Abstract
Context: Thespesia populnea Sol. ex Correa (Malvaceae), an indigenous tree species in India, is of interest to researchers because traditionally its heartwood is used in the treatment of ulcer and colic pain.
Objective: To validate its folk use in the treatment of ulcerative colitis (UC).
Materials and methods: Mice were administered intrarectal DNBS and then treated with different plant extracts (100 and 200 mg/kg), 30 min before and 24 and 48 h after DNBS infusion. Colonic mucosal injury was assessed by macroscopic and histological examination. Furthermore, malondialdehyde (MDA), myeloperoxidase (MPO), protease, and hemoglobin (Hb) contents were measured in tissue and blood samples.
Results: Administration of various extracts ameliorated macroscopic and microscopic scores which were altered due to DNBS treatment in mice. Hb concentration in blood was restored significantly by the aqueous extract to 17.20 ± 0.5, which was reduced to 13.80 ± 0.5 after treatment with DNBS. MDA level was increased to 10.82 nm/mg and 10.25 nm/ml in tissue and blood, respectively, due to DNBS treatment which was reduced to 2.69 nm/mg and 3.59 nm/ml in tissue and blood, respectively, by aqueous extract treatment. Similarly, MPO level was increased to 412 U/mg and 404 U/ml in tissue and blood, respectively, which was significantly reduced to 205 U/mg and 219 U/ml in tissue and blood, respectively, by aqueous extract treatment. Aqueous extract significantly reduced protease activity which was markedly increased in DNBS-treated animals.
Discussion and conclusion: Aqueous extract of heartwood of T. populnea is effective in the treatment of UC.
Introduction
Ulcerative colitis (UC) and Crohn’s disease are chronic, relapsing, immunologically mediated disorders that are collectively referred to as inflammatory bowel diseases (IBD). Etiology and pathogenesis of IBD remain obscure, although environmental factors, in combination with genetic factors, are suggested to be involved in its pathogenesis (Fiocchi, Citation1998; Loftus, Citation2004). The pathological findings associated with UC are an increase in certain inflammatory mediators, signs of oxidative stress, a deranged of the mucosa, abnormal glycosaminoglycan content of the mucosa, decreased oxidation of short chain fatty acids (SCFA’s), increased intestinal permeability, increased sulfide production, and decreased methylation (Kathleen & Jurenka, Citation2003; Kirsner & Shorter, Citation1982). The inflamed mucosa in UC produces high amount of prostaglandin, nitric oxide, and other oxidative stress products (Dudhgaonkar et al., Citation2007). Other inflammatory products secreted by inflamed mucosa are tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and leukotrien-B4 (LTB4) which lead to neutrophil chemotaxis (Razavi et al., Citation2008). Malondialdehyde (MDA) is an end product of the lipid peroxidation process. An increase in free radical causes overproduction of MDA which is commonly known as a marker of oxidative stress (Flohe et al., Citation1985). Myleoperoxidase (MPO) is an enzyme found predominantly in neutrophils and its activity in the colon is linearly related to infiltration of neutrophils. The assessment of MPO activity is well established for the quantification of intestinal inflammation. In inflammatory conditions like IBD, the level of neutrophils in inflamed tissues and, consequently, MPO enzyme level increases (Elson et al., Citation1995; Krawisz et al., Citation1984). Since protease levels are known to be elevated in IBD and thus may play a role in the extensive tissue damage in IBD (Hawkins et al., Citation1997). 5-Aminosalicyclic acid and salazosulphapyridine are the drugs of choice for current medical treatment. Corticosteroids, azathioprine, mercaptopurines, and cyclosporine are also used in more severe forms of the disease but these are not without side effects. Treatment of IBD using these drugs with minimum side effects is a continuous challenge (Hanauer, Citation1996). Owing to the lack of specific, curative treatments with limited toxicity, there is a pressing need for developing effective therapeutic approaches.
Thespesia populnea Sol. ex Correa (Malvaceae) is found in tropical shores from Bengal to Ceylon and is cultivated to some extent in Madras. The chemical constituents reported from this plant belong to different classes such as glycosides, tannins, flavonoids, volatile oils, steroids, resins, mucilage, and sugars. Thespesia populnea has a number of medicinal uses, many of which have been verified by scientific methods. Plant is alternative, stimulant, demulcent, phlegmatic, and generative of semen. Traditionally, heartwood of T. populnea has been reported in the treatment of ulcer and colic pain (Kirtikar & Basu, Citation1991; Nadkarni, Citation1982). Also the plant has proven its potential as antioxidant and anti-inflammatory properties which could be promising in the treatment of UC (Ilavarasan et al., Citation2003; Vasudevan et al., Citation2007). Root extracts of the plant are having antimicrobial activity (Senthil-Rajan et al., Citation2013), fruit pulp extracts are having antidiabetic and antihyperlipidemic activities (Belhekar et al., Citation2013), and the plant is having anti-tumor, anti-inflammatory (Mika & Guruvayoorappan, Citation2013), hepatoprotective (Yuvaraj & Subramoniam, Citation2009), and wound healing activities (Nagappa & Cheriyan, Citation2001). A new serin protease is isolated from the leaves of this plant (Ishwarya & Sangeetha, Citation2013). The objective of the present study is to validate the folk use of heartwood of T. populnea for the treatment of UC.
Materials and methods
Plant material
Heartwood of the plant T. populnea was collected from Ahmednagar district (M.S.) in the month May 2013 and authenticated by Mr. P. G. Diwakar, Deputy Director, Botanical Survey of India, Pune (Voucher specimen number BSI/WRC/Tec/2009/499).
Preparation of the extracts
Heartwood was dried under shade, powdered, and then subjected to extraction with ethanol in the Soxhlet apparatus. Marc left was extracted with water in the reflux condenser and both the extracts were vacuum dried to get ethanol and aqueous extracts. Ethanol extract was further partitioned with chloroform and ethyl acetate so as to obtain the respective extract.
Animals
Male Swiss mice (20–25 g) were used. The animals were housed under standard laboratory conditions and fed with standard rodent diet and water ad libitum. Rodent diet is composed of crude proteins 16%, crude fats 3.8%, crude fibers 2%, amino acids, vitamins, and minerals. The animals were kept in constant temperature (22 ± 2 °C), humidity (55%), and light–dark condition (12 h light/dark). The experimental protocol was approved by the institutional animal ethical committee (approval no. CPCSEA/C/01/448/09-10/05).
Chemicals
DNBS, amido black, isoflurane, chloroform, ethyl acetate, and ethanol were procured from Sigma-Aldrich (Bombay, India). Standard drug prednisolon was obtained from Nicholas Ltd, Mumbai, India.
Induction of experimental colitis
After 36 h of starving, colitis was induced by intrarectal administration of DNBS (5 mg/mouse) using a modification (Qiu et al., Citation1999) of the method first described for rats (Wallace et al., Citation1995). This dose of DNBS was found to induce reproducible colitis without mortality in mice. Briefly, mice were lightly anesthetized with isoflurane and then DNBS (5 mg in 100 μl of 50% ethanol) was infused into the rectum through a catheter (outer diameter 0.8 mm), and inserted 4–5 cm proximally to the anus.
Pharmacological treatments
Different plant extracts (100 and 200 mg/kg) were administered orally 30 min before and 24 and 48 h after DNBS infusion. Prednisolon (5 mg/kg) is used as a standard and is administered by the intraperitoneal route (Federico et al., Citation2004). Group I was treated with 100 µl DNBS (5 mg/mice in a 50% ethanol), group II was treated with standard drug predinisolon (5 mg/kg), groups III and IV were treated with chloroform extracts (100 and 200 mg/kg), respectively, groups V and VI were treated with ethyl acetate extracts (100 and 200 mg/kg), respectively, groups VII and VIII were treated with ethanol extracts (100 and 200 mg/kg), respectively, and groups IX and X were treated with aqueous extracts (100 and 200 mg/kg), respectively.
Assessment of colitis severity
Macroscopic and microscopic observations
Mice were dissected by cervical dislocation 3 d after DNBS treatment. The colon was removed and rinsed gently with saline solution and then opened by longitudinal incision and examined immediately. Colonic damage was assessed by a semi-quantitative scoring system originally established in rats (Morris et al., Citation1989) and adapted to mice for the present study. Blood samples of animals for each group after treatment were subjected to hematological studies (Ganjare et al., Citation2011).
Determination of Ulcer Index
After 48 h of colitis induction, mice were sacrificed by cervical dislocation and dissected to remove the colon. The entire colon was isolated, opened longitudinally, and rinsed with PBS. Histological scoring of the colon damage was performed and ulcer area was determined by summing the sizes of lesions measured macroscopically for each mouse. The total area of damage was expressed as the relative percentage of the total surface area of the colon (Zaware et al., Citation2011).
Determination of myeloperoxidase (MPO) activity in blood and colonic tissue
After macroscopic measurements, the excised colons (100–150 mg) were homogenized with PBS (pH 7.4) and centrifuged at 1000 rpm for 20 min at 4 °C. MPO activity of supernatants was then assayed by mixing the supernatant with citric phosphate buffer (pH 5.0) containing 0.4 mg/ml o-phenylene diamine and 0.015% hydrogen peroxide. The change in the absorbance at 492 nm was measured spectrophotometrically and compared with the standard dilution with horseradish peroxidase. Similarly the MPO level in blood was measured (Gholap et al., Citation2012).
Determination of MDA level in blood and colonic tissue
The reaction mixture contained 0.1 ml tissue sample, 0.2 ml 8.1% sodium dodecyl sulfate (SDS), 1.5 ml 2% acetic acid, and 1.5 ml 0.8% aqueous solution of thiobarbituric acid. The mixture pH was adjusted to 3.5 ml and the volume was finally made up to 4 ml with distilled water and 5 ml of mixture of n-butanol and pyridine (15%) was added. The mixture was shaken vigorously. After centrifugation at 4000 rpm for 10 min, the absorbance of organic layer was measured at 532 nm. MDA level was expressed as nmol/mg of protein. Similarly the MPO level in blood was measured and expressed as nmol/ml of protein (Nirmal et al., Citation2013).
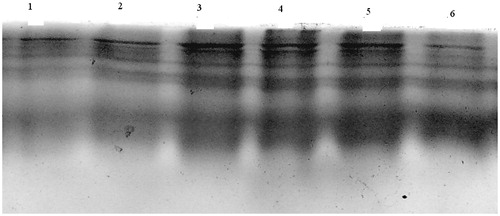
Analysis of protease in colon by SDS-PAGE
A small piece of colon tissue from the centre of the ulcer was homogenized for 10 s in cold PBS. The homogenate was centrifuged (14 000 rpm for 5 min at 41 °C) and the supernatant was analyzed for protease activity on gelatin zymograms according to Hawkins et al. (Citation1997). In short, 12% SDS–polyacrylamide gels were prepared containing 0.1% gelatin. An equal amount of protein from each sample (10–25 mg) was applied to the gel in standard SDS–gel loading buffer containing 0.1% SDS but lacking b-mercaptoethanol and samples were not boiled prior to loading. After electrophoresis, gels were soaked in 2% Triton X-100 in distilled water with shaking for 15 min. Gels were then incubated in 50 mM Tris–HCl, pH 8.0 containing 1 mM CaCl2 for 12 h at 37 °C, stained in amido black and subsequently destained.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) of six mice per experimental group. Statistical analysis was performed using the Prism Graphpad statistical software (GraphPad Software, Inc., La Jolla, CA). Parametric one-way analysis of variance (ANOVA) followed by Dunnet’s test was used to compare the control group with other group.
Results and discussion
Microscopic and histological score assessment
and depict the effect of various plant extracts at different dose levels and the standard drug prednisolon in comparison with the negative control group (receiving DNBS infusion in 50% ethanol only) on macroscopic and histological examination, respectively. The concentration of neutrophils, lymphocytes, and macrophages is increased in mice after treatment with DNBS. The most significant decrease in abnormal count of neutrophils, lymphocytes, and macrophages was observed in the group treated with aqueous extract at the dose of 200 mg/kg (). UC and Crohn’s disease are chronic inflammatory disorders of the GI tract, in which the predominant cells in inflamed mucosa are neutrophils and lymphocytes which are positive for CD4. There are also increases in the number of B cells, macrophages, dendritic cells, plasma cells, eosinophils, and perhaps mast cells (Roberts-Thomson et al., Citation2011).
Table 1. Effects of different extracts of heartwood of T. populnea (100 and 200 mg/kg, p.o.) and prednisolon (5 mg/kg, i.p.) on microscopic score lesion of mice with DNBS induced ulcerative colitis.
Table 2. Effects of different extracts of heartwood of T. populnea (100 and 200 mg/kg, p.o.) and prednisolon (5 mg/kg, i.p.) on histological score lesion of mice with DNBS-induced ulcerative colitis.
DNBS caused severe macroscopic edematous inflammation in the colon, as assessed by the high score of colonic damage and the goblet cell hyperemia. Oral administration of different extracts of heartwood of T. populnea (100 and 200 mg/kg) and prednisolon ameliorated DNBS effect significantly, while the effect of aqueous extract at 200 mg/kg dose level was comparable with that of the standard drug, prednisolon. In mice treated with aqueous extract colonic, macroscopic scores and the histological score were significantly reduced which in turn prevent the progression of colitis.
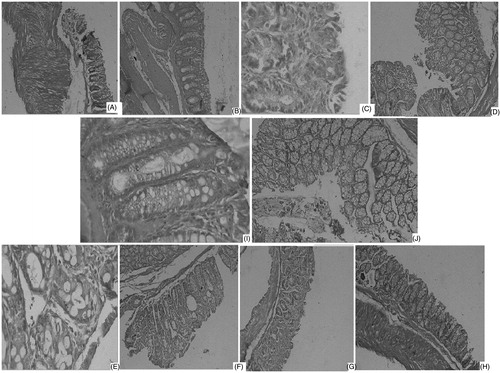
These findings were emphasized by the histopathological study presented in , where DNBS-treated mice showed transmural necrosis, edema, and diffuse inflammatory cell infiltration in the mucosa. There was focal ulceration of the colonic mucosa extending through the muscularis mucosa with the loss of epithelium. The architecture of the crypts was distorted, the lamina propria was thickened in peripheral areas of distorted crypts especially in basal areas and an infiltrate consisting of mixed inflammatory cells was observed in the control group (). Treatment with either 200 mg/kg of aqueous extract of T. populnea () or prednisolon () significantly attenuated the extent and the severity of the histological features of cell damage, an effect which was less promising in the other groups.
Figure 1. Histological colonic mucosal sections of mice receiving treatment of (A) chloroform extract (100 mg/kg), (B) chloroform extract (200 mg/ml), (C) ethyl acetate extract (100 mg/kg), (D) ethyl acetate extract (200 mg/ml), (E) ethanol extract (100 mg/kg), (F) ethanol extract (200 mg/ml), (G) aqueous extract (100 mg/kg), (H) aqueous extract (200 mg/ml) showing maximum beneficial effects in terms of attenuation of the morphological disturbance, reduction of the inflammatory cell infiltration, and mucosal edema associated with DNBS administration, (I) prednisolon 5 mg/kg showing attenuation of the extent and severity of cell damage, (J) DNBS infusion (5 mg/mice) showing inflammatory cell infiltration and mucosal edema, disorganized epithelial layer associated with DNBS administration.
Hemoglobin estimation
depicts the effect of various plant extracts and the standard drug prednisolon in comparison with the negative control group (receiving DNBS infusion in 50% ethanol) on hemoglobin concentration. Hemoglobin concentration was restored significantly by aqueous extract (200 mg/kg) to 17.20 ± 0.5 which was reduced up to 13.80 ± 0.5 after treatment with DNBS. Aqueous extract was found to be more significant and dose dependant to restore hemoglobin concentration than other extracts. One of the important symptoms of UC is bloody diarrhea, so there may be reduction in the hemoglobin content. Results showed that there was significant lowering of hemoglobin concentrations in the negative control group while treatment with different plant extracts and standard drug, prednisolon, reversed the lowering of hemoglobin concentration.
Table 3. Effects of different extracts of heartwood of T. populnea (100 and 200 mg/kg, p.o.) and prednisolon (5 mg/kg, i.p.) on hemoglobin concentration (g/10 ml) of mice in DNBS-induced colitis.
Determination of Ulcer Index
Ulcer Index includes inflammation extent, damage in crypt architecture, hyperemia, edema, and the extent of infiltration with inflammatory cells and goblet cell activation. DNBS causes elevation in the Ulcer Index which is prevented by the treatment with different extracts of heartwood of T. populnea. Aqueous extract (200 mg/kg) significantly reduced Ulcer Index up to 2.470 which was increased up to 7.20 by DNBS treatment ().
Table 4. Effects of different extracts of heartwood of T. populnea (100 and 200 mg/kg, p.o.) and prednisolon (5 mg/kg, i.p.) on Ulcer Index of mice in DNBS-induced colitis.
Determination of MPO activity in colon and blood sample
Treatment with DNBS increased the MPO level up to 412 U/mg and 404 U/ml in tissue and blood, respectively. Standard drug prednisolone significantly reduced this increased MPO level to 189 U/mg and 191 U/ml in tissue and blood, respectively. Similarly the most significant reduction in MPO level was found in mice treated with aqueous extract (200 mg/kg), i.e., 205 U/mg and 219 U/ml in tissue and blood, respectively ().
Table 5. Effects of different extracts of heartwood of T. populnea (100 and 200 mg/kg, p.o.) and prednisolon (5 mg/kg, i.p.) on MPO and MDA level in tissue and blood of mice.
A colonic inflammation was biochemically monitored by measuring the level of MPO activity both in the colonic tissue taken from the site of inflammation and in the blood. Since the intestine is in a constant state of controlled inflammation, thus amplification of the inflammatory response activates infiltration of inflammatory cells that trigger pathological responses and symptoms of IBD (Sartor, Citation1997). The colitis caused by DNBS was also characterized by an increase in the MPO activity, an indicator of the neutrophils accumulation in the colon. This fact is documented in both animal models (Akgun et al., Citation2005; Cetinkaya et al., Citation2005) and patients with IBD (Kruidenier et al., Citation2003). Indeed, the MPO activity has been shown to be proportional to the number of infiltrating granulocytes, mainly neutrophils, in the model of colonic inflammation (Krawisz et al., Citation1984). Therefore, slowdown of influx of neutrophils into the intestinal wall was suggested to be implicated in beneficial effect of the extract. A reduction in the activity of MPO enzyme can be interpreted as a manifestation of the anti-inflammatory activity of aqueous extract of heartwood of T. populnea (Veljaca et al., Citation1995).
Determination of MDA level in colon and blood samples
Treatment with DNBS increased the MDA level up to 10.82 nm/mg and 10.25 nm/ml in tissue and blood, respectively. Standard drug prednisolone significantly reduced this increased MDA levels up to 1.99 nm/mg and 2.23 nm/ml in tissue and blood, respectively. Similarly aqueous extract at the dose 200 mg/kg most significantly reduced the MDA level up to 2.69 nm/mg and 3.59 nm/ml in tissue and blood, respectively ().
In IBD, oxidative stress plays a role in disease initiation and progression (Kruidenier & Verspaget, Citation2002). ROS attack the cellular macromolecules, thus disrupting epithelial cell integrity and hindering mucosal recovery, especially in the case of impaired endogenous defense systems (Buffinton & Doe, Citation1995). Aqueous extract-treated groups significantly reduced the levels of MDA in colon and blood which significantly reduced lipid peroxidations and hence increase cellular membrane stability. Increased lipid peroxidation that occurs in colonic tissue can initiate a vicious cycle that generates more and more reactive metabolites, which exhausts cellular antioxidants and favors the consequent development of further inflammation. It is, therefore, reasonable to assume that treatment with aqueous extracts of heartwood of T. populnea improves colonic oxidative balance in animals on colitis because it was able to reduce the level of MDA, a good indicator of lipid peroxidation in colon as well as in blood (Ohkawa et al., Citation1979).
Analysis of protease in colon by SDS-PAGE
Since protease levels are known to be elevated in IBD and thus may play a role in the extensive tissue damage in IBD. Colon tissue from normal mice had little inherent protease activity, whereas this was markedly increased in DNBS-treated animals (lane 1 of ). Protease activity shows as clear bands (indicative of cleavage of the gelatin substrate) on a blue background (Ukil et al., Citation2003). Earlier studies on the experimental model of IBD using specific inhibitors of various proteases established that the majority of protease activity observed on the gelatin zymograms is because of serine proteases (Hawkins et al., Citation1997). However, treatment with different extracts of heartwood of T. populnea (200 mg/kg) greatly reduced the degree of protease activity in the colon of mice as seen by the presence of prominent bands in the corresponding lanes. This technique cannot be used for precise quantification of protease inhibitors (Hanspal et al., Citation1983). Aqueous, ethanol, and ethyl acetate extracts showed significant protease inhibitor activity as showed by prominent bands (lanes 3, 4, and 5 in ). Thus it correlates well with the attenuation of mucosal injury in DNBS-induced colitis.
Declaration of interest
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akgun E, Caliskan C, Celik HA, et al. (2005). Effects of N-acetylcysteine treatment on oxidative stress in acetic acid induced experimental colitis in rats. J Int Med Res 33:196–206
- Belhekar SN, Chaudhari PD, Saryawanshi JS, et al. (2013). Antidiabetic and antihyperlipidemic effects of Thespesia populnea fruit pulp extracts on alloxan-induced diabetic rats. Indian J Pharm Sci 75:217–21
- Buffinton GD, Doe WF. (1995). Depleted mucosal antioxidant defences in inflammatory bowel diseases. Free Radic Bio Med 19:911–18
- Cetinkaya A, Bulbuloglu E, Kurutas EB, et al. (2005). Beneficial effects of N-acetylcysteine on acetic acid-induced colitis in rats. Tohoku J Exp Med 206:131–9
- Dudhgaonkar SP, Tandan SK, Kumar D, et al. (2007). Influence of simultaneous inhibition of cyclooxygenase-2 and inducible nitric oxide synthase in experimental colitis in rats. Inflammopharmacology 15:188–95
- Elson CO, Sartor RB, Tennyson GS, Riddell RH. (1995). Experimental models of inflammatory bowel disease. Gastroenterology 109:1344–67
- Federico M, Giovanni M, Heike H, et al. (2004). The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 113:1202–9
- Fiocchi C. (1998). Inflammatory bowel disease: Etiology and pathogenisis. Gastroenterology 115:182–205
- Flohe L, Beckmann R, Gierlz H, Loschen G. (1985). Oxygen centered free radicals as mediators of inflammation. In: Sies H, ed. Oxidative Stress. London: Academic Press, 403–36
- Ganjare AB, Nirmal SA, Rub RA, et al. (2011). Use of Cordia dichotoma bark in the treatment of ulcerative colitis. Pharm Biol 49:850–5
- Gholap PA, Nirmal SA, Pattan SR, et al. (2012). Potential of Moringa oleifera root and Citrus sinensis fruit rind extracts in the treatment of ulcerative colitis in mice. Pharm Biol 50:1297–302
- Hanauer SB. (1996). Inflammatory bowel disease. N Engl J Med 334:841–8
- Hanspal JS, Bushell GR, Ghosh P. (1983). Detection of protease inhibitors using substrate-containing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem 132:288–93
- Hawkins JV, Emmel EL, Feuer JJ, et al. (1997). Protease activity in a hapten-induced model of ulcerative colitis in rats. Dig Dis Sci 42:1969–80
- Ilavarasan R, Vasudevan M, Anbazhagan S, Venkatraman S. (2003). Antioxidant activity of Thespesia populnea bark extracts against carbon tetrachloride-induced liver injury in rats. J Ethnopharmacol 87:227–30
- Ishwarya S, Sangeetha R. (2013). A new serine protease from the leaves of Thespesia populnea. Prep Biochem Biotechnol 43:95–107
- Kathleen A, Jurenka JS. (2003). Inflammatory bowel disease Part I: Ulcerative colitis – pathophysiology and conventional and alternative treatment options. Alternat Med Rev 8:247–78
- Kirsner JB, Shorter RG. (1982). Recent developments in nonspecific inflammatory bowel disease. (second of two parts). N Engl J Med 306:837–48
- Kirtikar KR, Basu BD. (1991). Indian Medicinal Plants, vol. I, 2nd ed. Dehradun, India: International Book Distributors
- Krawisz JE, Sharon P, Stenson WF. (1984). Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology 87:1344–50
- Kruidenier L, Kuiper I, Lamers C, Verspaget HW. (2003). Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J Pathol 201:28–36
- Kruidenier L, Verspaget HW. (2002). Review article: Oxidative stress as a pathogenic factor in inflammatory bowel disease – radicals or ridiculous? Alimen Pharmacol Ther 16:1997–2015.
- Loftus Jr EV. (2004). Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 126:1504–17
- Mika D, Guruvayoorappan C. (2013). Experimental study on anti-tumor and anti-inflammatory effect of Thespesia populnea phytochemical extract in mice models. Immunopharmacol Immunotoxicol 35:157–63
- Morris GP, Beck PL, Herridge MS, et al. (1989). Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96:795–803
- Nadkarni AK. (1982). Indian Materia Medica. Bombay: Bombay Popular Prakashan
- Nagappa AN, Cheriyan B. (2001). Wound healing activity of the aqueous extract of Thespesia populnea fruit. Fitoterapia 72:503–6
- Nirmal SA, Ingale JM, Pattan SR, Bhawar SB. (2013). Potential of Amaranthus roxburghianus root extract along with piperine in the treatment of ulcerative colitis in mice. J Integr Med 11:206–12
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Annal Biochem 95:351–8
- Qiu BS, Vallance BA, Blennerhassett PA, Collins SM. (1999). The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med 5:1178–82
- Razavi A, Khodadadi A, Eslami MB, et al. (2008). Therapeutic effect of sodium alginate in experimental chronic ulcerative colitis. Iran J Allergy Asthma Immunol 7:13–18
- Roberts-Thomson IC, Fon J, Uylaki W, et al. (2011). Cells, cytokines and inflammatory bowel disease: A clinical perspective. Expert Rev Gastroenterol Hepatol 5:703–16
- Sartor RB. (1997). Pathogenesis and immune mechanisms of chronic inflammatory bowel disease. Am J Gastroenterol 92:5S–11
- Senthil-Rajan D, Rajkumar M, Srinivasan R, et al. (2013). Investigation on antimicrobial activity of root extracts of Thespesia populnea Linn. Trop Biomed 30:570–8
- Ukil A, Maity S, Karmakar S, et al. (2003). Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol 139:209–18
- Vasudevan M, Gunnam KK, Parle M. (2007). Antinociceptive and anti-inflammatory effects of Thespesia populnea bark extract. J Ethnopharmacol 109:264–70
- Veljaca M, Lesch CA, Pllana R, et al. (1995). BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J Pharmacol Exp Ther 272:417–22
- Wallace JL, Le T, Carter L, et al. (1995). Hapten-induced chronic colitis in the rat: Alternatives to trinitrobenzene sulfonic acid. J Pharmacol Toxicol Methods 33:237–9
- Yuvaraj P, Subramoniam A. (2009). Hepatoprotective property of Thespesia populnea against carbon tetrachloride induced liver damage in rats. J Basic Clin Physiol Pharmacol 20:169–77
- Zaware BB, Nirmal SA, Baheti DG, et al. (2011). Potential of Vitex negundo roots in the treatment of ulcerative colitis in mice. Pharm Biol 49:874–8