Abstract
Context: Piper nigrum Linn (Piperaceae) (PnL) is used in traditional medicine to treat gastric ailments, dyslipidemia, diabetes, and hypertension.
Objective: The present study explores the possible protective effects of P. nigrum extracts on high-fat diet-induced obesity in rats.
Materials and methods: High-fat diet-induced obese rats were treated orally with 200 mg/kg bw of different extracts (hexane, ethylacetate, ethanol, and aqueous extracts) of PnL for 42 d. The effects of PnL extracts on body composition, insulin resistance, biochemical parameters, leptin, adiponectin, lipid profile, liver marker enzymes, and antioxidants were studied.
Results and discussion: The HFD control group rats showed a substantial raise in body weight (472.8 ± 9.3 g), fat% (20.8 ± 0.6%), and fat-free mass (165.9 ± 2.4 g) when compared with normal control rats whose body weight, fat%, and fat-free mass were 314.3 ± 4.4 g, 6.4 ± 1.4%, and 133.8 ± 2.2 g, respectively. Oral administration of ethyl acetate or aqueous extracts of PnL markedly reduced the body weight, fat%, and fat-free mass of HFD-fed rats. In contrast to the normal control group, a profound increase in plasma glucose, insulin resistance, lipid profile, leptin, thiobarbituric acid reactive substance (TBARS), and the activities of lipase and liver marker enzymes, and a decrease in adiponectin and antioxidant enzymes were noted in HFD control rats. Administration of PnL extracts to HFD-induced obese rats significantly (p < 0.05) restored the above profiles.
Conclusion: PnL extracts significantly reduced the body weight, fat%, and ameliorated HFD-induced hyperlipidemia and its constituents.
Introduction
Obesity is a chronic metabolic disorder which results from an imbalance between energy intake and expenditure and it is also contributed by altered lipid metabolic processes including lipogenesis and lipolysis. It is the most prevailing and common health dilemma to be solved throughout the world (Finucane et al., Citation2011). According to the World Health Organization (WHO) report, in the next 10 years, 30% of adults will be overweight with about 20% of them being obese (Browning et al., Citation2013). In both developed and developing countries, the prevalence of obesity has increased dramatically in recent decades principally due to increased intake of high-calorie diet and sedentary lifestyles (Canbakan et al., Citation2008; Kopelman, Citation2000).
Obesity therapies include reduction of nutrient absorption and administration of drugs that affect lipid mobilization and utilization. To date, pharmaco-therapeutics/medicines are unable to regulate the weight gain completely, which have limited tolerability and adverse effects. Therefore, a necessitation for alternative therapy especially based on natural products with minimal side effects has arisen. At present, the potential of natural products for the treatment of obesity has been explored and several studies on obese humans and animals suggest that phytochemicals have significant antiobesity effects (Birari et al., Citation2011).
From the beginning of the last century, evidence on the development of extracts and active molecules from natural origin for the treatment of obesity has been accumulating (Hsu et al., Citation2009). Indian traditional herbal medicine provides an effective solution without side effects for this possible risk. One such plant source is Piper nigrum Linn (Piperaceae) (PnL), commonly known as black pepper, which has been traditionally used for cholera, dyspepsia, gastric ailments, and diarrhea (Srinivasan, Citation2007). The chemical constituents of PnL include polyphenols, alkaloids, terpenoids, tannins, and oils; the major compounds are piperine, piperidine, pellitorine oil, cepharadione, piperolactum, paprazine, and sylvamide (Masood et al., Citation2013). Scientific data on the biological activities of PnL have been limited to date: PnL was found to have antidiabetic properties (Kumar et al., Citation2013), as well as hepatoprotective and antioxidant effects on rats (Singh et al., Citation2007). However, there has been no scientific literature available on the efficacy of PnL on body composition, hyperlipidemia, blood glucose, insulin resistance, and hormones of experimentally induced obese rats. In order to provide evidence as to whether PnL seeds possess antiobesity effects, we investigated with different extracts of PnL on HFD-induced obesity in rats.
Materials and methods
Animals
All experiments were carried out with male Sprague–Dawley rats (SD rats) weighing 170–180 g, obtained from National Institute of Nutrition (NIN), Hyderabad, India. After initial acclimatization, the animals were randomly divided into different groups as mentioned below. All procedures involving laboratory animals were in accordance with the Institute Animal Ethics Committee regulations approved by the committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA) (IAEC approval number: 36 a/2012(i)/a/-CPCSEA/-IAEC/SVU/MB; dt.01.07.2012).
Chemicals
To measure plasma glucose and insulin, kits were procured from Stanbio Laboratory, Boerne, TX, and Bio-Merieux, RCS, Lyon, France, respectively. Orlistat (Cat no. 04139) was obtained from Sigma- Aldrich (St. Louis, MO). All other reagents used in the experiments were of analytical grade and of high purity.
Preparation of extracts
Dried Piper nigrum seeds were obtained from the local market in the month of June, their identity was authenticated by a taxonomist (Dr K. Madhava Chetty), Department of Botany, Sri Venkateswara University, India, voucher number 2271, and a specimen has been preserved at the departmental herbarium. Seeds were shade dried and made into course powder. The powder (2.5 kg) was soaked sequentially with hexane, ethylacetate, ethanol, and double distilled water each 6 d at room temperature in a 10 L aspirator jar to collect the extracts. These extracts were evaporated by using a rotavapor to obtain respective dry extracts to be used for further studies. The extracts were dissolved in Tween 80 for pharmacological studies (Gayasuddin, Citation2013).
Induction of obesity
Normal- and high-fat diets were obtained from NIN, Hyderabad. Normal diet contained pellet chow of standard composition containing all the recommended macro- and micronutrients (56% carbohydrate, 18.5% protein, 8% fat, 12% fiber, and adequate levels of minerals and vitamins). HFD contained 29.5% beef tallow, 22.0% casein, 23.0% starch, 17.9% cellulose, 4.0% l-cysteine, 0.3% choline chloride, 1.8% vitamin mixture (AIN-93 ViX), and 1.5% salt mixture. During the course of the experimental period (14 weeks), the rats were fed with either normal diet or freshly prepared HFD (15 g/rat/d) as mentioned below and water ad libitum.
Experimental design
To test the antiobesity activities, PnL extracts (200 mg/kg bw, suspended in 0.5% carboxymethyl cellulose) were orally administered to the below-mentioned HFD-fed rats for 42 d (9–14th week). The rats were divided into following groups:
Group I – Normal diet control
Group II – HFD control
Group III – HFD + Orlistat (5 mg/kg bw)
Group IV – HFD + PnL hexane extract
Group V – HFD + PnL ethyl acetate extract
Group VI – HFD + PnL ethanolic extract
Group VII – HFD + PnL aqueous extract
Acute toxicity studies
The acute oral toxicity studies were performed in overnight fasted rats as per OECD (Organization for Economic Co-operation and Development) guidelines. The rats did not show any abnormal behavior or mortality up to 2000 mg/kg bw of each extract. Hence its 1/10th of the concentration (200 mg/kg bw) was used as the therapeutic dose in the study.
Measurement of body composition
The body weight of each rat was measured once a week. Body composition of experimental animals was assessed at the end of the experiment by total body electrical conductivity (TOBEC) using the small animal body composition analysis system (EM-SCAN, Model SA-3000 Multi-detector, Springfield, Springfield, IL). Lean body mass, fat-free mass, total fat, and total body fat percent were calculated as described previously (Venu et al., Citation2004).
Estimation of glucose, insulin, and insulin resistance
At the end of the experiment, blood was collected from overnight fasted rats after inhaling anesthesia by the retro-orbital puncture method, and plasma was separated by centrifugation at 2500 rpm for 15 min. Plasma glucose was estimated using kits (Cat no. 1060-500, Stanbio Laboratory, Boerne, TX). Plasma level of insulin was determined using kits from Bio-Merieux, RCS, Lyon, France. Insulin resistance was calculated using the homeostasis model assessment (Mattews et al., Citation1985).
Measurement of leptin and adiponectin
Plasma leptin and adiponectin levels were measured by enzyme-linked immunosorbent assay (Crystal Chem, Downers Grove, IL), performed in duplicate and were expressed in nanograms per milliliter (ng mL−1).
Oral glucose tolerance test (OGTT)
At the end of the experiment, after overnight fasting, glucose was orogastrically administered to experimental rats at a dose of 2.0 g/kg bw and blood samples were collected from supra orbital sinus at 0, 30, 60, 90, and 120 min and glucose levels were estimated at all time intervals (Prasad et al., Citation2012).
Plasma lipid analysis
Plasma was analyzed for total cholesterol, triglycerides, phospholipids, free fatty acids, LDL-C, VLDL-C, and HDL-C by enzymatic colorimetric methods using kits (Nicholas Piramal, India Ltd., Mumbai, India).
Tissue lipid analysis
Tissue lipids were extracted from the experimental animals by the method of Floch et al. (Citation1957). The liver tissues were rinsed with ice-cold physiological saline, dried, and homogenized in cold chloroform–methanol (2:1, v/v), and contents were extracted after 24 h. The extraction was repeated four times. The combined filtrate was washed with 0.7% KCl and the aqueous layer was discarded. The organic layer was made up to a known volume with chloroform and used for tissue lipid analysis.
Liver antioxidants analysis
Blood samples were collected after sacrificing the experimental rats. Then liver was excised and rinsed in ice-cold normal saline followed by ice-cold 10% KCl solution, blotted, dried, and weighed. A 10% w/v homogenate was prepared in ice-cold 10% KCl solution and centrifuged at 2000 rpm for 10 min at 4 °C. The supernatant thus obtained was used for the estimation of thiobarbituric acid reactive substances (TBARS) (Fraga et al., 1988), glutathione (GSH) (Ellman, Citation1959), superoxide dismutase (SOD) (Kakkar et al., Citation1984), catalase (CAT) (Aebi, Citation1984), and GSH peroxidase (GPx) (Paglia & Valentine, Citation1967).
Assay of lipase, AST, ALT, and ALP
Plasma levels of lipase (Bioclin®, Minas Gerais, Brazil) and liver marker enzymes, aspartate aminotransferase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) (Span Diagnostics, Mumbai, India) were determined by the kinetic method using the commercial kits.
Adiposity index and fat pad analysis
Adiposity index reflects the intensity of obesity. It was measured by using the formula: sum of the total body fat pad weights/body weights × 100 (Venu et al., Citation2004).
Statistical analysis
All the results are expressed as mean ± SD of six animals in each group. All the grouped data were statistically evaluated with SPSS 10.0 software (SPSS Inc., Chicago, IL). Hypothesis testing methods included one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. Significance levels at p < 0.05 and 0.001 were considered to indicate statistical significance.
Results
Phytochemical analysis of different extracts of PnL revealed the presence of flavonoids, saponins, glycosides, terpenoids, amino acids, alkaloids, carbohydrates, phenolic compounds, and proteins. Qualitative analysis of the extracts confirmed the prominent presence of piperine, a potent antioxidant.
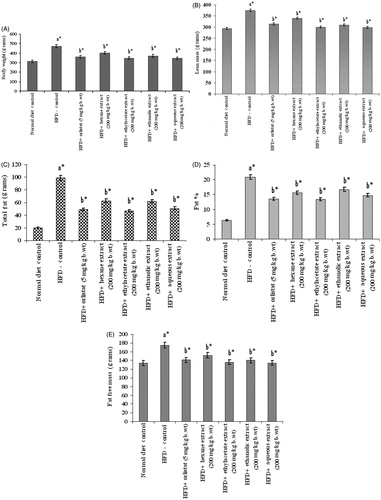
Consumption of HFD for 14 weeks produced a substantial change in the body composition of experimental rats. The body weight, lean mass, total fat, fat-free mass, and fat percentage of HFD-fed control rats were 472.8 ± 9.3 g, 374.1 ± 6 g, 98.7 ± 4.34 g, 165.9 ± 2.42 g and 21%, respectively, which are very considerably higher when compared with normal diet fed rats as shown in . However, oral administration of ethylacetate and aqueous extracts of PnL (200 mg/kg bw) for 42 d (from 9th to 14th week) when compared with other PnL extracts, significantly (p < 0.05) reduced body weight, lean mass, total fat, fat percentage, and fat-free mass of HFD-fed obese rats.
Figure 1. Effect of Piper nigrum on body weight (A), lean mass (B), total fat (C), fat% (D), and fat free mass (E) in normal and experimental obese rats. Values are mean ± SD, n = 6. Values are statistically significant at *p < 0.05. a*Significantly different from control. b*Significantly different from HFD control.
The levels of plasma glucose, insulin, insulin resistance, leptin, and adiponectin in control and experimental obese rats are shown in and . There was a marked elevation in plasma glucose, insulin, insulin resistance, leptin, and lipase levels and decrease in adiponectin in HFD-induced obese rats compared with the normal control group. However, oral administration of PnL extracts (200 mg/kg bw) or orlistat (5 mg/kg bw) has significantly (p < 0.05) revered these changes. Ethylacetate and aqueous extracts exhibited better performance in reversing the above parameters when compared with other extracts.
Figure 2. Effect of Piper nigrum on leptin, adiponectin, and lipase in control and experimental obese rats. Values are mean ± SD, n = 6. Values are statistically significant at *p < 0.05. a*Significantly different from control. b*Significantly different from HFD control.
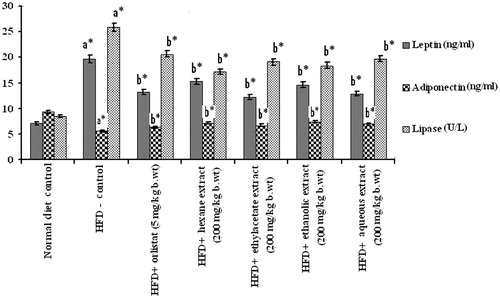
Table 1. Effect of Piper nigrum Linn. on plasma glucose, insulin and insulin resistance in normal and experimental obese rats.
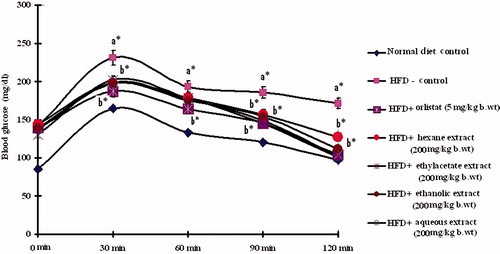
When OGTT was performed, in normal control rats, blood glucose level reached its maximum value at 60 min after glucose load and declined to near basal levels at 120 min. In contrast, in HFD-fed obese rats, the peak increase in blood glucose level was noticed even after 60 min and remained high over the next 60 min. Oral administration of PnL extracts (200 mg/kg bw) or orlistat to obese rats elicited a significant decrease in the blood glucose level at 60 min and beyond, when compared with HFD control rats (). Ethylacetate and aqueous extracts showed better performance when compared with the remaining ones.
Figure 3. Oral glucose tolerance test. Effect of Piper nigrum on oral glucose tolerance test in control and experimental obese rats. Values are mean ± SD, n = 6. Values are statistically significant at *p < 0.05. a*Significantly different from control. b*Significantly different from HFD control.
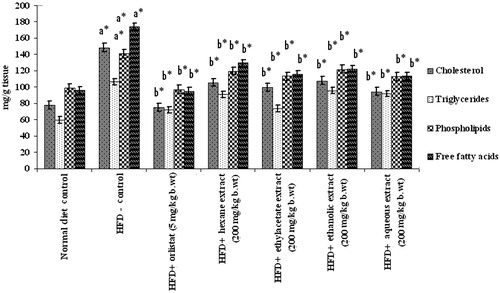
The levels of total cholesterol, FFAs, TGs, PLs, HDL-C, LDL-C, and VLDL-C in plasma and liver of control and HFD-fed rats are depicted in and . Except HDL-C, there was a large increase in tissue and plasma lipid profiles in HFD-fed obese rats which was significantly (p < 0.05) attenuated by oral administration of PnL extracts, more particularly by ethylacetate and aqueous extracts (200 mg/kg bw).
Figure 4. Effect of Piper nigrum on lipid profile in plasma of normal and experimental obese rats. Values are mean ± SD, n = 6. Values are statistically significant at *p < 0.001. a*Significantly different from control. b*Significantly different from HFD control.
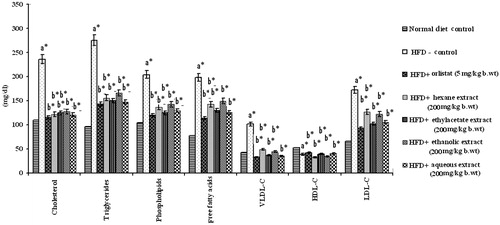
Figure 5. Effect of Piper nigrum on liver lipid profile of normal and experimental obese rats. Values are mean ± SD, n = 6. Values are statistically significant at **p < 0.05. a*Significantly different from control. b*Significantly different from HFD control.
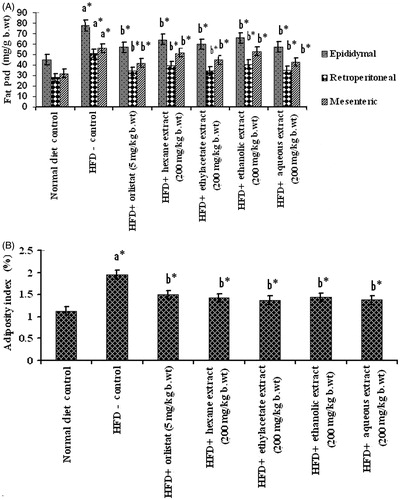
Changes in fat pad weights and adiposity index of control and HFD fed obese rats are summarized in . Feeding on HFD resulted in increased fat pad (epididymal, retroperitoneal, and mesenteric) weights and adiposity index. However, following treatment with PnL extracts (200 mg/kg bw) or orlistat, fat pad weight, and adiposity index were brought back to near normal. Ethylacetate and aqueous extracts showed more efficiency in this regard than other extracts.
Figure 6. (A) and (B) Effect of Piper nigrum on fat pad weights and adiposity index in normal and experimental obese rats. Values are mean ± SD, n = 6. Values are statistically significant at *p < 0.05. a*Significantly different from control. b*Significantly different from HFD control.
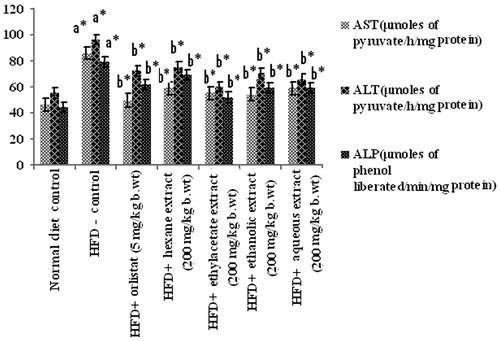
Assay of liver marker enzymes showed a steep raise in the activities of AST, ALT, and ALP in HFD-induced obese rats when compared with corresponding normal control rats. Administration of ethylacetate and aqueous extracts of PnL (200 mg/kg bw) significantly (p < 0.05) decreased plasma levels of AST, ALT, and ALP in HFD-fed obese rats ().
Figure 7. Effect of Piper nigrum on liver marker enzymes in plasma of normal and experimental obese rats. Values are mean ± SD, n = 6. Values are statistically significant at *p < 0.05. a*Significantly different from control. b*Significantly different from HFD control.
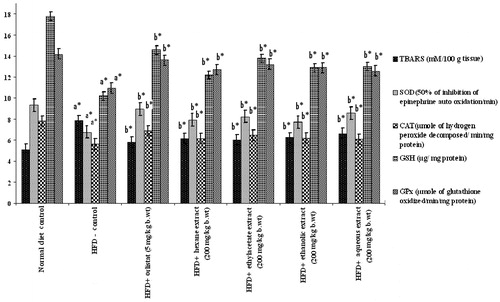
shows the levels of TBARS, GSH, SOD, CAT, and GPx in the liver homogenate of control and experimental groups of rats. The HFD control group showed a significant increase in the level of TBARS with a significant decrease in the level of GSH, SOD, CAT, and GPx. Oral administration of PnL extracts (200 mg/kg bw) or orlistat to obese rats significantly reversed the above biochemical changes. The degree of reversal effect was maximum with ethylacetate and aqueous extracts.
Figure 8. Effect of Piper nigrum on liver antioxidants in normal and experimental obese rats. Values are mean ± SD, n = 6. Values are statistically significant at *p < 0.05. a*Significantly different from control. b*Significantly different from HFD control. Activity is expressed as 50% inhibition of epinephrine auto oxidation per min for SOD; μmoles of hydrogen peroxide decomposed per min per mg of protein for catalase; μmoles of glutathione oxidized per min per mg of protein for GPx; μg/mg protein for GSH; mM/100 g tissue for TBARS.
Discussion
HFD-induced obesity in animals is considered to be a good model as they have been reported to bear close resemblance to human obesity (Shah et al., Citation2011). Supplementation of HFD to rodents caused visceral adiposity, hyperglycemia, dyslipidemia, hyper-insulinemia, and hepatic steatosis, which are distinctly linked with human obesity (Duiyan et al., Citation2013). Rodents fed with HFD had obesity since it facilitates the development of a positive energy balance leading to an increase in visceral fat deposition; this led to abdominal obesity in particular. The present study clearly depicted that rats fed with high-fat foods elicited a significant increase in body weight, lean mass, total fat, fat percentage, and fat-free mass.
Administration of PnL extracts, more particularly ethyl acetate and aqueous extracts (200 mg/kg bw/d), significantly suppressed the increase in body weight in dietary obese rats after 6 weeks. Thus, the reduced body weight, lean mass, total fat, fat percentage, and fat free mass, which could be attributed partially to a decrease in the mass of the fat pad in the pepper-supplemented groups, reflected a marked antiobesity effect of pepper. This might be due to the possible role of pepper to alter energy expenditure by increasing the expression of fat burning proteins (Kyung et al., Citation2011) and also inhibition of lipogenesis mediated by decrease in gene expression of FAS and malic enzyme (Yamashita et al., Citation2007).
The criterion for the predictive diagnosis of metabolic disorder is based on the glyco-metabolic features. Many reports have shown that long-term exposure to HFD in experimental animals resulted in hyperlipidemia, increased body weight, mild hyperglycemia, and a symptom of insulin resistance (Axen et al, Citation2003; Duiyan et al., Citation2013). In the present study, HFD-induced obese rats developed a hyperglycemic state associated with insulin resistance and/or glucose intolerance. This is in line with previous reports (Woods et al., Citation2003).
The OGTT is advantageous as it is performed under rather physiological conditions, as glucose is given per oral thus preserving the effect of entero hormone response and the physiological kinetics of glucose absorption (Belfiore & Iannello, Citation1998). The OGTT stimulates a post-absorptive state in which the production and the release of insulin and its responsiveness are necessary (Woods et al., Citation2003). Administration of pepper extracts in our study significantly lowered plasma glucose, insulin level, and insulin resistance in HFD-fed obese rats when compared with HFD control rats. Pepper extract promotes insulin sensitivity, thus lowering insulin resistance in obese rats, possibly by regulating the cell energy metabolism or reducing free fatty acids (Obici et al., Citation2002).
High-fat diet-induced obesity is associated with dyslipidemia characterized by an increase in triglycerides, VLDL-C, total cholesterol, phospholipids, free fatty acids, and LDL-C and a decrease in serum level of HDL-C (Duiyan et al., Citation2013). In the present study, feeding on HFD increased the plasma lipid profiles of experimental rats. During the period of HFD intake, the hepatic tissue converts glucose into FFAs, from which TGs are made, transported to blood stream as VLDL-C and stored as fat in the adipose tissue (Fungwe et al., Citation1993). Elevated total cholesterol and LDL-C are the risk factors for coronary heart disease, while high HDL-C is helpful in transporting excess cholesterol to liver for excretion in the bile (Saravanan & Ponmurugan, Citation2012). During obesity, the elevated level of phospholipids may be due to elevated levels of FFAs (Duiyan et al., Citation2013) and TC which can promote the synthesis of PLs. Oral supplementation of different extracts of PnL to HFD-induced obese rats significantly lowered plasma TC, TGs, FFAs, PLs, and LDL-C levels. The suppression of increment of plasma lipids by pepper extracts might result from the reduced absorption of fat and cholesterol due to inhibition of the activity of pancreatic lipase (Yongqi et al., Citation2009). Further, these Piper species have earlier been reported to prevent the oxidation of LDL-C (Agbor et al., Citation2007) and the progression of atherosclerosis in the diet-fed hamsters through the enhancement of an antioxidant protection mechanism (Agbor et al., Citation2012).
Leptin and adiponectin, the circulating hormones, are mainly secreted by adipose tissue and play a very important role in maintaining energy homeostasis. During the development of obesity, elevated level of leptin and concomitant decreased level of adiponectin were observed (Arita et al., Citation1999; Kamohara et al., Citation1997). Leptin acts both centrally and peripherally, with a major role in the regulation of food intake, body weight, and energy balance (Friedman, Citation2004) and its secretion levels are usually positively correlated with the extent of TGs stores in adipocytes (Staiger & Haring, Citation2005). Adiponectin is known to contribute to insulin sensitivity and fatty acid oxidation (Rosen & Spiegelman, Citation2006). In the present study, increased leptin and decreased adiponectin levels were observed in plasma of the HFD control group compared with the normal control group. Oral treatment with pepper extracts decreased leptin and increased adiponectin in plasma of HFD-induced obese rats. These observations suggest that the decreased leptin and increased adiponectin levels after administration of pepper extracts, especially ethyl acetate and aqueous extracts of PnL, may have ameliorated insulin resistance in obese rats, resulting in decreased plasma lipid levels and weight loss.
In the present study, elevated levels of TGs and lipase were observed in obese rats. Oral supplementation of pepper extracts inhibited the activity of pancreatic lipase in HFD-fed rats compared with HFD control rats. Many antilipemic drugs including orlistat act as lipase inhibitors and clinically employed to prevent obesity and hyperlipidemia (NICE Clinical Guidelines, Citation2006) by decreasing the digestion and absorption of dietary fat in blood and increasing fat excretion in the feces. It has been reported that the effect of piperine, an active compound of Piper longum on energy intake, is similar to orlistat (Kumar et al., Citation2013). The decreased absorption of ingested fat leads to an overall decreased caloric absorption, in turn leading to weight loss (Tsujita et al., Citation2006).
Enlarged adipocytes size is the prompt character of obesity, which correlates with insulin resistance. Lipid deposition in non-adipose tissues such as liver has deleterious consequences on organ function, leading to resultant cell dysfunction or cell death. Excess lipid is esterified and stored as TGs in lipid droplets (Schaffer, Citation2003). During obesity, an elevated level of TGs content in adipose tissue is possible (Van Herpen & Schrauwen-Hinderling, Citation2008). Amplified importation of fatty acids, elevated synthesis within the tissue, and/or decreased fatty acid oxidation (Jump, Citation2004) promote the conversion of excess fatty acids into phospholipids and cholesterol which results in lipid accumulation in non-adipose tissues. SREBP-1c, a gene transcription factor, has the critical role in scheming the transcription of genes involved in fatty acid synthesis, especially in liver (Jump, Citation2004). Several lipogenic genes, such as FAS and acetyl-CoA carboxylase, were regulated by SREBP-1c at the transcriptional level (Sato, Citation2010). Suppression of lipogenic enzymes including FAS and ACC can attenuate body fat accumulation (Okamoto et al., Citation2011). In our work, HFD-fed rats that received PnL extracts or orlistat showed a decrease in liver cholesterol, triglycerides and phospholipids, and FFAs. This might be due to regulation of lipogenic genes by specific transcription factors. This is in line with previous findings (Hyejeong et al., Citation2012). In the present study, HFD-fed rats showed increased adiposity index and it might be due to the consumption of calorie-rich diet in the form of fats which later get deposited in various body fat pads (retroperitoneal, epididymal, and mesenteric fat) (Oben et al., Citation2006; Srinivasan et al., Citation2004), and also leads to excessive growth of adipose tissue. However, on treatment with PnL extracts, there was a significant decrease in adiposity index, which proved its antiobese action.
The liver function test is essential since liver is the vital organ in detoxification of compounds. During diet-induced obesity, the liver of obese rats displayed characteristic features of hepatic steatosis such as fat accumulation and swelling of rough endoplasmic reticulum and mitochondria in hepatocytes (Das et al., Citation2012). In general, enzymes are excellent diagnostic tools of tissue damage and their levels alter in blood stream in diseased conditions. Many reports showed elevated activities of AST, ALT, and ALP in blood during hepatic damage (Saravanan et al., Citation2009) which indicates the hepatotoxic effect of HFD on rats (Seoyoon et al., Citation2013). In the present study, HFD-fed rats showed increased activity of these enzymes. These manifestations are end result of metabolic changes, with an increase of gluconeogenesis and of cetogenesis and/or of hepatic lesions that occur in metabolic syndrome (Sharma et al., Citation2012). Oral administration of PnL extracts caused a reduction in the activity of these enzymes when compared with the HFD control group and accordingly might alleviate hepatic damage caused by HFD-induced obesity. This is in agreement with previous report (Sharma et al., Citation2012).
Cellular oxidative damage is always accompanied with derangement of multiple enzymatic and non-enzymatic antioxidant defense systems present in cells. Enzymatic antioxidants such as SOD, CAT, and GPx synergistically scavenge reactive oxygen species and prevent lipid peroxidation. SOD, the natural cellular antioxidant enzyme, plays a pivotal role in oxygen defense metabolism by intercepting and reducing superoxide to hydrogen peroxide (Winterbourn, Citation1995). Catalase, a heme-containing ubiquitous enzyme, is known to be involved in the detoxification of high H2O2 concentrations and protects the tissues from highly reactive hydroxyl radicals. GPx, selenium-containing tetrameric glycoprotein, present in significant concentrations, detoxifies H2O2 into water and molecular oxygen through the oxidation of reduced GSH (Saravanan & Ponmurugan, Citation2011). GPx has been shown to be an important adaptive response to a condition of increased peroxidative stress. Earlier studies showed that during HFD-induced obesity, enzymatic antioxidants levels got considerably reduced. In the present study, decreased hepatic SOD, CAT, and GPx activities were found in HFD-fed rats. This is in line with Sharma et al. (Citation2012) who reported declined level of antioxidant enzymes in HFD-fed animals which could be as a result of increased production of free radicals. Oral administration of PnL extracts improved SOD, CAT, and GPx activities and decreased TBARS significantly in HFD-fed rats. This indicates the potential of pepper as an effective inhibitor of HFD-induced oxidative stress.
Conclusion
In conclusion, the present study suggests that hexane, ethyl acetate, ethanolic, and aqueous extracts of PnL have anti-obesity and antihyperglycemic activities. Among the four extracts, ethyl acetate and aqueous extracts have been found to be more efficacious in obesity treatment. The therapeutic efficacy can be attributed to its phytochemicals such as alkaloids, tannins, and polyphenols.
Acknowledgements
The authors are thankful to Dr. C. Venkatarao, Department of Chemistry, Sri Venkateswara University for his suggestions.
Declaration of interest
The authors report that they have no potential conflicts of interest. Authors are thankful to Department of Biotechnology, New Delhi, India (Grant No: BT/PR7799/PBD/17/849/2013) for providing Junior Research Fellowship and financial assistance to carry out this research work.
References
- Aebi H. (1984). Catalase in vitro. Methods Enzymol 105:121–6
- Agbor GA, Luli A, Julianne S. (2012). Piper species protect cardiac, hepatic and renal antioxidant status of atherogenic diet fed hamsters. Food Chem 134:1354–9
- Agbor GA, Vinson JA, Patel S, et al. (2007). Effect of selenium and glutathione-enriched yeast supplementation on a combined atherosclerosis and diabetes hamster model. J Agric Food Chem 55:8731–6
- Arita Y, Kihara S, Ouchi N. (1999). Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
- Axen KV, Dikeakos A, Sclafani A. (2003). High dietary fat promotes syndrome X in non-obese rats. J Nutr 133:2244–9
- Belfiore F, Iannello S. (1998). Insulin resistance in obesity: Metabolic mechanisms and measurement methods. Mol Gen Met 65:121–8
- Birari RB, Gupta S, Mohan CG, Bhutani KK. (2011). Antiobesity and lipid lowering effects of glycyrrhizachalcones: Experimental and computational studies. Phytomedicine 18:795–801
- Browning K, Fortna SR, Hajnal A. (2013). Roux-en-Y gastric bypass reverses the effects of diet induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol 9:2357–72
- Canbakan B, Tahan V, Balci H, et al. (2008). Leptin in nonalcoholic fatty liver disease. Ann Hepatol 207:249–54
- Centre for Public Health Excellence at NICE (UK); National Collaborating Centre for Primary Care (UK). (2006). Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children [Internet]. London: National Institute for Health and Clinical Excellence (UK); Dec (NICE Clinical Guidelines, no. 43.) Available from: http://www.ncbi.nlm.nih.gov/books/NBK63696/ [last accessed 26 Mar 2015]
- Das N, Sidkar K, Santinath G. (2012). Moringa oleifera Lam. leaf extract prevents early liver injury and restores antioxidant status in mice. Ind J Exp Biol 50:404–12
- Duiyan J, Yi X, Xin M, et al. (2013). Antiobesity and lipid lowering effects of the aflavins on high-fat diet induced obese rats. J Funct Foods 5:1142–50
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–7
- Finucane MM, Stevens GA, Cowan MJ, et al. (2011). National, regional and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377:557–67
- Floch J, Lee M, Stanley GHS. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
- Fraga CG, Leibouitz BE, Toppel AL. (1988). Lipid peroxidation measured as TBARS in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radic Biol Med 4:155–61
- Friedman JM. (2004). Modern science versus the stigma of obesity. Nat Med 10:563–9
- Fungwe TV, Cagen LM, Cook GA. (1993). Dietary cholesterol stimulated hepatic biosynthesis of triglyceride and reduces oxidation of fatty acids in the rat. J Lipids Res 34:933–41
- Gayasuddin Md, Parvez Md, Mohd I, Venkataiah G. (2013). Effect of ethanolic extract of Piper nigrum L. fruits on midazolam induced hypnosis in rats. Int Pharmacol Toxicol 3:5–8
- Hsu CL, Wu CH, Huang SL, Yen GC. (2009). Phenolic compounds rutin and O-coumaric acid ameliorate obesity induced by high fat diets in rats. J Agric Food Chem 57:425–31
- Hyejeong J, Youngshim C, Ui-Hyun P. (2012). Piperine, an LXRa antagonist, protects against hepatic steatosis and improves insulin signaling in mice fed a high-fat diet. Biochem Pharm 84:1501–10
- Jump DB. (2004). Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci 41:41–78
- Kakkar B, Das P, Viswanathan N. (1984). A modified spectrophotometric assay of SOD. Ind J Biochem Biophys 21:130–2
- Kamohara S, Burcelin R, Halaas JL, et al. (1997). Acute stimulation of glucose metabolism in mice by leptin treatment. Nature 389:374–7
- Kopelman PG. (2000). Obesity as a medical problem. Nature 404:635–43
- Kumar S, Sharma S, Vsudeva N. (2013). Screening of antidiabetic and antihyperlipidemic potential of oil from Piper longum and piperine with their possible mechanism. Expert Opin Pharmacother 13:1723–36
- Kyung JK, Myoung SL, Keunae J. (2011). Piperidine alkaloids from Piper retrofractum Vahl. protect against high-fat diet-induced obesity by regulating lipid metabolism and activating AMP-activated protein kinase. Biochem Biophys Res Commun 411:219–25
- Masood SB, Imran P, Muhammad TS, et al. (2013). Black pepper and health claims: A comprehensive treatise. Crit Rev Food Sci Nutr 53:875–86
- Mattews Dr, Hosker JP, Rudenski AS, Naylor BA. (1985). Homeostasis model assessment: Insulin resistance beta cell functions from plasma insulin concentrations in man. Diabetologia 28:412–19
- Oben J, Kuate D, Agbor G, et al. (2006). The use of a Cissus quadrangularis formulation in the management of weight loss and metabolic syndrome. Lipids Health Dis 5:24–9
- Obici S, Feng Z, Karkanias G, et al. (2002). Decreasing hypothalamic insulin receptors causes hyper phagia and insulin resistance in rats. Nat Neurosci 5:566–72
- Okamoto M, Irii H, Tahara Y, et al. (2011). Synthesis of new [6]-gingerol analog and its protective effect on the development of metabolic syndrome in high-fat diet-fed mice. J Med Chem 1:1–31
- Paglia D, Valentine W. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–69
- Prasad SSS, Sukapaka M, Prathipati VK, et al. (2012). Carbenoxolone treatment ameliorated metabolic syndrome in WNIN/Ob obese rats, but induced severe fat loss and glucose intolerance in lean rats. PLoS One 12:e50216
- Rosen ED, Spiegelman BM. (2006). Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444:847–53
- Saravanan G, Ponmurugan P, Senthilkumar GP, Rajarajan T. (2009). Antidiabetic properties of S-allylcysteine, a garlic component on streptozotocin-induced diabetes in rats. J Appl Biomed 7:151–9
- Saravanan G, Ponmurugan P. (2011). Ameliorative potential of S-allyl cysteine on oxidative stress in STZ induced diabetic rats. Chem-Biol Inter 189:100–6
- Saravanan G, Ponmurugan P. (2012). Ameliorative potential of S-allylcysteine: Effect on lipid profile and changes in tissue fatty acid composition in experimental diabetes. Exp Toxicol Pathol 64:639–44
- Sato R. (2010). Sterol metabolism and SREBP activation. Arch Biochem Biophys 501:177–81
- Schaffer JE. (2003). Lipotoxicity: When tissues over eat. Curr Opin Lipidol 14:281–7
- Seoyoon C, Youngshim C, Yeji C. (2013). Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chem 141:3627–35
- Shah SS, Gaurang B, Sabdeer SD. (2011). Effect of piperine in the regulation of obesity induced dyslipidemia in high fat diet rats. Ind J Pharm 43:296–9
- Sharma AK, Bharti S, Bhatia J. (2012). Sesamol alleviates diet-induced cardiometabolic syndrome in rats via up-regulating PPARγ, PPARα and e-NOS. J Nutr Biochem 23:1482–9
- Singh R, Singh N, Saini BS, Rao HS. (2007). Hepatoprotective and antioxidant properties of methanolic extract of Piper nigrum Linn. in rats. Phcog Mag 3:251–8
- Srinivasan K, Patole PS, Kaul CL, Ramarao P. (2004). Reversal of glucose intolerance by pioglitazone in high-fat diet fed rats. Exp Clin Pharmacol 26:327–33
- Srinivasan K. (2007). Black pepper and its pungent principle – Piperine: A review of diverse physiological effects. Crit Rev Food Sci Nutr 47:735–48
- Staiger H, Haring HU. (2005). Adipocytokines: Fat-derived humoral mediators of metabolic homeostasis. Exp Clin Endocrinol Diabetes 113:37–79
- Tsujita T, Takaichi H, Takaku T. (2006). Antiobesity action of E-polylysine, a potent inhibitor of pancreatic lipase. J Lipid Res 47:1852–8
- Van Herpen NA, Schrauwen-Hinderling VB. (2008). Lipid accumulation in non-adipose tissue and lipotoxicity. Phys Behav 94:231–41
- Venu L, Harishankar N, Prasanna KT, Raghunath M. (2004). Maternal dietary vitamin restriction increases body fat content but not insulin resistance in WNIN rat offspring up to 6 months of age. Diabetologia 47:1493–501
- Winterbourn CC. (1995). Concerted antioxidant activity of glutathione and superoxide dismutase. In: Packer L, Fuchs J, eds. Biothiols in Health and Disease. New York: Marcel Dekker Inc, 117–34
- Woods SC, Seeley RJ, Rushing PAA. (2003). Controlled high fat diet induces an obese syndrome in rats. J Nutr 133:1081–7
- Yamashita H, Fujisawa K, Ito E. (2007). Improvement of obesity and glucose tolerance by acetate in type 2 diabetic otsuka long-evans tokushima fatty (oletf) rats. Biosci Biotech Biochem 71:1236–43
- Yongqi G, Guanzhong W, Xin S, et al. (2009). Antiobesity action of a daidzein derivative on male obese mice induced by a high-fat diet. Nutr Res 29:656–63
