Abstract
Context: The fruit of Xanthium strumarium L. (Asteraceae) has been used for the treatment of various inflammatory diseases.
Objective: This study investigates the protective effect of caffeoylxanthiazonoside (CYXD) isolated from fruits of X. strumarium on sepsis mice in vitro and in vivo.
Materials and methods: Cecal ligation and puncture (CLP) operation was used to establish the sepsis mice model, and sham mice were also performed. CYXD was administered by intraperitoneal injection (10, 20, and 40 mg/kg/d), then the survival rate was measured in 96 h. Additionally, sepsis mice were induced by injection LPS (2 mg/kg); CYXD was administered by intraperitoneal injection (10, 20, and 40 mg/kg/d), then mice were sacrificed, and serum levels of TNF-α and IL-6 were determined by ELISA assay. Furthermore, the ability of CYXD to neutralize LPS was measured by using the LAL test, and expressions of TNF-α, IL-6 were determined by using real-time fluorogenic PCR.
Results: Results indicated that CYXD significantly elevated survival rates of sepsis mice induced by CLP (p < 0.05) with survival rates of 35%, 45%, and 65%. Furthermore, the LPS level was decreased obviously by CYXD (1, 2, and 4 mg/L) (p < 0.05). Additionally, CYXD (10, 20, and 40 mg/kg) can not only significantly decrease TNF-α and IL-6 levels induced by LPS in mice's serum (p < 0.05), but also inhibit mRNA expressions of TNF-α and IL-6 induced by LPS in RAW 264.7 cells at doses of 20, 40, and 80 μg/mL (p < 0.05).
Conclusion: Our study demonstrated that CYXD has significant protective effects on sepsis mice.
Introduction
Sepsis, which is a systemic host response to micro-organisms, is a serious complex clinical syndrome correlated to severe infections, and sepsis might result in septic shock, multiple organ dysfunction syndrome (MODS), and even death (Huttunen & Aittoniemi, Citation2011; Wang et al., Citation2007). Therefore, the sepsis currently has become a major clinical serious medicinal problem (Callaghan & Redmond, Citation2006; Zheng et al., Citation2010). Recently, hospital mortality rate induced by septic shock and severe sepsis also remain a pessimistic condition (the mortality range from 25 to 80%), although broad improvements had been made in heath care (Setoguchi et al., Citation2011; Wang et al., Citation2007). Therefore, finding new antisepsis drugs is very urgent and important.
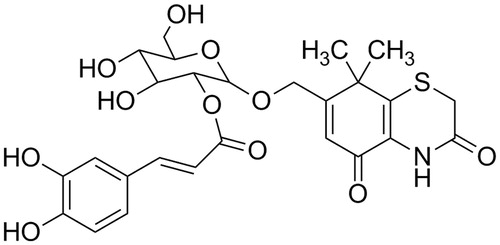
Xanthium strumarium L. (Asteraceae) has been used for the treatment of various inflammatory diseases, such as nasal sinusitis, rhinitis, tympanitis, and arthritis (Peng et al., Citation2014; Xue et al., Citation2014); the fruit of X. strumarium is called Fructus Xanthii (Cangerzi in Chinese). Caffeoylxanthiazonoside (CYXD, ) is one of the major thiazinedione heterocyclic compounds of the fruit of X. strumarium (Li et al., Citation2012; Qin et al., Citation2006), and the CYXD had a significant anti-allergic rhinitis effect on rodent animals with allergic rhinitis (Peng et al., Citation2014). In our present investigation regarding the fruit of X. strumarium, a large quantity of CYXD was also isolated. Then, as part of our continuing investigation for finding anti-sepsis agents from natural compounds, we further investigated anti-sepsis activity of CYXD on sepsis mice induced by cecal ligation and puncture (CLP) and injection of LPS, which provided a scientific basis for the clinical use of this compound in the future.
Material and methods
Plant material
Dried Fructus Xanthii was purchased from Tong-ren-tang Pharmaceutical Group, and it was identified by Professor Ben-Quan Wu. The voucher specimen of the plant was deposited at our hospital (No. FX20130804).
Animals
Experimental groups consisted of Institute of Cancer Research (ICR) mice (20 ± 2 g), purchased from the Shanghai Laboratory Animal Centre (Shanghai, China), and each animal was used only once in our experiment.
Ethics statement
All animal treatments were strictly in accordance with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals, and the experiments were carried out with the approval of the Animal Experimentation Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University.
Chemicals
Lipopolysaccharide (LPS) was purchased from Sigma Chemicals (St. Louis, MO). Mouse TNF-α, and IL-6 ELISA kits were purchased from eBioscinence (San Diego, CA). The quantitative real-time RT-PCR reaction kit and Trizol reagents were purchased from the TOYOBO Biotech Ltd. Co (Osaka, Japan). The dimethyl sulfoxide (DMSO) was purchased from the Sino Pharm Chemical Reagent Co. (Shanghai, China). Silica-gel (100–200 and 200–300 mesh) was purchased from Qingdao Haiyang Chemical Co. (Qingdao, China). Sephadex LH-20 was purchased from GE Healthcare Co. (Totowa, NJ). All other chemicals used in this study were of analytical reagent grade.
Extraction, isolation, and preparation of pure compounds
Dried Fructus Xanthii was powdered and extracted with 75% ethanol by reflux three times, and each extraction period lasted 1.5 h. The solvent was evaporated under vacuum to obtain crude extract. Then the extract was suspended in water and partitioned with petroleum ether, chloroform, ethyl acetate, and n-butanol successively. The n-butanol fraction was subjected to repeated column chromatography over silica gel (100–200 mesh) column chromatography and eluted with ethyl acetate–methanol (20:1–1:2). Combination of similar fractions on the basis of TLC analysis afforded seven fractions (I–VII). By using a series of chromatographic techniques, such as silica gel column chromatography (200–300 mesh) and Sephadex LH-20 chromatography, the compound was isolated from fractions IV and identified as 7-hydroxymethyl-8,8-dimethyl-4,8-dihydrobenzol (caffeoylxanthiazonoside, CYXD) (Peng et al., Citation2014; Qin et al., Citation2006).
Preparation of cecal ligation and puncture model of mice
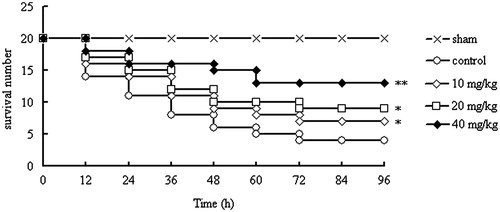
Cecal ligation and puncture (CLP) mice were prepared following the method described previously (Rittirsch et al., Citation2009; Yang et al., Citation2013). Briefly, mice were general anesthetized with isoflurane (3 mL/kg), and an abdominal midline incision (approximately 1.5 cm) was made after disinfection. After the cecum was isolated and exteriorized from the left side of the abdomen, ligate midpiece of the cecum. Then, the cecum was punctured twice with an 18-guage needle, and the cecum was returned to the peritoneal cavity. After relocating the cecum into the abdominal cavity, the abdominal incision was closed. For the sham group, mice underwent the same surgical procedures, but no ligation or puncture was done. Furthermore, survival analysis after CLP surgery was performed and observed during 96 h after CLP surgery. About 100 CLP mice were divided into five groups (n = 20): sham, control, CYXD (10, 20, and 40 mg/kg/d), and the vehicle (control, 10 mL/kg) and CYXD were administered by intraperitoneal injection (i.p.).
Plasma TNF-α and IL-6 levels in LPS-induced sepsis mice
A total of 40 ICR mice were randomly divided into four groups (n = 10). Group 1 was given LPS (2 mg/kg), while groups 2, 3, and 4 were given 10, 20, and 40 mg/kg of CYXD by intraperitoneal injection (i.p.), respectively, followed by injection with LPS (2 mg/kg). Mice were sacrificed at 12 h after LPS injection, and blood sample was collected from their heart. Then, TNF-α and IL-6 levels were determined by using ELISA kits.
Neutralization of endotoxin
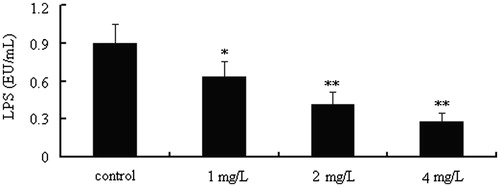
The ability of CYXD to neutralize LPS was measured by using the LAL test, which is described previously and commonly considered as an extremely sensitive indicator of the presence of free, non-neutralized LPS (Fu et al., Citation2008). Briefly, a series of concentrations of the CYXD (0–4.0 mg/L) were incubated with LPS (0.1 μg/L) for 30 min at 37 °C. Then, 100 μL of the mixture was added to an equal volume of the LAL reagent, and the kinetic turbidity was determined by using the EDS-99 Tube Reader (Beijing Jinshan Biotech Ltd., Xuzhou, China).
Cell culture and cell viability assay
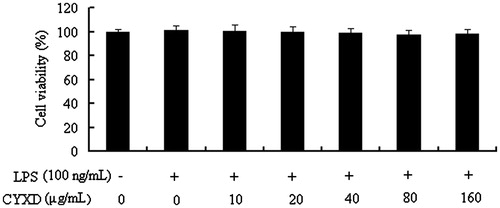
The RAW264.7 cells were cultured in plastic dishes containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS). The RAW 264.7 cells were plated at a density of 5 × 105 and pre-incubated for 24 h in a CO2 incubator (5% CO2) at 37 °C. Then RAW 264.7 cells were cultured with CYXD (0, 10, 20, 40, 80, and 160 μg/mL) in the presence of 100 ng/mL LPS for 24 h at 37 °C. After that, the cells were washed twice with phosphate-buffered saline (PBS) and incubated with 100 μL of 0.5 mg/mL MTT for 2 h to measure the cell viability. The medium was then discarded, and 100 μL DMSO was added. After 30 min incubation, the absorbance at 570 nm was read using a microplate reader (Bio-Rad, Hercules, CA).
Determination of mRNA expressions of pro-inflammatory cytokines using real-time fluorogenic PCR
RAW 264.7 cells were harvested, and total RNA was extracted using the Trizol reagents. Total RNA was used for cDNA synthesis by reverse transcription using quantitative real-time PCR (Bio-Rad, Hercules, CA), and cDNAs of TNF-α, IL-6, and β-actin were amplified. All mRNA primers were designed by Primer Premier 5.0 and synthesized by Shengong Biotech Co., Ltd (Shanghai, China). The primers used for the real-time PCR are shown in . Reverse transcription was performed according to the manufacturer's recommendation of quantitative real-time fluorogenic PCR reaction kit.
Table 1. The primer used for the real-time PCR.
Statistical analysis
Data are presented as mean ± SEM. The Chi-squared of exact test was used to analyze the significance of mice mortality differences among groups. For the other investigations, statistical analyses were performed using the two-tailed Student's t-test with a significance level of p < 0.05.
Result
Effect of CYXD on survival of CLP mice
In the results of our present study, for the control group, there were only four mice alive at 96 h after CLP, and the survival rate was 20%. However, mice treated with CYXD (10, 20, and 40 mg/kg) can significantly increase the survival rate of CLP mice compared with the control group (p < 0.05, p < 0.05, and p < 0.01, respectively), and the survival rates were 35%, 45%, and 65%, respectively (). Furthermore, for the sham mice, no death was observed in 96 h. The results above indicated that CYXD can significantly increase the survival rate of mice after CLP surgery.
Figure 2. Effect of CYXD on survival rate of CLP mice. Mice were divided into five groups (n = 20): sham, control, and CYXD (10, 20, and 40 mg/kg/d). The vehicle (control, 10 mL/kg) and CYXD were administered by intraperitoneal injection. Each column represented the mean ± SEM (n = 20). *p < 0.05 and ** p < 0.01, compared with the control.
Effect of levels of CYXD on TNF-α and IL-6 in CLP mice
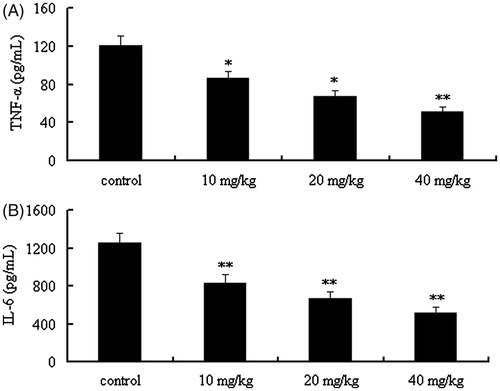
As can be seen from , the pro-inflammatory cytokines (including TNF-α and IL-6) in serum can be decreased significantly by treating with the CYXD at the doses of 10, 20, and 40 mg/kg, compared with the control group (p < 0.05).
Figure 3. Effect of CYXD on TNF-α and IL-6 in the serum of CLP mice. Mice were divided into five groups (n = 10): sham, control, and CYXD (10, 20, and 40 mg/kg). The vehicle (control, 10 mL/kg) and CYXD were administered by intraperitoneal injection. Each column represented the mean ± SEM (n = 10). *p < 0.05 and **p < 0.01, compared with the control.
CYXD neutralizes LPS activity in vitro
In order to determine the ability of CYXD to neutralize LPS in vitro, LPS levels in vitro treated with or without CYXD were measured. As can be seen from , the LPS level can be decreased obviously by treatment with CYXD (1, 20, and 40 mg/L) compared with the control group (p < 0.05).
Effects of CYXD on LPS-induced cell viability, TNF-α, and IL-6 expressions
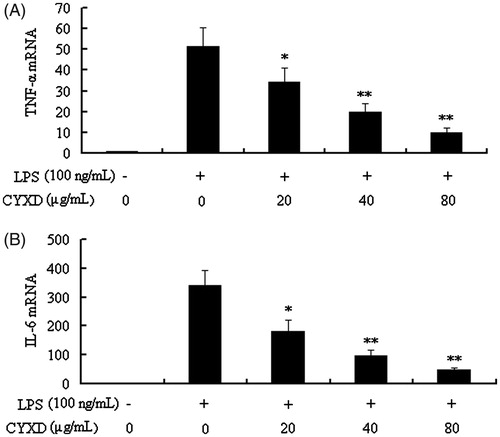
In the results of our present study, CYXD at doses of 20, 40, and 80 μg/mL can significantly suppress the expressions of TNF-α and IL-6 induced by LPS in RAW 264.7 (p < 0.05) (). To exclude the possibility that the inhibitive effects of CYXD on the pro-inflammatory cytokines production mentioned above were due to cytotoxcity of CYXD, the MTT assay was performed, and when the RAW 264.7 cells were treated with CYXD and LPS, there was no obvious change in the cell viability ().
Figure 5. Effects of CYXD on TNF-α and IL-6 mRNA expressions in RAW 264.7 cells induced by LPS (100 ng/mL). TNF-α and IL-6 mRNA expressions were normalized by β-actin, and fold changes in mRNA expression are shown and expressed as mean ± SEM (n = 4). *p < 0.05 and **p < 0.01, compared with the control.
Discussion
Clinically, sepsis is one of the major causes of mortality in intensive care unit (ICU), and the incidence of sepsis has been obviously increased over the last decades (Dellinger et al., Citation2008; Georgopoulou et al., Citation2011; Yang et al., Citation2013). It is no doubt that traditional Chinese medicine (TCM) and its related constituents are very useful to protect human being's health (Qiu, Citation2007). Recently, lots of investigations reported that natural compounds coming from TCMs showed dramatic protective effects on sepsis (Bae et al., Citation2011; Fu et al., Citation2008; Gao et al., Citation2009; Jiang et al., Citation2011; Ma et al., Citation2006). In our present study, we reported that an anti-sepsis natural compound, which is called the caffeoylxanthiazonoside (CYXD), was isolated from the Fructus Xanthii. The CYXD can not only neutralize LPS and inhibit the release of pro-inflammatory cytokines but also increase the survival rate of mice after CLP operation. To the best of our knowledge, our investigation is the first report to demonstrate the anti-sepsis activity of CYXD.
The CLP model is a classic self-infection model characterized the start of sepsis secondary to local abdominal infection induced by operation of ligation and puncture on the cecum. The CLP mice model has become as a gold standard animal model in common sepsis investigation based on its simple operation and high degree of similarity to human sepsis (Dejager et al., Citation2011; Rittirsch et al., Citation2009; Wang et al., Citation2013). In our present investigation, the survival rate of CLP mice was determined to represent the severity of sepsis and protective effect of the CYXD. Administering CYXD at the testing doses (10, 20, and 40 mg/kg/d, i.p.) can significantly increase the survival rate of mice within 96 h, demonstrating that CYXD has obvious protective effects on sepsis mice.
It is well known that LPS is a common trigger of sepsis, and previous investigations have demonstrated that LPS can induce inflammatory response, resulting in severe pro-inflammatory reaction with subsequent acute hypotension, MOF, and even death (Fu et al., Citation2008; Kolgazi et al., Citation2006). Furthermore, previous investigations indicated that neutralization or removal LPS would be beneficial for the treatment of sepsis (Cruz et al., Citation2009). In the results of our present study, we demonstrated that the CYXD at the testing doses can significantly neutralize LPS in vitro.
The pathophysiology of sepsis, which is characterized by systemic inflammation, and multiple organ failure (MOF), is extremely complex (Gao et al., Citation2009). Pro-inflammatory cytokines, such as TNF-α and IL-6, play important effects in the sepsis process (Fioretto et al., Citation2008). It is reported that TNF-α is a key mediator in the initiation of systemic inflammatory response, and IL-6 is the early and later cytokines. Both the two cytokines are related to the activation of cytokine cascade reaction in sepsis (Fioretto et al., Citation2008; Setoguchi et al., Citation2011). In the results of our present study, CYXD can not only significantly decrease the TNF-α and IL-6 levels induced by LPS in mice serum but also inhibit mRNA expressions of TNF-α and IL-6 induced by LPS in RAW 264.7 cells, which is another valid evidence for demonstrating anti-sepsis effects of CYXD.
In conclusion, the results of our present work are noteworthy, and our investigation demonstrated that the CYXD have the favorable protective effects on sepsis mice. Thus, CYXD can be considered as an effective and safe disease preventive or therapeutic candidate against sepsis. However, more laboratory and clinical investigations are necessary to elucidate the mechanism of action of CYXD in the treatment of sepsis.
Declaration of interest
The authors report that they have no conflicts of interest. This work was supported in part by the Natural Science Foundation of Guangdong (No. S2013040014504).
References
- Bae GS, Seo SW, Kim MS, et al. (2011). The roots of Nardostachys jatamansi inhibits lipopolysaccharide-induced endotoxin shock. J Nat Med 65:63–72
- Callaghan AO, Redmond HP. (2006). Treatment of sepsis: Current status of clinical immunotherapy. Surgeon 4:355–61
- Cruz DN, Antonelli M, Fumagalli R, et al. (2009). Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 301:2445–52
- Dejager L, Pinheiro I, Dejonckheere E, Libert C. (2011). Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol 19:198–208
- Dellinger RP, Levy MM, Carlet JM, et al. (2008). Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
- Fioretto JR, Martin JG, Kurokawa CS, et al. (2008). Interleukin-6 and procalcitonin in children with sepsis and septic shock. Cytokine 43:160–4
- Fu JF, Cao HW, Wang N, et al. (2008). An anti-sepsis monomer, 2′,5′,6′,7-tetrahydroxyflavanonol (THF), identified from Scutellaria baicalensis Georgi neutralizes lipopolysaccharide in vitro and in vivo. Int Immunopharmacol 8:1652–7
- Gao XH, Xu XX, Pan R, et al. (2009). Saponin fraction from Astragalus membranaceus roots protects mice against polymicrobial sepsis induced by cecal ligation and puncture by inhibiting inflammation and upregulating protein C pathway. J Nat Med 63:421–9
- Georgopoulou AP, Savva A, Giamarellos-Bourboulis EJ, et al. (2011). Early changes of procalcitonin may advise about prognosis and appropriateness of antimicrobial therapy in sepsis. J Crit Care 26:331.e1–331.e7
- Huttunen R, Aittoniemi J. (2011). New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. J Infect 63:407–19
- Jiang N, Xin WY, Wang T, et al. (2011). Protective effect of aescin from the seeds of Aesculus hippocastanum on liver injury induced by endotoxin in mice. Phytomedicine 18:1276–84
- Kolgazi M, Sener G, Çetinel S, et al. (2006). Resveratrol reduces renal and lung injury caused by sepsis in rats. J Surg Res 134:315–21
- Li H, Min YS, Park KC, Kim DS. (2012). Inhibition of melanogenesis by Xanthium strumarium L. Biosci Biotechnol Biochem 76:767–71
- Ma HY, Kou JP, Zhu DN, et al. (2006). Liu-Shen-Wan, a traditional Chinese medicine, improves survival in sepsis induced by cecal ligation and puncture via reducing TNF-α levels, MDA content and enhancing macrophage phagocytosis. Int Immunopharmacol 6:1355–62
- Peng W, Ming QL, Han P, et al. (2014). Anti-allergic rhinitis effect of caffeoylxanthiazonoside isolated from fruits of Xanthium strumarium L. in rodent animals. Phytomedicine 21:824–9
- Qin LP, Han T, Li HL, et al. (2006). A new thiazinedione from Xanthium strumarium. Fitoterapia 77:245–6
- Qiu J. (2007). Traditional medicine: A culture in the balance. Nature 448:126–8
- Rittirsch D, Huber-Lang MS, Flierl MA, Award P. (2009). Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4:31–6
- Setoguchi D, Nakamura M, Yatsuki H, et al. (2011). Experimental examination of anti-inflammatory effects of a 5-HT3 receptor antagonist, tropisetron, and concomitant effects on autonomic nervous function in a rat sepsis model. Int Immunopharmacol 11:2073–8
- Wang LX, Jiang WW, Ding GF, et al. (2007). The newly identified CpG-N ODN208 protects mice from challenge with CpG-S ODN by decreasing TNF-α release. Int Immunopharmacol 7:646–55
- Wang N, XLiu N, Zheng XC, et al. (2013). Ulinastatin is a novel candidate drug for sepsis and secondary acute lung injury, evidence from an optimized CLP rat model. Int Immunopharmacol 17:799–807
- Xue LM, Zhang QY, Han P, et al. (2014). Hepatotoxic constituents and toxicological mechanism of Xanthium strumarium L. fruits. J Ethnopharmacol 152:272–82
- Yang C, Wu K, Li SH, You Q. (2013). Protective effect of curcumin against cardiac dysfunction in sepsis rats. Pharm Biol 51:482–7
- Zheng XC, Yang D, Liu X, et al. (2010). Identification of a new anti-LPS agent, geniposide, from Gardenia jasminoides Ellis, and its ability of direct binding and neutralization of lipopolysaccharide in vitro and in vivo. Int Immunopharmacol 10:1209–19