid in the plate was then removed, and DMSO was added to dissolve the MTT–formazan complexes that were formed. Finally, the optical density was measured at 540 nm.
Statistical analysis
The data are expressed as the means ± SD. Intergroup differences were determined by the significant analysis method using ANOVA and Duncan’s multiple range test. p < 0.05 was considered statistically significant.
Results
Maysin and its derivatives were isolated from CG
The HPLC profile of the methanol extracts from CG (EA-CG) showed various compounds with strong absorption at 360 nm (). Eleven peaks were isolated by their unique retention times using specific mobile phase. Our results suggested that maysin is the main material in the EA-CG fraction of CG extract and is 19.28% in 70% methanol fraction (70% MeOH) from ethyl acetate (EtOAc) extracts. Furthermore, the EA-CG fraction had four types of maysin derivatives, such as luteolin (yield: 1.68 mg/100 mg 70% MeOH from EtOAc extracts), isoorientin (yield: 13.99 mg/100 mg 70% MeOH from EtOAc extracts), rhamnosylisoorientin (yield: 13.65 mg/100 mg 70% MeOH from EtOAc extracts), and derhamnosylmaysin (yield: 9.44 mg/100 mg 70% MeOH from EtOAc extracts). Maysin was then isolated and purified using different types of purification apparatuses, e.g., gel filtration, and identified. We depict the natural plant image of maysin and its structure in and , respectively.
Figure 1. Maysin and its derivatives found in centipedegrass. (A) Overflow of the cascade for extraction and purification of maysin from CG. CGE, centipedegrass extract; EA-CG, ethyl acetate extract. (B) Compound contents (µg/g extract) of maysin and its derivatives in the centipedegrass fraction (EA-CG). The values are presented as the means ± SD (n = 3) and are given as mg/L of the investigated bitter gourd fractions.
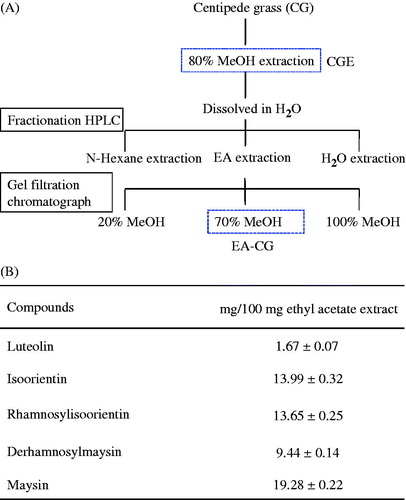
Figure 2. EA-CG inhibited Aβ oligomerization. (A) Chemical structure of maysin. (B) Oligomeric Aβ species were prepared by incubating Aβ42 and Aβ oligomers with or without EA-CG compounds. The oligomers species, including monomer, oligomer, and highly aggregated Aβ, were separated following electrophoresis by 12% Tris-Tricine SDS-PAGE. The representative Western blot from one of the three independent experiments is shown.
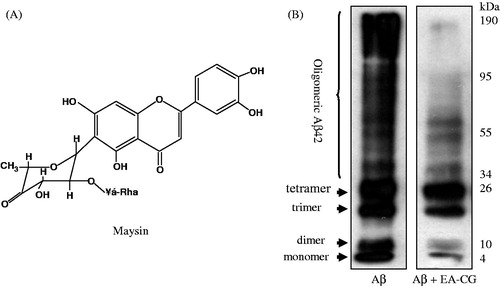
Maysin and its derivatives from CG-inhibited Aβ oligomerization
To determine if CGs inhibit the formation of monomers and oligomers, we carried out western blotting to analyze Aβ oligomerization in the presence and absence of EA-CG. To visualize each oligomeric form of Aβ and its different oligomeric types after treatment of the Aβ peptide with EA-CG in an in vitro oligomeric procedure, we used the 6E10 antibody to detect each of those molecules from the monomer to the highly aggregated, fibrillar form. Interestingly, EA-CG inhibits more significantly the monomer, dimer, and higly oligomeric forms of Aβ rather than trimer, tetramner compared with control (solvent only). We found that several Aβ oligomeric forms such as the monomer, dimer, trimer, and highly aggregated oligomeric forms were remarkably inhibited in the presence of 50 µg/mL EA-CG compared with the control group (). These results indicated that oligomerized Aβ was decreased to several oligomeric forms, except the tetrameric form, in the presence of 50 µg/mL EA-CG. Therefore, the results suggested that maysin and its derivatives inhibited Aβ oligomer formation.
Maysin and its derivative from CG-inhibited BACE1 enzyme activity
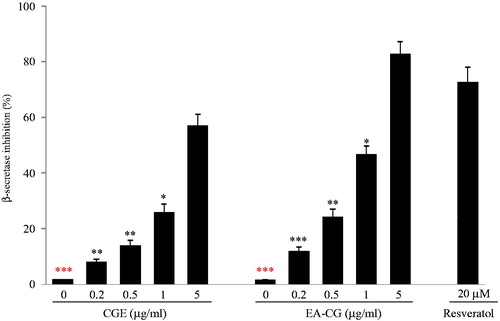
Next, we investigated whether CGE or EA-CG could inhibit BACE1 activity. CGE or EA-CG was assayed as BACE1 inhibitors using a FRET assay, which uses BACE1 and a specific substrate (Rh-EVNLDAEFK-quencher), which was based on a Swedish mutation of APP. The rate of proteolysis of BACE1 enzyme using FRET peptide substrate linearly represents to increase fluorescent upon enzymatic cleavage. Using this FRET assay, CGE and EA-CG inhibited BACE1 enzyme activity in a dose-dependent manner. The fluorescence intensity of BACE1 enzyme activity was decreased by 11.7, 24.2, 46.5, and 82.5% by various doses (0.2, 0.5, 1, and 5 µg/mL, respectively) of EA-CG (). Similarly, the intensity of BACE1 enzyme activity fluorescence was decreased by 7.9, 13.7, 25.6, and 56.7% for several doses (0.2, 0.5, 1, and 5 µg/mL, respectively) of CGE (). Together, EA-CG has been shown to more strongly inhibit BACE1 enzyme activity than CGE. Accordingly, all the above-mentioned results indicated that maysin and its derivatives function as BACE1 inhibitors and could efficiently reduce β-secretase activity.
Figure 3. EA-CG dose-dependently inhibited BACE1 activity. The BACE1 enzyme activity was determined by incubating recombinant human BACE1 and its substrate, Rh-EVNLDAEFK, which was also used as a quencher in the presence or in the absence of CGE or EA-CG. The fluorescence activity was analyzed by dose response with CGE or EA-CG, and resveratrol was used as the positive control. Comparison of BACE1 inhibition was performed, and the results were described as the inhibition rate (% of control). Representative results are based on three independent experiments, and the values are presented as the means ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with 5 µg/mL CGs-treated cells.
Maysin and its derivatives from CG reduced Aβ42-induced cell death in PC12 cells
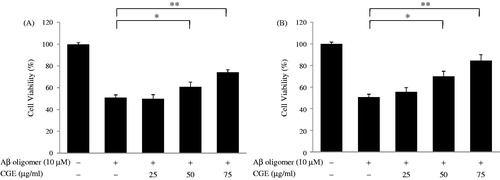
To examine whether maysin compounds in CG could protect cells from Aβ-mediated cell toxicity, we used PC12 cells to perform a MTT assay. Treatment with Aβ (10 µM) had significant inhibitory effects on cell viability. However, treatment of Aβ with CGE revealed a gradual reduction in the cytotoxicity as the concentration of CGE was increased (). Moreover, EA-CG reduced Aβ-induced cell toxicity, and the 75-µg/mL EA-CG treatment significantly increased cell viability up to 82.5% (). Based on this observation, our results suggest that maysin and its derivatives are highly effective inhibitors of Aβ42 oligomer-mediated cellular toxicity.
Figure 4. Maysin and its derivatives inhibited Aβ-mediated neuronal cell toxicity in a dose-dependent manner. Neuronal PC12 cells were cultured with Aβ oligomer (10 µM) for 24 h in the presence of various doses of CGE or EA-CG, and cell toxicity was determined using the MTT assay. These data were presented as relative cell viability values. (A) The effect of CGE on Aβ-mediated neuronal cell toxicity. Representative results are expressed as the means ± SD following three independent experiments. *p < 0.05, significantly different from the Aβ oligomer-treated group; **p < 0.01, significantly different from the Aβ oligomer-treated group. (B) The effect of EA-CG on Aβ-mediated, neuronal cell toxicity. Representative results are expressed as the means ± SD following three independent experiments. *p < 0.05, **p < 0.01.
Discussion
The progressive accumulation of Aβ aggregates via an oligomerization or fibrillation process is widely believed to be fundamental to initialization of neurodegenerative pathology and to trigger a cascade of events that include neurotoxicity, oxidative stress, and inflammation during the progression of AD (Hardy & Selkoe, Citation2002). Aβ42 is one of the most toxic Aβ species, and its neurotoxic effects are initiated by the subsequent activation by proteases such as β-secretase and γ-secretase (Opazo et al., Citation2002) following its interaction with the β-amyloid precursor protein (β-APP). Many studies support that Aβ accumulation results due to the lack of interaction inhibition between soluble Aβ oligomers, which leads to neuronal cell death.
Much evidence has emerged suggesting that various resources of natural products exert an anti-Aβ effect with regard to decreasing soluble Aβ production. Here, we identified a natural substance based on CG, which contains maysin and maysin derivatives such as luteolin, isoorientin, and rhamnosylisoorientin. Maysin and its derivatives of CG showed potential inhibitory effects on β-secretase enzyme activity. Moreover, EA-CG inhibited the formation of Aβ monomers and oligomers, indicating that its maysins could potentiality intervene with Aβ monomeric or oligomeric forms. Recent studies on Aβ support the idea that non-fibrillar aggregates or soluble oligomers, rather than mature amyloid fibrils, are the pathogenic components that drive neurodegeneration and neuronal cell death (Paleologou et al., Citation2009). Kang et al. (Citation2011) reported that brown algae Ecklonia cava was shown to have anti-Aβ effects such as reduced Aβ secretion, inhibited Aβ oligomerization, particularly mid-size oligomer formation. Therefore, considering the importance of soluble oligomeric forms of Aβ or Aβ aggregation in AD pathogenesis, our results clearly indicated that maysin of CG diminished the formation of Aβ monomers or oligomers and is a potential inhibitor of the Aβ oligomerization.
As APP processing occurs by sequential proteolysis that is carried out by BACE1 and leads to the generation of Aβ and ultimately neurotoxicity, recent research has focused on preventing Aβ production. One way to prevent AD is to block β-secretase and thus prevent proliferation of APP-induced amyloid plaques. In this study, we further investigated the potential ability of CG to interrupt an activated enzyme such as BACE1. We found that the natural compound maysin and its derivatives that were isolated from CG significantly inhibited BACE1 enzyme activity and functioned as a specific BACE1 inhibitor. The most promising link between BACE1 and AD is the fact that the expression and activity of BACE1 were elevated in the brains of AD patients (Li et al., Citation2004). Interestingly, Aβ generation was so completely abolished in mice that were deficient for BACE1 that the brain became the primary source of Aβ (Cai et al., Citation2001). To date, several types of natural-product BACE1 inhibitors have been reported to lower Aβ. These findings are supported by results indicating that natural compounds such as p-coumaric acid, gallic acid, or ursolic acid isolated from corni fructus significantly block BACE1 enzyme activity (Youn et al., Citation2012). It was also reported that the natural phenol compounds, resveratrol dimer and vitisinol (E), that were isolated from the stem bark extract of Vitis vinifera L. (Vitaceae) inhibited BACE enzymic activity (Choi et al., Citation2009). Taken together, our results suggest that maysin from CG has the potential ability to inhibit the Aβ oligomerization process and inhibit BACE1 enzyme activity. These results suggest that maysin could be an important inhibitor of BACE1 and is an effective candidate for attenuating the progression of AD.
Amyloid plaques are prominent pathological signatures of AD, and AD patients progressively accumulate primarily Aβ in their brains due to increased synthesis of the toxic Aβ42 peptide. To explore whether CG maysin has the ability to prevent Aβ-induced neuronal cell death, we investigated the protection effect of CG maysin in Aβ42-induced cell death. In the present study, exposure of PC12 cells to Aβ induced a significant decrease in cell viability, while CG maysin increased cell viability in a dose-dependent manner. The present data indicated that CG maysin was effective in attenuating Aβ-induced apoptosis in PC12 cells, a process that may be relevant to neurodegenerative events that occur in AD. Increasing evidence suggests that Aβ is generated by oxidative stress and its harmful neurotoxicity leads to neuronal cell loss and, consequently, disabled memory function, which is linked to a wide spectrum of pathophysiologies due to lesions of the brain. In addition, the fact that the brains of AD patients contain a 50-fold increase in apoptosis, when compared with age-matched controls, highlights the importance of anti-apoptotic agents in treating AD-associated neurodegeneration (Colurso et al., Citation2003). Taken together, the ability of CG maysin to inhibit the formation of Aβ oligomers suggested that it is useful in blocking Aβ-mediated cellular toxicity. Because the prevention of Aβ oligomerization is critical for impeding disease progression and protecting neuronal cell death and maysin showed strong neuroprotective effects against Aβ-induced toxicity, maysin itself might be a potent neuroprotective and anti-neurotoxicity agent.
Natural compounds usually tend to be safer and have fewer adverse effects than chemically synthesized drugs. Several plants and plant compounds with antioxidant activity have shown favorable effects toward the central nervous system. We found that CGE contained total phenolic and flavonoid contents and exhibited potent DPPH radical scavenging activity or hydroxyl radical scavenging activity (data not shown). Moreover, luteolin, orientin, and isoorientin, which are all found in CG, are flavone C-glucosides that protect plants by deterring harmful insects from feeding and perform bioactive roles by acting as anti-inflammatory agents, anti-nociceptives, antimicrobials, and inhibitors of myolytic activity (Lee et al., Citation2012). Taken together, the results suggest that CG could potentially exhibit anti-AD effects by inhibiting BACE1 and Aβ-induced toxicity through its considerable antioxidant capacities.
In conclusion, these data suggested that maysin and its derivatives of CG protected neuronal cells against Aβ-induced toxicity. Furthermore, CG maysin directly inhibited BACE1 activity and reduced Aβ oligomerization. Therefore, maysin may be effective against AD and may serve as a lead compound for the development of a therapeutic drug for AD.
Declaration of interest
The authors report that there are no declaration of interest. This project was supported by the Nuclear R & D Program of the Ministry of Science and Technology and also supported by the Ministry of Education, National Research Foundation, Korea (No. 2013066165).
References
- Barampuram S, Chung BY, Lee SS, et al. (2009). Development of an embryogenic callus induction method for centipedegrass (Eremochlo aophiuroides Munro) and subsequent plant regeneration. In Vitro Cell, Dev Biol Plant 45:155–61
- Byrne PF, Darrah LL, Snook ME, et al. (1996). Maizesilk-browning, maysin content, and antibiosis to the corn earworm, Helicoverpa zea (Boddie). Maydica 41:13–18
- Caesar I, Jonson M, Nilsson KP, et al. (2012). Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic Drosophila. PLoS One 7:e31424
- Cai H, Wang Y, McCarthy D, et al. (2001). BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci 4:233–4
- Caltagirone C, Ferrannini L, Marchionni N, et al. (2012). The potential protective effect of tramiprosate (homotaurine) against Alzheimer's disease: A review. Aging Clin Exp Res 24:580–7
- Campbell VA, Gowran A. (2007). Alzheimer's disease; taking the edge off with cannabinoids? Br J Pharmacol 152:655–62.
- Chami L, Checler F. (2012). BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer's disease. Mol Neurodegener 7:52
- Chishti MA, Yang DS, Janus C, et al. (2001). Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 276:21562–70
- Choi YH, Yoo MY, Choi CW, et al. (2009). A new specific BACE-1 inhibitor from the stem bark extract of Vitis vinifera. Planta Med 75:537–40
- Colurso GJ, Nilson JE, Vervoort LG. (2003). Quantitative assessment of DNA fragmentation and beta-amyloid deposition in insular cortex and midfrontalgyrus from patients with Alzheimer's disease. Life Sci 73:1795–803
- Dahlgren KN, Manelli AM, Stine WB Jr, et al. (2002). Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 277:32046–53
- Hardy J, Selkoe DJ. (2002). The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297:353–6
- Kang IJ, Jeon YE, Yin XF, et al. (2011). Butanol extract of Ecklonia cava prevents production and aggregation of beta-amyloid, and reduces beta-amyloid mediated neuronal death. Food Chem Toxicol 49:2252–9
- Karuppagounder SS, Pinto JT, Xu H, et al. (2009). Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int 54:111–18
- Kasajima N, Ito H, Hatano T, Yoshida T. (2008). Phloroglucinoldiglycosides accompanying hydrolysable tannins from Kunzeaambiqua. Phytochemistry 69:3080–6
- Lee EM, Bai HW, Lee SS, et al. (2012). Stress-induced increase in the amounts of maysin and maysin derivatives in world premium natural compounds from centipedegrass. Radiat Phys Chem 81:1055–8
- Lee EM, Lee SS, Chung BY, et al. (2010). Pancreatic lipase inhibition by C-glycosidic flavones isolated from Eremochloa ophiuroides. Molecules 15:8251–9
- Li R, Lindholm K, Yang LB, et al. (2004). Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer's disease patients. Proc Natl Acad Sci USA 101:3632–7
- Luo Y, Bolon B, Kahn S, et al. (2001). Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci 4:231–2
- Ma QL, Yang F, Rosario ER, et al. (2009). Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: Suppression by omega-3 fatty acids and curcumin. J Neurosci 29:9078–89
- Opazo C, Huang X, Cherny RA, et al. (2002). Metalloenzyme-like activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H2O2. J Biol Chem 277:40302–8
- Paleologou KE, Kragh CL, Mann DM, et al. (2009). Detection of elevated levels of soluble alpha-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain 132:1093–101
- Park HJ, Chung BY, Lee MK, et al. (2012). Centipede grass exerts anti-adipogenic activity through inhibition of C/EBPβ, C/EBPα, and PPARγ expression and the AKT signaling pathway in 3T3-L1 adipocytes. BMC Complement Altern Med 12:230
- Rezai-Zadeh K, Shytle D, Sun N, et al. (2005). Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci 25:8807–14
- Vassar R, Bennett BD, Babu-Khan S, et al. (1999). Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–41
- Wiseman BR, Gueldner RE, Lynch RE, Severson RF. (1990). Biochemical activity of centipedegrass against fall armyworm larvae. J Chem Ecol 16:2677–90
- Youn K, Jun M. (2012). Inhibitory effects of key compounds isolated from Cornifructus on BACE1 activity. Phytother Res 26:1714–18
