Abstract
Context: Allergic rhinitis (AR) is a global health problem that affects a large number of population. Piperine (PIP) has been reported to exhibit anti-inflammatory, anti-histaminic, and immunomodulatory activities; however, its antiallergic profile has not been studied.
Objective: The objective of the study was to investigate the antiallergic potential of PIP in ova-albumin (OVA)-induced AR, mast cell degranulation (MSD), and OVA-induced paw edema.
Materials and methods: Mice were sensitized with OVA alternately on 1, 3, 5, 7, 9, 11, and 13th day. They were treated with either vehicle, PIP (10, 20, and 40 mg/kg, p.o.), or montelukast (10 mg/kg, p.o.) from the 14th to 20th day. On the 21st day, intranasal (OVA: 5% µl) challenge was done. Animals were evaluated for physiological parameters, biochemical parameters, spleen weight, expression of interleukins (IL-6 and IL-1β), and immunoglobin-E (IgE). Histopathology of nasal mucosa, lungs, and spleen was carried out. MSD and paw edema studies were made to understand the mechanism of action.
Results: PIP (10, 20, and 40 mg/kg, p.o.) showed a significant dose-dependent protection with respect to nasal rubbing, redness of nose, and sneezing (p < 0.001) following nasal challenge. PIP dose dependently reduced histamine, NO concentration (p < 0.001), as well as reduced expression of IL-6, IL-1β, and IgE (p < 0.001) as compared with the control group. Histopathology showed inhibition of infiltration of eosinophils and hyperplasia. It dose dependently reduced MSD and paw edema (p < 0.001).
Discussion and conclusion: PIP acts by mast cell-stabilizing activity, exhibits immunomodulatory and anti-inflammatory activity, thereby providing an effective treatment for AR.
Introduction
Allergic rhinitis (AR) is a global health problem that causes major illness and disability worldwide (Ozdoganoglu & Songu, Citation2012). Patients from all countries, all ethnic groups, and of all ages suffer from AR. It affects social life, sleep, school, and work. The economic impact of AR is often underestimated because the disease does not induce elevated direct costs. However, the indirect costs are substantial (Brożek et al., Citation2010).
AR, an inflammatory condition of the nasal mucosa mediated by an IgE-associated response to indoor and outdoor environmental allergens, has traditionally been classified as being seasonal or perennial, depending on whether an individual is sensitized to cyclic pollens or year round allergens, such as dust mites, pets, cockroaches, and moulds (Greiner & Meltzer, Citation2006). Patients with AR have symptoms such as sneezing, watery nose, marked increase in eosinophils in the nasal submucosa, and epithelium as well as chronic inflammation of the nasal mucosal membrane (Okubo et al., Citation2011; Wang & Clement, Citation2000).
Antihistamines, corticosteroids, and mast cell stabilizers are used mainly in the treatment of AR but they produce potentially dangerous effects (Okubo et al., Citation2011; Ramírez et al., Citation2011; Walter et al., Citation2011). Commonly used first-generation antihistamines (diphenhydramine, brompheniramine, and chlorpheniramine) have poor selectivity for the H1-receptor and, as such, inhibit muscarinic receptors, which might result in various degrees of mucous membrane drying, vision blurring, constipation, urinary retention, tachycardia, and sedation (Okubo et al., Citation2011). Specific H1 antagonists like rupatadine, although provide benefit, but interfere with the metabolism of many drugs (Saint-Martin et al., Citation2003). The use of glucocorticosteroids (Al Suleimani & Walker, Citation2007) and anticholinergics is questionable due to long-term adverse effects (Nathan, Citation2007). Prophylactic use of mast cell stabilizers has the disadvantage of frequent administration due to the redundancy, synergy, and pleiotropism that exists for the mediators of AR (Al Suleimani & Walker, Citation2007; Gelfand, Citation2004).
Piper nigrum L. (Piperaceae) fruit is traditionally used for upper respiratory tract disorders such as cough, AR, sinusitis, and bronchitis (Agarwal & Agarwal, Citation2004; Szelenyi & Brune, Citation2002). PIP, an active alkaloid present in Piper nigrum, exhibits a wide variety of biological effects including anti-tumor (Lai et al., Citation2012; Makhov et al., Citation2012), anti-apoptotic (Muthukumar & Vanisree, Citation2011; Pathak & Khandelwal, Citation2009; Shrivastava et al., Citation2012), antibacterial (Chen et al., Citation2010; Karsha & Lakshmi, Citation2010), antioxidant (Kumar et al., Citation2010; Mehta et al., Citation2012), immunomodulatory (Alamgir & Uddin, Citation2010; Chuchawankul et al., Citation2012; Pathak & Khandelwal, Citation2009), anti-inflammatory (Bang et al., Citation2009), and antihistaminic activities (Gohil et al., Citation2011; Mehmood & Gilani, Citation2010). PIP has also been reported to reduce T helper cell type 2 (Th2) cytokines (IL-4 and IL-5). It has also been reported to lower the levels of eosinophil, expression of interterleukin-13 MRNA in lung tissue as well as histamine and IgE production in serum (Kim & Lee, Citation2009). Therefore, the present study was aimed to evaluate the potential of PIP against OVA-induced AR in mice.
Materials and methods
Drugs and chemicals
PIP and OVA (grade V) were procured from Sigma Aldrich (St. Louis, MO), histamine (Loba Chemie, Pune, India), cetirizine–HCl, and montelukast sodium (Cipla Pvt. Ltd, Aurangabad, India), clonidine (Neon Laboratories Ltd., Mumbai, India), and disodium cromoglyacate (DSCG) (Ahlcon Parentrals, Pune, India) were obtained as gift samples. O-Thalaldialdehyde (Thomas Baker Ltd., Mumbai, India), IL-6 kit (Quantikine, R&D Systems, Minneapolis, MN), and RPMI (Sigma Aldrich, St. Louis, MO) were purchased. Bordetella bronchiseptica (Cat. no. NCIM2267, batch no. ATCC-14455) was obtained from the National Collection of Industrial Micro-organisms (NCIM), National Chemical Laboratory (NCL), Pune, India.
Experimental animals
Swiss albino mice (25–35 g) were procured from the Sinhgad Institute of Pharmaceutical Sciences, Lonavala, Pune, India. Animals were housed in diurnal lighting conditions (12 h/12 h) and allowed free access to food and water for 7 d prior to the experiment. The experiments were performed according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and were approved by the Institutional Animal Ethical Committee (IAEC) of Sinhgad Institute of Pharmacy, Pune, India (approval no. IAEC/SIOP/2012/30).
Experimental protocol induction of rhinitis
Experiments were carried out in rooms free of noise and strong odors. The temperature and the relative humidity were also controlled in the range of 23 ± 2 °C and 55 ± 5%, respectively. The animals were placed 40 min in advance in the perspex observation boxes (26 × 26 × 26 cm3) for acclimatization. Observation boxes were thoroughly cleaned after each observation for every new animal.
Sensitization protocol
The sensitization solution (SS) consisted of 50 mg of ovalbumin, 1000 mg of aluminum hydroxide dissolved in 500 ml of saline, and 0.5 ml of 1 × 1010 Bordetella bronchiseptica (Alcaligenaceae). Briefly, on days 1, 3, 5, 7, 9, 11, and 13, mice were sensitized by i.p. injection of 500 μl of SS, except Group I – normal (vehicle). The remaining mice were then divided into five groups (n = 6). Group II – AR control; Group III – standard drug, montelukast (10 mg/kg, p.o.); Groups IV, V, and VI – PIP (10, 20, and 40 mg/kg, p.o.), followed by treatment with PIP or montelukast up to the day 20th. On 21st day, all groups were challenged with intranasal OVA (0.5 µl of SS) administration. Animals were evaluated for physiological and biochemical parameters, spleen weight, and histopathology (Wang et al., Citation2007).
Evaluation of parameters
Body weight
To monitor the effect of test drug on body weight, animals were weighed periodically on 0th, 7th, 14th, and 21st day.
Physiological parameters
Symptoms of rhinitis were observed and scored as following: the number of sneezing and rubbing/unit times was counted continuously in a randomized blind fashion directly for 40 min following nasal challenge. Sneezing is characterized by an explosive expiration just after a deep inspiration and nose rubbing is characterized by an external perinasal scratch with either one or both forelimbs of animal (Zhao et al., Citation2005). Redness of the nose was evaluated by observing the intensity in redness of nostrils and scoring them as the following: 0 – normal, 1 – faint, 2 – medium, and 3 – dark (Suleimani et al., Citation2008).
Biochemical parameters
Estimation of nitric oxide [nitrite concentration (NO)]
Nitric oxide is one of the important mediators in regulating patency of airways and its concentration increases in allergies related to the upper respiratory tract. The nitric oxide concentration in plasma samples was measured by the Griess reagent method, the absorbance was measured at 548 nm using the UV–visible spectrophotometer (V630, Jasco Analytical Instrument, Lakewood, NJ). The concentrations of nitrite were determined from standard curves constructed with serial dilutions of sodium nitrite (Iijima et al., Citation2001).
Measurement of histamine concentration
Histamine is a biogenic amine, noted for manifestation of certain allergic reactions and implicated as a mediator of hypersensitivity. Thus, histamine concentration in serum by an OPA spectrofluorometric procedure was estimated. The fluorescence intensity was measured at 460 nm (an excitation at 355 nm) using a spectrofluorometric method (Patange et al., Citation2005).
Measurement of IL-6, IL-1β, and IgE
IL-6, IL-1β, and IgE are the important inflammatory mediators released to stimulate immune response. The estimation of IL-6, IL-1β, and IgE in the serum was carried out immediately after challenge. The concentrations were estimated as per the instructions and procedure given in ELISA kit (Quantikine ELISA kit, R&D Systems, Minneapolis, MN).
Spleen weight
After 24 h, followed by the last intranasal challenge, animals were sacrificed and their spleen was separated, moisture was removed by blotting with the filter paper, and the organ weight was taken using a digital weighing balance.
Histopathological evaluation
Mice were euthanized after the intranasal OVA challenge test. The lung, spleen, and nose tissues were immersed in the freshly prepared 10% neutral buffered formalin for a period of 48 h. Following this, the tissue was rinsed in running tap water and decalcified in 10% nitric acid solution. After rinsing in tap water, the tissue processed for dehydration through graded alcohols and embedded in paraffin, sectioned, and stained with hematoxylin–eosin stains. Sections were examined by a light electron microscope (40 × ) (Motic digital microscope with the image plus 2.0 software, Olympus Corporation, Tokyo, Japan). The intensity of inflammatory cell recruitment in nasal mucosa was graded using an arbitrary scale by the histopathologist: Grade 0 – not present or very slight, Grade 1 – mild, Grade 2 – moderate, and Grade 3 – severe disruption of epithelial observed in individual slides were also noted. All slides were coded before analysis and read blind to avoid observer bias (Zhao et al., Citation2005).
Measurement of paw edema
In the second set of animals, OVA-induced paw edema was carried out to study the antihistaminic activity of PIP. The procedure described by Medeiros et al. (Citation2008) was followed with slight modification. Swiss albino mice were divided in following groups. Group I, normal: vehicle treated without challenge; Group II, control: vehicle treated and challenged; Group III, the standard group treated with cetrizine (10 mg/kg); and Groups IV, V, and VI were treated with test drug PIP (10, 20, and 40 mg/kg, p.o.). The standard and test drugs were given orally for 4 d. On the fourth day, all mice except Group I were challenged with SS-containing OVA (10 µg/0.1 ml), by sub-plantar injection in the hind paw. The collateral paw received only vehicle. Paw thickness was measured at 1, 3, and 24 h after challenge using the plethysmometer (VJ Instruments, Karanja, India). The inhibition was calculated using the formula:
Mast cell degranulation (MSD)
MSD study was carried out using the reported method (Nirmal et al., Citation2012; Venkatesh et al., Citation2009). The Swiss albino mice were divided into five groups (n = 6), the groups were divided as the following: Group I: control, Group II: standard, DSCG (50 mg/kg, p.o.), Groups III, IV, and V treated orally with PIP (10, 20, and 40 mg/kg, p.o.). The treatment was given for 4 d, on the fourth day, all groups were injected with 5 ml of cold phosphate-buffer i.p. After gentle abdominal massage for 10 s, the peritoneal fluid was collected in centrifuge tubes, containing RPMI-1640 media (pH 7.2–7.4). The cell viability was checked using trypan blue dye (0.4%). Cells were washed three times with RPMI-1640 media by centrifugation at a low speed (500–600 rpm), after discarding the supernatant, the pellets of mast cells were resuspended in the medium. Mast cells were incubated with a mast cell degranulating agent, clonidine (50 µg/ml), at 37 °C for 10 min in the incubator. Then the cells were stained with toludine blue (1%) and the numbers of degranulated and intact mast cells were counted from the total number of 100 cells observed under high-power microscope. The percent protection of mast cells was calculated using the following formula:
Statistical analysis
Data for each parameter were analyzed by one-way ANOVA followed by Dunnet's post hoc test, body weight was analyzed using two-way ANOVA followed by the Bonferroni post hoc test using a GraphPad, prism software, version 4.03 (GraphPad Software, Inc., La Jolla, CA).
Results
Effect of PIP on body weight
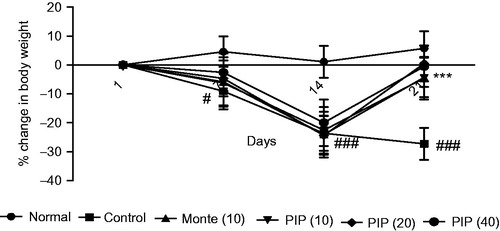
shows the periodic observation in body weight of normal, AR control, standard drug montelukast (10 mg/kg), and test drug PIP (10, 20, and 40 mg/kg) treated groups. There was a significant decrease in the body weight from the seventh day onwards in all the treatment groups (p < 0.05 and p < 0.001) except normal. Treatment with montelukast as well as PIP (10, 20, and 40 mg/kg) significantly attenuated the loss in body weight as observed on day 21.
Figure 1. Effect of piperine (10, 20, and 40 mg/kg) and montelukast (10 mg/kg) treatment on body weight in allergic rhinitis-induced mice (n = 6). Values are expressed in mean ± SEM. #p < 0.05, ###p < 0.001 compared with the normal group, ***p < 0.001 compared with the AR control group. Figure in parentheses indicates dose in mg/kg, p.o. PIP, piperine; Monte, montelukast.
Effect of PIP on nasal symptoms
shows the effect of PIP (10, 20, and 40 mg/kg) on OVA-induced nasal symptoms in mice. The AR control group showed a significant increase in sneezing, nasal rubbing, as well as nasal redness. PIP dose dependently reduced sneezing and nasal rubbing (p < 0.001). The effects were comparable with that of the standard. Nasal redness as observed by the unbiased observer was very high in the AR control group. The redness was significantly attenuated by PIP as well as by montelukast.
Table 1. Effect of piperine (10, 20, and 40 mg/kg, p.o.) and montelukast (10 mg/kg, p.o.) on sneezing, nasal rubbing, and nasal redness in allergic rhinitis-induced mice.
PIP ameliorates allergic and inflammatory mediators
shows the marked increase in NO level in the AR control group as compared with the normal value (p < 0.001). PIP (10, 20, and 40 mg/kg) as well as montelukast (10 mg/kg) dose dependently reduced the level of NO in serum samples. The reduction in NO level was significant (p < 0.001) as compared with the AR control group.
Figure 2. Effect of piperine (10, 20, and 40 mg/kg) and standard drug montelukast (10 mg/kg) on nitric oxide (μg/ml) (A), histamine concentration (μg/ml) (B), average spleen weight (mg) (C), IL-1β concentration (pg/ml) (D), Ig-E concentration (ng/ml) (E), and IL-6 concentration (pg/ml) (F) in allergic rhinitis-induced mice (n = 6). Values are expressed in mean ± SEM. ###p < 0.001 compared with the normal group, *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the AR control group. Figure in parentheses indicated dose in mg/kg, p.o. PIP, piperine; Monte, montelukast.
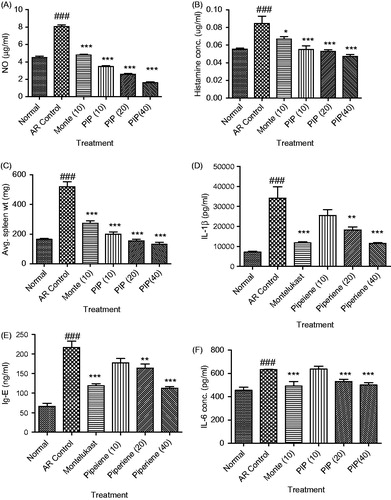
Stimulation by nasal allergen induces sneezing and nose rubbing due to the release of histamine. In the present study, significant increase in histamine level was observed in the AR control group (p < 0.001). PIP (10, 20, and 40 mg/kg) significantly reduced the histamine level (p < 0.001) as compared with the AR control. Montelukast also decreased the histamine level to a slight extent (p < 0.05) (). Serum IL-1β and IgE were significantly increased in the AR control group as compared with the normal mice (p < 0.001). Treatment with PIP (20 and 40 mg/kg) and montelukast significantly reduced it (p < 0.01 and p < 0.001). We further investigated the ability of PIP to inhibit the production of Th2 cytokine, IL-6, in order to understand its role in the last phase of AR. Significant rise in serum IL-6 was observed in the AR control group indicating inflammatory immune response. PIP (10, 20, and 40 mg/kg) as well as montelukast (10) decreased the IL-6 level in a dose-dependent manner (p < 0.001) ().
PIP normalized spleen weight
The spleen is a part of the mononuclear phagocyte system and its enlargement usually characterizes infection or immune response. Sensitization with OVA has shown to cause a marked increase in the spleen weight in the AR control group as compared with the normal. Animals treated with PIP (10, 20, and 40 mg/kg) as well as montelukast (10 mg/kg) have shown significant (p < 0.001) decrease in the spleen weight as compared with the AR control group ().
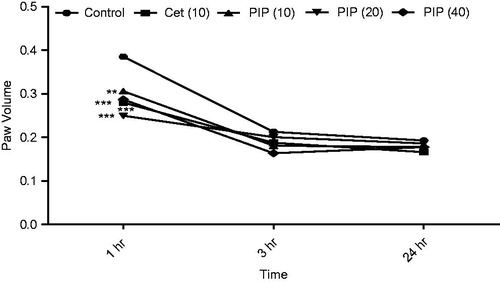
Figure 3. Effect of piperine treatment (10, 20, and 40 mg/kg) and cetrizine (10 mg/kg) on OVA-induced paw edema (n = 6). Values are expressed in mean ± SEM. **p < 0.01 and ***p < 0.001 compared with the control group. Figure in parentheses indicated dose in mg/kg, p.o. PIP, piperine; Cet, cetrizine.
Anti-histaminic activity of PIP
Prior treatment with PIP (10, 20, and 40 mg/kg) as well as standard drug cetrizine (10 mg/kg) showed significant inhibition (p < 0.001) of paw edema induced by OVA as compared with the control group at 1 h () suggesting anti-histaminic activity of PIP.
PIP stabilizes mast cells
and show the protective effect of PIP (10, 20, and 40 mg/kg) on mast cell degranulation. The control group exhibited a higher number of degranulated cells. PIP (20 and 40 mg/kg) (p < 0.001) showed maximum protection against degranulation by clonidine than PIP (10 mg/kg) (p < 0.05). The results of the higher dose of PIP were comparable with that of DSCG (50 mg/kg).
Figure 4. Effect of piperine treatment (10, 20, and 40 mg/kg) and disodium cromoglyacate (50 mg/kg) on mast cell degranulation. (a) The normal group showing intact mast cells, (b) the AR control group showing degranulation of mast cells, (c) montelukast (10 mg/kg) treated group section showing partial cell degranulation, (d) PIP (10 mg/kg), (e) PIP (20 mg/kg), and (f) the PIP (40 mg/kg) treated group shows stabilization of mast cells in a dose-dependent manner.
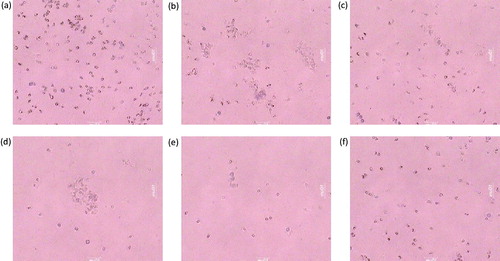
Table 2. Effect of piperine (10, 20, and 40 mg/kg, p.o.) and disodium chromoglycate (50 mg/kg, p.o.) treatment on mast cell degranulation.
Histopathology
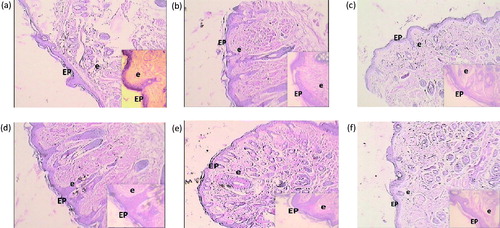
Photomicrograph representative sections of the lungs are shown in . The normal group showed intact alveoli, bronchioles, and epithelial cell linings. Treatment with OVA resulted in the degeneration of the alveoli and inflammation of the bronchial epithelium. Treatment with test as well as standard drug showed protection as there was comparatively less damage to alveoli, bronchioles, and epithelial cell linings. Prominent effects were obtained with PIP (40 mg/kg). shows histopathology of the spleen. The normal group showed intact red and white pulps. Red pulp is a reservoir of monocytes and induces an innate response while white pulps are rich in lymphocytes and induce an immune response through humoral cell-mediated pathways. The AR control group showed complete degeneration of white and red pulps, and protection was offered by all the treatment groups in a dose-dependent manner. represents photomicrograph of nasal mucosa. The nasal mucosa of the AR control group showed increased infiltration of eosinophils and disruption of nasal epithelium as compared with the normal mice (Grade 3). PIP (10 and 20 mg/kg) showed more intact epithelium and reduced eosinophil infiltrations (Grade 2) while montelukast and higher dose of PIP showed intact epithelium and very few eosinophil infiltrations (Grade 1).
Figure 5. Histological alteration in lung tissue of mice: photomicrograph of lung sections stained with hematoxylin and eosin: (a) the normal group shows alveoli and bronchioles having normal architecture. Epithelial cells linings are intact and inflammation is absent. (b) The AR control group section shows abnormal bronchiole epithelium and degenerated alveolar sacs. (c) Montelukast (10 mg/kg) treated group section shows partial cell disruption in alveoli and bronchioles. The epithelial cell linings show less damage. (d) The PIP (10 mg/kg) treated group demonstrates intact epithelial cell linings; partial protection of bronchioles and alveoli, with minimum Inflammation. (e) The PIP (20 mg/kg) treated group shows moderate intact alveoli and bronchiolar structures. (f) The PIP (40 mg/kg) treated group sections showed more protective effect on alveoli and bronchiolar epithelial membrane. A, alveoli; E, epithelium; B, bronchiole; PV, pulmonary vein; PIP, piperine; Monte, montelukast. Figure in parentheses indicates dose in mg/kg, p.o. (40 × ).
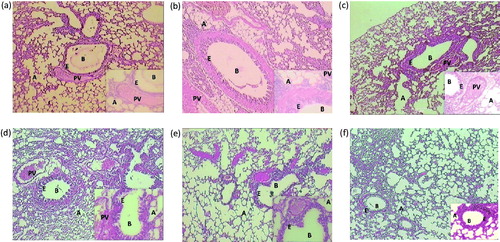
Figure 6. Histological alteration of spleen in mice: (a) the normal group shows intact white and red cell pulps. (b) The AR control group shows the complete disruption in white and red pulp cells, hyperplasia is observed. (c) Montelukast (10 mg/kg) group shows more protective effect on red and white pulps and less hyperplasia is observed. (d) PIP (10 mg/kg) shows protective effect on red and white pulp cells. (e) PIP (20 mg/kg) shows more protection of both cells and intact structure with less hyperplasia. (f) PIP (40 mg/kg) total protection of both pulp and hyperplasia is not observed. RP, red pulp; WP, white pulp; H, hyperplasia; PIP, piperine; Monte, montelukast. Figure in parentheses indicates dose in mg/kg, p.o. (40 × ).
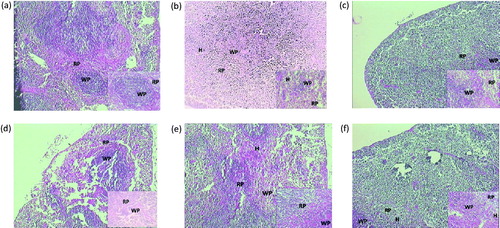
Figure 7. Histological alteration of nasal mucosa in mice: (a) the normal group shows intact epithelium and very few eosinophil infiltrations. (b) The AR control group shows abnormal epithelium, thickened subepithelial smooth muscle layer, and infiltration of eosinophil. (c) The montelukast (10 mg/kg) group shows intact epithelium and reduced eosinophil infiltration. (d) PIP (10 mg/kg) shows less intact epithelium and moderate number of eosinophil infiltrations. (e) PIP (20 mg/kg) shows more intact epithelium and reduced eosinophil infiltration. (f) PIP (40 mg/kg) shows intact epithelium and few eosinophil infiltration. EP, epithelium; e, eosinophiles; PIP, piperine; Monte, montelukast. Figure in parentheses indicates dose in mg/kg, p.o. (40 × ).
Discussion and conclusion
AR is characterized by symptoms such as sneezing, itchy nose, rhinorrhea, lacrimation, and nasal congestion. It is presumed to be triggered by multiple mediators released from the mast cells and other inflammatory cells (Al Suleimani & Walker, Citation2007). AR comprises two phases of allergic response. Prior phase involves the activation of mast cells and basophiles resulting in the production of histamine, prostaglandins, leukotriens, and cytokines, which causes sneezing, nasal itching, nasal congestion, and redness. The later phase involves migration of eosinophils, mast cells, and basophiles into the nasal tissue (Wang et al., Citation2007). The available treatment intends either to reduce the effect of release of mediators from activated cell or end organ effects of released mediators. Commonly used pharmacological agents are H1 receptor antagonist, mast cell stabilizer, corticosteroids, anti-leukotrienes, and anticholinergic agents (Greiner et al., Citation2012).
Piperine present in the long and black pepper has been used as a dietary factor in herbal medicine for centuries in the world. In India, it is widely used traditionally either in powdered or in decoction form, as a remedy for sore throat, cough, and nasal congestion. Piperine has already been reported for antihistaminic, immune-modulatory, anti-inflammatory, anti-apoptotic, and antioxidant activities (Meghwal & Goswami, Citation2013).
OVA-induced AR in mice shows similar nasal allergic symptoms as observed in humans (Wang et al., Citation2007). The objective of the present study was to evaluate antiallergic profile of piperine using OVA-induced AR, OVA-induced paw edema, and clonidine-induced mast cell degranulation. Administration of SS for 14 d showed induction of rhinitis in mice as observed by the episodes of nasal irritation on the challenge. Piperine significantly ameliorated sneezing, rubbing, and redness induced by sensitization of nerve endings resulted from histamine released in response to antigen–antibody reaction. Histamine being the major mediator of early-phase allergic response was also analyzed in the serum of sensitized mice. The level of histamine in sensitized animals was higher when compared with normal animals. Treatment with piperine dose dependently reduced histamine level in serum. The experiment demonstrated that piperine had antiallergic potential.
The cascade leading to AR includes production of IgE on antigen exposure. The acquisition of IgE by mast cell and basophils via the high affinity IgE receptor (FcεR1) promotes cross linking of bound IgE–FcεR1 complex with multivalent allergen. This results in release of proinflammatory and inflammatory mediators such as IL-6 and IL1β. The proinflammatory cytokine IL-6 has been recognized as an important mediator of allergy as it is secreted by cells of innate immunity. IL-6 has been reported to be released on allergen challenge in patients (Ohkubo et al., Citation1998). It is an important Th2 type of cytokine involved in the induction of IgE synthesis as well as in mast cell proliferation and maturation (Bachert et al., Citation1998; Guzmán et al., Citation2010). The beneficial effects of piperine can be ascribed to its attenuating effect on IgE, IL-6, and IL-1β concentration in serum. Our result corroborates with the previous study (Kim & Lee, Citation2009). The reduced level of these cytokines and immunoglobin suggests a potential use of piperine as antiallergic, due to its potent anti-inflammatory and immunomodulatory activities.
AR is associated with increased production of nitric oxide (Arnal et al., Citation1997; Yamada et al., Citation2012). Iijima et al. (Citation2001) have reported eosinophils as a measurable source of nitric oxide. In present study, exposure to OVA increased NO in AR control mice. There was significant reduction in the NO level in the entire treatment group; moreover, large numbers of eosinophils were observed in histopathological section of nasal mucosa in AR control mice. Piperine-treated group showed comparatively less migration of eosinophil into epithelial tissue. A similar effect was observed in histopathology of lung and spleen. The lung tissue of the AR control group showed complete disruption of alveoli and bronchiole morphology, inflammation, and redness in epithelial cell linings. Montelukast as well as piperine showed protection in epithelial cell lining and partial protection in the structure of bronchioles and alveoli. It also showed less inflammation and redness, thus the histological results obtained corroborate with the physiological, biochemical, and immunological findings.
The local injection of OVA into the paw of the animal results in acute allergic response due to the release of histamine from mast cells, release of proteoglycans (heparin and chondroitin sulfate E), proteases, and other inflammatory mediators (e.g., prostaglandins and leukotriens) at the later hours. In the present study, piperine inhibited the paw edema at first hour indicating its efficacy in attenuating histamine; moreover, the anti-inflammatory effects of PIP (40 mg/kg) were sustained till the third hour advocating its anti-inflammatory potential at higher dose. Earlier reports also support the potent anti-inflammatory activity of piperine (Bang et al., Citation2009; Sudjarwo, Citation2005).
The mast cell degranulation study was designed to understand the mechanism of action of piperine. Mast cells are important mediators of inflammatory response such as allergy and anaphylaxis (Kim et al., Citation2013), where histamine remains the best characterized mediator implicated in the acute phase of allergic response (Petersen et al., Citation1996). Mast cell degranulation can be induced by clonidine, a mast cell degranulator which leads to histamine release. The results showed increased degranulated cells in the control group. Piperine-treated animal showed prevention of granulation of mast cells when exposed to clonidine. Piperine dose dependently acted as an antiallergic by stabilizing the mast cells.
In conclusion, the above results showed that piperine prevented allergic responses by mast cell stabilization thereby inhibiting the release of mediators like histamine, IL-6, IL-1β, and IgE. The anti-inflammatory activity obtained provides additional benefits. The present study reveals the protective role of piperine, in OVA-induced AR, therefore, it is anticipated that piperine can be developed as a drug for clinical use.
Acknowledgements
The authors would like to acknowledge Prof. M. N. Navale, Founder, STES and Principal, SIOP, for providing the necessary facilities to carry out the research work.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Agarwal RK, Agarwal A. (2004). Herbal composition having antiallergic properties and a process for the preparation there of. US Patent 6730332
- Al Suleimani YM, Walker MJ. (2007). Allergic rhinitis and its pharmacology. Pharmacol Therapeut 114:233–60
- Alamgir M, Uddin SJ. (2010). Recent advances on the ethnomedicinal plants as immunomodulatory agents. In: Debprasad Chattopadhyay (ed). Ethnomedicine: A Source of Complementary Therapeutics. India: Research Signpost, 227–44
- Arnal JF, Didier A, Rami J, et al. (1997). Nasal nitric oxide is increased in allergic rhinitis. Clin Exp Allergy 27:358–62
- Bachert C, Wagenmann M, Holtappels G. (1998). Cytokines and adhesion molecules in allergic rhinitis. Am J Rhinol 12:3–8
- Bang JS, Choi HM, Sur BJ, et al. (2009). Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther 11:R49
- Brożek JL, Bousquet J, Baena-Cagnani CE, et al. (2010). Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immun 126:466–76
- Chen WX, Hu YY, Ge C, et al. (2010). Optimization technology for extracting antibacterial substances from black pepper. Sci Tech Food Ind 11:074
- Chuchawankul S, Khorana N, Poovorawan Y. (2012). Piperine inhibits cytokine production by human peripheral blood mononuclear cells. Genet Mol Res 11:617–27
- Gelfand EW. (2004). Inflammatory mediators in allergic rhinitis. J Allergy Clin Immun 114:S135–8
- Gohil P, Mehta K, Chauhan S, et al. (2011). Phytochemical and pharmacological screening of novel polyherbal formulations. Asian J Pharm Biol Res 1:112–22
- Greiner AN, Hellings PW, Rotiroti G, Scadding GK. (2012). Allergic rhinitis. Lancet 378:2112–22
- Greiner AN, Meltzer EO. (2006). Pharmacologic rationale for treating allergic and nonallergic rhinitis. J Allergy Clin Immun 118:985–96
- Guzmán C, Hallal-Calleros C, Lopez-Griego L, Morales-Montor J. (2010). Interleukin-6: A cytokine with a pleiotropic role in the neuroimmunoendocrine network. Neuroendocrinology 3:152–60
- Iijima H, Duguet A, Eum SY, et al. (2001). Nitric oxide and protein nitration are eosinophil dependent in allergen-challenged mice. Am J Respir Crit Care 163:1233–40
- Karsha PV, Lakshmi OB. (2010). Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J Nat Prod Resour 1:213–15
- Kim HH, Bae Y, Kim SH. (2013). Galangin attenuates mast cell-mediated allergic inflammation. Food Chem Toxicol 57:209–16
- Kim SH, Lee YC. (2009). Piperine inhibits eosinophil infiltration and airway hyperresponsiveness by suppressing T cell activity and Th2 cytokine production in the ovalbumin-induced asthma model. J Pharm Pharmacol 61:353–9
- Kumar S, Saravana Kumar M, Raja B. (2010). Efficacy of piperine, an alkaloidal constituent of pepper on nitric oxide, antioxidants and lipid peroxidation markers in L-NAME induced hypertensive rats. Int J Res Pharm Sci 1:300–7
- Lai LH, Fu QH, Liu Y, et al. (2012). Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharm Sin 33:523–30
- Makhov P, Golovine K, Canter D, et al. (2012). Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate 72:661–7
- Medeiros K, Figueiredo C, Figueredo T, et al. (2008). Antiallergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitized mice. J Ethnopharmacol 119:41–6
- Meghwal M, Goswami T. (2013). Piper nigrum and piperine: An update. Phytother Res 27:1121–30
- Mehmood MH, Gilani AH. (2010). Pharmacological basis for the medicinal use of black pepper and piperine in gastrointestinal disorders. J Med Food 13:1086–96
- Mehta A, Kaur G, Chintamaneni M. (2012). Piperine and quercetin enhances antioxidant and hepatoprotective effect of curcumin in paracetamol induced oxidative stress. Int J Pharmacol 8:101–7
- Muthukumar V, Vanisree A. (2011). Molecular interaction of survivin and piperine by computational docking analyses for neuroblastoma targeting. Ann Neurosci 18:145–7
- Nathan RA. (2007). The burden of allergic rhinitis. Allergy Asthma Proc 28:3–9
- Nirmal S, Patel A, Bhawar S, Pattan S. (2012). Antihistaminic and antiallergic actions of extracts of Solanum nigrum berries: Possible role in the treatment of asthma. J Ethnopharmacol 142:191–7
- Ohkubo K, Ikeda M, Pawankar R, et al. (1998). Mechanisms of IL-6, IL-8, and GM-CSF release in nasal secretions of allergic patients after nasal challenge. Rhinology 36:156–61
- Okubo K, Kurono Y, Fujieda S, et al. (2011). Allergic Rhinitis. Allergol Int 60:171–89
- Ozdoganoglu T, Songu M. (2012). The burden of allergic rhinitis and asthma. Ther Adv Respir Dis 6:11–23
- Patange S, Mukundan M, Ashok Kumar K. (2005). A simple and rapid method for colorimetric determination of histamine in fish flesh. Food Control 16:465–72
- Pathak N, Khandelwal S. (2009). Immunomodulatory role of piperine in cadmium induced thymic atrophy and splenomegaly in mice. Environ Toxicol Pharm 28:52–60
- Petersen LJ, Mosbech H, Skov PS. (1996). Allergen-induced histamine release in intact human skin in vivo assessed by skin microdialysis technique: Characterization of factors influencing histamine releasability. J Allergy Clin Immun 97:672–9
- Ramírez L, Urbinelli R, Allaert FA, Demoly P. (2011). Combining H1-antihistamines and nasal corticosteroids to treat allergic rhinitis in general practice. Allergy 66:1501–2
- Saint-Martin F, Dumur J, Pérez I, Izquierdo I. (2003). A randomized, double-blind, parallel-group study, comparing the efficacy and safety of rupatadine (20 and 10 mg), a new PAF and H1 receptor-specific histamine antagonist, to loratadine 10 mg in the treatment of seasonal allergic rhinitis. J Invest Allergy Clin 14:34–40
- Shrivastava P, Vaibhav K, Tabassum R, et al. (2012). Anti-apoptotic and anti-inflammatory effect of piperine on 6-OHDA induced Parkinson's Rat model. J Nutr Biochem 24:680–7
- Sudjarwo SA. (2005). The potency of piperine as antiinflammatory and analgesic in rats and mice. Folia Med Indo 41:190–4
- Suleimani YMA, Dong Y, Walker MJ. (2008). Differential responses to various classes of drugs in a model of allergic rhinitis in guinea pigs. Pulm Pharmacol Ther 21:340–8
- Szelenyi I, Brune K. (2002). Herbal remedies for asthma treatment: Between myth and reality. Drugs Today 38:265–303
- Venkatesh P, Mukherjee PK, Nema NK, et al. (2009). Mast cell stabilization and antihistaminic potentials of Curculigo orchioides rhizomes. J Ethnopharmacol 126:434–6
- Walter M, Kottke T, Stark H. (2011). The histamine subreceptor: Targeting inflammatory disorders. Eur J Pharmacol 668:1–5
- Wang D-Y, Clement P. (2000). Pathogenic mechanisms underlying the clinical symptoms of allergic rhinitis. Am J Rhinol 14:325–33
- Wang H, Zhang J, Gao C, et al. (2007). Topical levamisole hydrochloride therapy attenuates experimental murine allergic rhinitis. Eur J Pharmacol 577:162–9
- Yamada T, Yamamoto H, Kubo S, et al. (2012). Efficacy of mometasone furoate nasal spray for nasal symptoms, quality of life, rhinitis-disturbed sleep, and nasal nitric oxide in patients with perennial allergic rhinitis. Allergy Asthma Proc 33:e9–16
- Zhao Y, Woo J, Leung P, et al. (2005). Symptomatic and pathophysiological observations in a modified animal model of allergic rhinitis. Rhinology 43:47–54
