Abstract
Context: Moringa oleifera Lam. (Moringaceae) is a rich source of antioxidants. All parts of the plant are medicinally important and have been used as traditional medicine for a variety of human ailments in India.
Objective: Therapeutic efficacy of adjuvants with M. oleifera (MO) root extract was investigated against beryllium-induced oxidative stress.
Materials and methods: Hydroalcoholic (50% v/v) root extract of M. oleifera (150 mg/kg, p.o.) alone and combinations of M. oleifera with either piperine (2.5 mg/kg, p.o.) or curcumin (5.0 mg/kg, p.o.) daily for 1 week were administered in experimental rats against beryllium toxicity (1.0 mg/kg, i.p. daily for 5 weeks). Oxidative stress parameters including blood sugar, G-6-Pase in liver, and DNA damage were analyzed. Histopathological changes in liver and kidney were also observed.
Results: Beryllium enhanced lipid peroxidation (LPO), depleted reduced glutathione (GSH) and antioxidant enzymes activities, decreased blood sugar and G-6-Pase activity, and did not damage DNA. Histologically, liver was observed with structural loss and disintegration of hepatocytes, heavy vacuolation in hepatocytes, and kidney was observed with constriction of glomeruli and hypertrophy in epithelial cells of uriniferous tubules. Therapy of M. oleifera with piperine was effective; however, combination of M. oleifera with curcumin showed better therapeutic effect by reduction of LPO, elevated GSH level, maintained antioxidant enzymes activities, restored blood sugar, and G-6-Pase activity in liver together with almost normal histoarchitecture of liver and kidney.
Discussion and conclusion: Curcumin enhanced therapeutic efficacy of M. oleifera root extract and showed better antioxidant potential against beryllium toxicity.
Introduction
Exposure to toxic metals remains an occupational and environmental problem in the world due to their widespread use in human activities. Beryllium is one of the most hazardous metals that posses a great threat to the present day world. In Europe, Be and its compounds are classified as category 2 carcinogens (ESIS, Citation2008). Beryllium has highly desirable and exceptional material properties (enhancing metal-hardening capacity, high electrical and thermal conductivity, high melting and boiling points, and is non-corrosive); it has become an essential element used in various industries (NTP, Citation2011). Beryllium is continuously used as an alloying agent in producing beryllium copper alloy, which is extensively used in springs, electrical contacts, spot welding electrodes, and non-sparking tools. It is used in the manufacture of instruments and structural components such as aircraft and space shuttle brakes, satellite mirrors, X-ray windows, electrical circuits, and computer components; it serves as a neutron moderator in nuclear reactors, and nuclear weapons, and is also used in home appliances, dental applications, golf sticks, bicycle frames, and many other applications (Jaskula, Citation2010).
Due to wide range of its applications, beryllium is continuously extracted from mines and workers are exposed to it during mining and processing of beryllium and its compounds. Beryllium also found in bauxite ore, the primary source of aluminum (Taiwo et al., Citation2010). Due to exposure to beryllium, a number of diseases such as bronchitis, dermatitis, acute pneumonitis, hepatomegaly, chronic pulmonary granulomatosis, berylliosis, and chronic beryllium diseases (CBD) have been reported that depend on the doses and duration of exposure (Cummings et al., Citation2009). Beryllium sensitization may occur as a result of skin contact with fine particulate beryllium (Cooper & Harrison, Citation2009). Inhalation of high doses of soluble compounds is known to cause acute beryllium disease, which is an inflammatory obstructive lung disease after high-dose exposure for short duration (Cummings et al., Citation2009). The LD50 value of beryllium nitrate is 3.16 mg/kg (Mathur et al., Citation1985). In a study of 362 employees from a Norwegian aluminum smelter who were evaluated for BeS, one individual had confirmed sensitization or a BeS rate of 0.28% (Nilsen et al., Citation2010). Exposure to beryllium and its compounds provokes oxidative stress and leads to various pathological consequences and apoptosis (Sawyer et al., Citation2005). Thus, inhibition of oxidative stress may be an approach in the prevention of beryllium-related diseases.
A number of synthetic compounds have been investigated against beryllium intoxication but they have certain limitations. Increasing interest towards natural products for the treatment of a variety of diseases attracted us to deal beryllium toxicity with some plant extracts having polyphenolic constituents in it and the use of adjuvants that may enhance the therapeutic efficacy of natural products. Moringa oleifera Lam. (Moringaceae) is locally known as Shahjna. Different parts of this plant are used in the indigenous system of medicine as an anti-inflammatory (Kooltheat et al., Citation2014), anthelmintic (Rastogi et al., Citation2009), analgesic (Manaheji et al., Citation2011), antioxidant (Chang et al., Citation2012), hepatoprotective (Hamza, Citation2010), antiurolithiatic (Fahad et al., Citation2010) antitoxic (Agrawal et al., Citation2013b), and for the treatment of a variety of human ailments such as heart complaints (Panda et al., Citation2013), eye disease, dyspepsia, enlargement of spleen (Singh & Sharma, Citation2012), and ulcers (Choudhary et al., Citation2013).
Piperine (1-piperoylpiperidine) is an alkaloid obtained from the fruits of black pepper (Piper nigrum Linn.), long pepper (Piper longum Linn.), and other Piper species (Piperaceae). It has bioavailability enhancing activity for some drugs (Nirala et al., Citation2007, Citation2008). Piperine is known to exhibit a variety of biological activities including anti-inflammatory (Ying et al., Citation2013), antioxidant (Jain & Mishra, Citation2011), antithyroid (Vijayakumar & Nalini, Citation2006), antimutagenic (Srinivasan, Citation2007), antitumor (Li et al., Citation2011), antidepressant (Mao et al., Citation2011), and hepatoprotective activity (Sahu et al., Citation2012). Curcumin is a hydrophobic polyphenol derived from the rhizome of the herb Curcuma longa has a wide spectrum of biological and pharmacological activities, including antioxidant (Sehgal et al., Citation2012), anti-inflammatory (Abarikwu et al., Citation2014), antimicrobial and anticarcinogenic (Jagadeesh et al., Citation2009), hepatoprotective (Rajesh et al., Citation2010), and nephro-protective (Tarasub et al., Citation2011). Thus, present study investigates the antioxidant potential of M. oleifera root extract with and without piperine and curcumin in the treatment of beryllium-induced oxidative stress and pathological consequences.
Materials and methods
Chemicals
Beryllium nitrate, piperine, and curcumin were purchased from Sigma-Aldrich Company (St. Louis, MO). All the therapeutic agents were stored and refrigerated in a desiccator to avoid oxidation and thermal decomposition. All other chemicals used in this study were of pure and analytical grade and procured from standard chemical dealers.
Maintenance of animals and their feeding
Adult female albino rats of Wistar strain (10–12 weeks old having body weight of 160 ± 10 g, were randomly selected from departmental animal facility. Animals were housed under standard husbandry conditions (25 ± 2 °C temp., 60–70% relative humidity and 14 h light and 10 h dark). Animals were fed on standard commercially available pellets of animal (Pranav Agro Industries Ltd., New Delhi, India) and drinking water ad libitum. Animals used in this study were treated and cared for in accordance with the guidelines recommended by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India, and experimental protocols were approved by Institutional Animal Ethics Committee (CPCSEA/501/01/A) of Jiwaji University, Gwalior, India.
Identification of plant material and preparation of extract
Moringa oleifera was collected in October 2009, from the university campus, identified by Dr. A. K. Jain (Professor and Head), Department of Botany, Jiwaji University, Gwalior (MP), India. Small roots from the identified plants were cut by digging at the bottom of the plants. Some roots were preserved in herbarium (specimen no. JU/DB/2002) at the Department of Botany, Jiwaji University, Gwalior (MP) for future reference. The fresh roots of M. oleifera were washed with fresh water, cut, and shade dried. The dry roots were crushed and ground to a fine powder. This fine powder was socked in 50% ethyl alcohol for 7 d with vigorously shaking; supernatant was collected and filtered with Whatman No. 1 paper. The filtrate was evaporated at room temperature and the obtained extract was stored at 4 °C for further study.
Preparation of doses and treatments
Beryllium nitrate (35% w/v; Sigma-Aldrich, St. Louis, MO) was diluted in triple distilled water making up doses of 1 mg/2 ml/kg and administered intraperitoneally (Nirala et al., Citation2007). The doses of M. oleifera root extract (150 mg/kg/5 ml) were prepared in 1% gum acacia, and the doses of piperine (2.5 mg/kg/3 ml) and curcumin (5 mg/kg/3 ml) were prepared in olive oil and administered orally with the help of an intragastric rubber catheter. Forty-two adult female rats were divided into seven groups of six animals in each as follows:
Group 1: Normal control
Group 2: Be(NO3)2 (1.0 mg/kg i.p. daily) for 5 weeks
Group 3: Be(NO3)2 (as in group 2) + M. oleifera (150 mg/kg p.o.) after beryllium toxicity daily for 1 week
Group 4: Be(NO3)2 (as in group 2) + piperine (2.5 mg/kg p.o.) after beryllium toxicity daily for 1 week
Group 5: Be(NO3)2 (as in group 2) + curcumin (5.0 mg/kg p.o.) after beryllium toxicity daily for 1 week
Group 6: Be(NO3)2 (as in group 2) + M. oleifera (150 mg/kg p.o.) + piperine (2.5 mg/kg p.o.) after beryllium toxicity daily for 1 week
Group 7: Be(NO3)2 (as in group 2) + M. oleifera (150 mg/kg p.o.) + curcumin (5 mg/kg p.o.) after beryllium toxicity daily for 1 week
Twenty-four hours after final administration, the animals were sacrificed under mild ether anesthesia, and blood was drawn by puncturing retro-orbital venous sinus to isolate serum. The liver and kidney were immediately excised, blotted free of adhering fluid, and processed for biochemical studies. Standard techniques were applied to assay various biochemical parameters.
Oxidative stress parameters
Lipid peroxidation (LPO) was determined by measuring TBARS (Sharma & Krishnamurthy, Citation1968). Reduced glutathione (GSH) was determined by dithionitrobenzoic acid (DTNB) (Brehe & Burch, Citation1976). The activities of superoxide dismutase (SOD) (Misra & Fridovich, Citation1972), catalase (Aebi, Citation1984), glutathione reductase (GR) (Tayarani, Citation1989), glutathione-S-transferase (GST) (Habig et al., Citation1974), glucose-6-phosphate dehydrogenase (G6PDH) (Askar et al., Citation1996) were determined in liver and kidney. Activity of glucose-6-phosphatase (G-6-Pase) (Baginski et al., Citation1974) was determined in liver. Blood sugar was determined by the kit method as per instructions provided by the company (E-Merck, Mumbai, India).
Comet assay
It was performed by taking small pieces of liver and kidney (Singh et al., Citation1988).
Histological preparations
Liver and kidney samples were fixed in Bouin’s fixative and processed to obtain 5 µm thick paraffin sections and stained with hematoxylin and eosin (H&E) for histological observation.
Statistical analysis
Results are presented as mean ± SE of six animals used in each group. Data were subjected to statistical analysis through one-way analysis of variance (ANOVA) taking significant at 5% level of probability followed by Student’s t-test taking significant at p≤0.05 (Snedecor & Cochran, Citation1994). Percent protection was calculated by the following formula:
where D is the drug, N is the normal, T is the toxicant.
Results
Biochemical studies
Therapeutic effect of M. oleifera, piperine, and curcumin alone and combinations of M. oleifera with either piperine or curcumin were evaluated against beryllium-induced oxidative stress and histopathological alterations in rats. The extent of LPO (measured as thiobarbituric acid reactive substance; TBARS), GSH, activity of catalase, and SOD was monitored () as an indication of the oxidative stress. Biochemical analysis revealed tremendous rise in the production of TBARS (p≤0.001) along with a decrease in GSH (p≤0.001, p≤0.05) and activities of catalase (p≤0.01) and SOD (p≤0.001) in liver and kidney after beryllium exposure. Therapy with M. oleifera, piperine, and curcumin alone was found to be effective in depleting the TBARS level and maintaining the level of GSH, activity of catalase, and SOD in liver and kidney. Co-therapy with M. oleifera with piperine showed more improvement in these variables over M. oleifera alone; however, combination of M. oleifera with curcumin showed more pronounced effect in restoring the level of these markers of oxidative stress in liver and kidney which was confirmed statistically by one-way analysis of variance (ANOVA).
Table 1. Combination effect of Moringa oleifera along with piperine and curcumin against beryllium induced oxidative stress.
Enzymatic activities of glutathione reductase (GR) (p≤0.001, p≤0.05) and GST (p≤0.01) were significantly decreased and activity of glucose-6-phosphate dehydrogenase (G-6-PDH) was significantly (p≤0.01) increased after exposure to beryllium nitrate (). Therapy with M. oleifera, piperine, and curcumin alone significantly (p≤0.05) restored the activity of GR in liver only. Therapy with M. oleifera along with piperine showed significant improvement in the activities of GR and GST (p≤0.01, p≤0.05) in liver and kidney. Combination therapy of M. oleifera along with curcumin showed significant recovery in activities of GR, GST, and G-6-PDH (p≤0.01, p≤0.05) in liver and kidney. Beryllium nitrate significantly decreased blood sugar level (p≤0.001) and activity of glucose-6-phosphatase (p≤0.01) in liver (). Therapy with M. oleifera, piperine, and curcumin alone did not significantly improve blood sugar level and glucose-6-phosphatase activity, whereas combination of M. oleifera with piperine showed significant improvement in blood sugar level and glucose-6-phosphatase activity (p≤0.05). Combination therapy of M. oleifera with curcumin showed significant recovery in blood sugar level (p≤0.01), and glucose-6-phosphatase activity (p≤0.05) more towards normal control. The extent of recovery by therapeutic agents was calculated as percent protection and is presented in and .
Table 2. Combination effect of Moringa oleifera along with piperine and curcumin against beryllium induced oxidative stress and disturbance in carbohydrate metabolism.
Histopathology
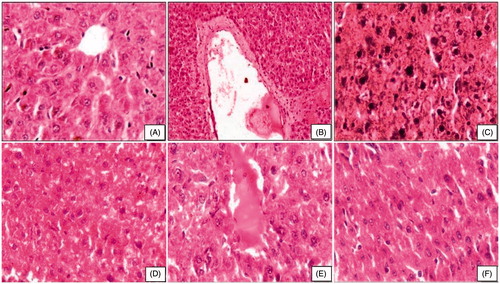
Histological sections of control liver showed well-formed cord arrangement of hepatocytes having conspicuous/prominent nucleus and normal appearance of central vein (). After beryllium toxicity, liver showed structural loss and disintegration of hepatocytes, massive hepatic necrosis, hyperchromatia of nuclei, and vacuolation in hepatocytes (). Moringa oleifera root extract at 150 mg/kg showed recovery in liver with better cord arrangement, and hepatocytes had large vesicular nuclei; however, some had cytoplasmic vacuoles (). Moringa oleifera with piperine showed better cord arrangement. The hepatocytes had large vesicular nuclei; however, some had cytoplasmic vacuoles (). Moringa oleifera along with curcumin maintained cord arrangement towards control. The hepatocytes were well formed with vesicular nuclei and cananiculi proliferation was good ().
Figure 1. Photomicrograph of liver after subchronic exposure to beryllium followed by combination therapy of moringa oleifera. (A) Control rats showing well-formed chord arrangement of hepatocytes and central vein (HE, 400×). (B and C) Beryllium-treated rats showing central camal with debris, loss of chord arrangement, nuclei of hepatocytes are irregular, and hyperchromatic (HE, 100×, 400×). (D) Therapy with MO 150 mg showing large vesicular nuclei and proliferation of camamiculi (HE, 400×). (E) Therapy with MO+Pip showing congested central camal (HE, 400×). (F) Therapy with MO+Cur showing histoarchitecture of liver (HE, 400×).
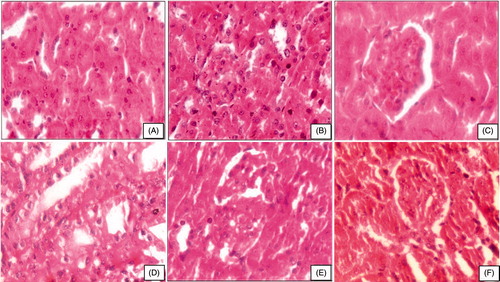
Kidney of control rats showed well-formed Bowman’s capsule with normal glomeruli and uriniferous tubules (). Beryllium administration showed wide space between glomerulus and capsule wall due to the constriction of glomeruli and uriniferous tubules with hypertrophy, heavy vacuolation, and lymphocytic infiltrations (). Treatment with M. oleifera root extract showed slight improvement in kidney with better formed glomerulus structure (). Combined therapy with M. oleifera and piperine could not improve glomeruli; however, they were better than beryllium per se administration and Moringa treatment alone. Some of the uriniferous tubules had debris in the lumen, whereas some tubules showed irregular distribution of hyperchromatic nuclei (). Combination therapy of M. oleifera and curcumin showed significant recovery with better formed glomeruli. The epithelial cells and medullary region were also better formed. However, recovery was observed in kidney histoarchitecture, still some hyperchromatic nuclei and some large nuclei in the epithelial cells persist ().
Figure 2. Photomicrograph of kidney after subchronic exposure to beryllium followed by combination therapy of moringa oleifera. (A) Control rats showing well-formed uriniferous tubules (HE, 400×). (B and C) Beryllium-treated rats showing obliteration of lumen of uriniferous tubules, exfoliation of nuclei, and contraction of glomerulus (HE, 400×). (D) Therapy with MO 150 mg showing uriniferous tubules with lumen and epithelial cell are vacuolated (HE, 400×). (E) Therapy with MO+Pip showing well-formed glomerulus and better lumen of uriniferous tubules (HE, 400×). (F) Therapy with MO+Cur showing well-formed glomeruli (HE, 400×).
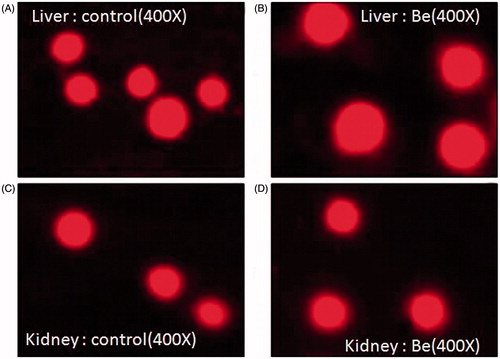
Comet assay
On observation of the slide under fluorescent microscope, no difference in the shape of nuclei was found. Beryllium-treated liver and kidney group did not show comet tail-like structure; still they appear as intact nuclei as observed in the liver and kidney of the control group ().
Discussion
Beryllium exposure induced oxidative stress as indicated by alteration in various hepatorenal biochemical parameters and histopathological alterations in rats. In a previous study, four different doses (50, 100, 150, and 200 mg/kg) of M. oleifera root extract were widely screened against beryllium toxicity and M. oleifera at doses of 150 mg/kg was found to be better against beryllium-induced toxicity (Agrawal et al., Citation2013b). The present study reports the antioxidant potential of M. oleifera root extract alone and in combination with piperine and curcumin against beryllium-induced oxidative stress. Oxidative stress is generated due to over production of reactive oxygen species (ROS). Beryllium induces oxidative stress by the excess production of hydrogen peroxide and superoxide free radicals; however, mechanism is unknown (Sawyer et al., Citation2005). MnTBAP is a novel class of catalytic antioxidants that have been shown to scavenge superoxide, H2O2, peroxynitrite, lipid peroxides, and also inhibits beryllium-induced ROS-dependent macrophage apoptosis (Sawyer et al., Citation2005). Be induces oxidative stress through its ability to deplete endogenous thiol antioxidants (Dobis et al., Citation2008).
Excess production of ROS depletes GSH (major internal antioxidants) and initiates membrane damage of vital organs of the body. Metal toxicity results in the production of ROS, which causes peroxidation of membrane lipids and induces a plethora of alterations in the structure and function of cellular membranes (Nirala et al., Citation2009). Increased TBARS after beryllium administration indicates enhanced LPO due to failure of the antioxidant defense mechanism and reduction in GSH. Moringa oleifera, piperine, and curcumin act as antioxidants and possibly could decrease pro-oxidant effect of beryllium up to some extent. Combination therapy with M. oleifera and curcumin increased the enzymatic and non-enzymatic antioxidant defense mechanisms more effectively, thus prevented LPO as well as maintained the GSH level more towards normal in liver and kidney, which is also evidenced by better formed cord arrangement of hepatocytes in liver and in the intact membrane structure of liver and kidney.
Active oxygen species and free radicals are involved in a variety of pathological events, including cancer. The antioxidant defense enzymes have been suggestive of playing an important role in maintaining physiological levels of oxygen and hydrogen peroxide and eliminating peroxides generated from inadvertent exposure to xenobiotics and drugs. Any natural compound with anti-oxidant properties may help in maintaining health when continuously taken as components of dietary foods, spices, or drugs (Singh et al., Citation2000). DPPH assay of hydro-alcoholic root extract of M. oleifera (Sultana et al., Citation2009) reported 61.8% free radical scavenging activity, which is slightly more than the pure alcoholic extract (56.6%). They also concluded that aqueous alcoholic extract showed more free radical scavenging effect than pure alcoholic extract. Curcumin is well-known antioxidant molecule, act synergistically with M. oleifera, and maintain the cellular integrity more effectively by decreasing LPO and maintaining the GSH in liver and kidney.
Beryllium reduces the activities of major antioxidant enzymes namely SOD, catalase, GR, and GST, whereas activity of G-6-PDH increases significantly. SOD and catalase are the major antioxidant enzymes, which catalyze ROS in most cells. Both enzymes play an important role in the elimination of ROS and act as the key component of cellular defense system against oxidative stress. SOD converts superoxide (O2−) to hydrogen peroxide (H2O2) and is a major defense system for aerobic cells in combating toxic effects of superoxide radical (Mishra et al., Citation2009). Catalase decomposes hydrogen peroxide (H2O2) into H2O and O2 and protects the tissue from highly reactive hydroxyl radicals. These antioxidant enzymes depend on various essential trace elements (bivalent metal ion) and prosthetic groups for proper molecular organization and enzymatic action. Beryllium may bind to the enzymatic active site and inhibit mRNA expression of catalase and SOD (El-Beshbishy et al., Citation2012), thus decreasing enzymatic activities. Increased SOD activity by therapeutic agents, along with that of catalase, signifies inhibition in LPO. Reduced LPO by the combination therapy in the present study is in correlation with the induction of antioxidant enzymes above the basal level. It may be due to an increase in the mRNA expression of catalase and SOD by the combination therapy of M. oleifera and curcumin.
GR is concerned with the maintenance of cellular level of GSH by affecting fast reduction of oxidized glutathione to reduced form. GST is phase II xenobiotic-metabolizing enzyme and plays a major role in cellular detoxification from oxidative, genotoxic, and carcinogenic xenobiotics (Hayes et al., Citation1999). GSTs are a family of soluble proteins, which conjugate xenobiotics with glutathione. Metabolites after glutathionylation are more hydrophilic and thus biologically inactive. Therefore, they are readily excreted in bile or urine as conjugates. This action is thus believed to be a major mechanism for the detoxification of xenobiotics (Henderson et al., Citation1998). The GST works together with glutathione peroxidase (GPx) and GSH in the decomposition of H2O2 or other organic hydroperoxides to non-toxic products.
Inhibition of GR and GST in the present study might be due to binding of beryllium to enzymatic active site and inhibited its action. Roots of M. oleifera are rich in gallic tannins, catechol tennins, steroids and triterpenoids, flavanoids, saponins, anthraquinones, alkaloids, and reducing sugars (Kasolo et al., Citation2011) and acts as antioxidants. Administration of M. oleifera root extract provides antioxidants, which could neutralize free radicals that may prevent binding of beryllium to enzymatic active sites and helps in the excretion of beryllium from body, thus the activity of GR and GST reversed towards control. Piperine and curcumin are the alkaloids and act as antioxidant increases the antioxidative potential of M. oleifera root extract when administered simultaneously. Combination of M. oleifera with piperine could maintain activity of GR and GST in liver and kidney. However, combination of M. oleifera and curcumin showed more pronounced therapeutic effect in reversal of these variables, which might be due to combinatorial action of M. oleifera and curcumin.
Glucose-6-phosphate dehydrogenase (G-6-PDH) catalyzes the first step in the pentose phosphate pathway (PPP) and that pathway supplies reducing energy to cells by maintaining the level of NADPH, which in turn supplies hydrogen for the production of GSH by the action of GR and helps in the protection of vital organs. Administration of toxic doses of beryllium nitrate enhances the activity of G-6-PDH. It might be due to increased production of glucose-6-phosphate by the breakdown of glycogen and inhibition of glucose-6-phosphatase activity. Decrease in GSH and reducing equivalent (NADPH) dramatically increase G-6-PDH activity for more production of NADPH, maintenance of GSH, and the energy level of body.
Beryllium administration decreases blood sugar level and glucose-6-phosphatase activity in liver. Decrease in blood sugar due to beryllium toxicity provides the signal for glycogenolysis thus the level of hepatic glycogen decreases (Agrawal et al., Citation2013a). Beryllium inhibits the key regulatory enzymes of carbohydrate metabolism (Nirala et al., Citation2008) thus glucose and its derived product (glucose-6-phosphate) enter into pentose phosphate pathway for the production of body energy and reducing equivalent. The G-6-Pase is a SER membrane enzyme involved in glycogenolysis. Beryllium administration decreased enzymatic activity possibly due to binding of beryllium ions to its phosphate group (Aldridge & Thomas, Citation1966). Depleted enzymatic activity also directly reflects the degradation of SER membrane, which was also evident by ultra morphology of the liver and kidney (Nirala et al., Citation2007). Treatment with aqueous doses of M. oleifera helps in maintaining integrity of cellular membrane of hepatocytes as shown in histological sections and restored activity of glucose-6-phosphatase. The antioxidants present in M. oleifera prevent the harmful effect of beryllium as well as beryllium-induced ROS. Combination therapy of M. oleifera root extracts along with piperine and curcumin could maintain the level of GSH as well as reducing equivalent, G-6-Pase activity, and the level of glucose-6-phosphate towards control in liver and kidney thus the activity of G6PDH reversed towards control.
Beryllium does not induce DNA damage at these doses, which is also evidenced by Strupp (Citation2011), who performed in vitro assay and proved that beryllium did not induce DNA damage. He also reported that treatment with beryllium metal extracts did not induce DNA repair synthesis, indicative of no DNA damaging potential of beryllium metal. However, beryllium inhibits the synthesis of nuclear proteins and influences the steps leading to DNA synthesis and selectively interferes with regulatory mechanisms controlling transcriptional events in gene expression, which may provide insight into the mechanism of beryllium-induced carcinogenicity (Perry et al., Citation1982) as well as chromosomal aberrations (Nikiforova & Voronin, Citation1989). Thus, it can be suggested that beryllium interferes with biochemical metabolic pathway and induces oxidative stress and histopathological lesions which lead to hepatorenal dysfunction.
Conclusion
Moringa oleifera root extract is a rich source of procyanidins, β-carotene, vitamin A, and vitamin E and showed the hepatorenal protection against beryllium toxicity, as evidenced by maintained histoarchitecture and oxidative stress parameters of liver and kidney. Curcumin with M. oleifera acts synergistically to abrogate beryllium-induced hepatorenal abnormality at biochemical and histopathological levels indicating that mix therapy is much more useful than individual application of therapeutic agents. However, more compounds of root extract of M. oleifera need to be identified, which are responsible for detoxification of beryllium.
Declaration of interest
The authors report no declarations of interest. Authors are thankful to University Grants Commission (New Delhi), India for financial assistance (F-33-349/2007 SR).
References
- Abarikwu SO, Akiri OF, Durojaiye MA, Alabi AF. (2014). Combined administration of curcumin and gallic acid inhibits gallic acid-induced suppression of steroidogenesis, sperm output, antioxidant defenses and inflammatory responsive genes. J Steroid Biochem Mol Biol 143:49–60
- Aebi H. (1984). Catalase in vitro. Meth Enzymol 105:121–6
- Agrawal ND, Agrawal K, Shukla S, et al. (2013a). Ameliorative effect of Aloe vera against beryllium induced hepatorenal toxicity. J Cell Tiss Res 13:3585–90
- Agrawal ND, Agrawal K, Gupta S, et al. (2013b). Therapeutic potential of Moringa oleifera root extract in attenuation of beryllium induced oxidative stress, biochemical and histopathological alterations in rat. Int J Curr Res 5:1677–83
- Aldridge WN, Thomas M. (1966). The inhibition of phosphoglucomutase by beryllium. Biochem J 98:100–4
- Askar MA, Sumathy K, Baquer NJ. (1996). Regulation and properties of glucose-6 phosphate dehydrogenase from rat brain. Ind J Biochem Biophys 33:512–18
- Baginski ES, Foa PP, Zak B. (1974). Glucose-6-phosphate. In: Hans Ulrich Bergmeyer, Verlag Chemie Weinheim, eds. Methods of Enzymatic Analysis, 2nd ed, vol. 2. New York: Academy Press Inc, 876–80
- Brehe JE, Burch HB. (1976). Enzymatic assay for glutathione. Anal Biochem 74:189–97
- Chang CL, Lin CS, Lai GH. (2012). Phytochemical characteristics, free radical scavenging activities, and neuroprotection of five medicinal plant extracts. Evid Based Complement Alternat Med 2012:984295
- Choudhary MK, Bodakhe SH, Gupta SK. (2013). Assessment of the antiulcer potential of Moringa oleifera root-bark extract in rats. J Acupunct Meridian Stud 6:214–20
- Cooper RG, Harrison AP. (2009). The uses and adverse effects of beryllium on health. Ind J Occup Environ Med 13:65–76
- Cummings KJ, Stefaniak AB, Virji MA, Kreiss K. (2009). A reconsideration of acute beryllium disease. Environ Health Perspect 117:1250–6
- Dobis DR, Sawyer RT, Gillespie MM, et al. (2008). Modulation of lymphocyte proliferation by antioxidants in chronic beryllium disease. Am J Respir Crit Care Med 177:1002–11
- El-Beshbishy HA, Hassan MH, Aly HAA, et al. (2012). Crocin ‘‘saffron’’ protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotox Environ Safe 83:47–54
- European Chemical Substances Information System (ESIS). (2008). Available from: http://ecb.jrc.it/esis/ [last accessed 12 Aug 2008]
- Fahad J, Vijayalakshmi MC. Sanjeeva SK, et al. (2010). Antiurolithiatic activity of aqueous extract of bark of Moringa oleifera (Lam.) in rats. Health 2:352–5
- Habig WH, Pabst MJ, Jacoby WB. (1974). Glutathione-S-transferases – The first step in mercapturic acid formation. J Biol Chem 249:7130–9
- Hamza AA. (2010). Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem Toxicol 48:345–55
- Hayes JD, Ellis EM, Neal GE, et al. (1999). Cellular response to cancer chemopreventive agents: Contribution of the antioxidant responsive element to the adaptive response to oxidative and chemical stress. Biochem Soc Symp 64:141–68
- Henderson CJ, Smith AG, Ure J, et al. (1998). Increased skin tumorigenesis in mice lacking pi class glutathione-S-transferases. Proc Natl Acad Sci USA 9:5275–80
- Jagadeesh MC, Sreepriya M, Bali G, Manjulakumari D. (2009). Biochemical studies on the effect of curcumin and embelin during N-nitrosodiethylamine/phenobarbital induced-hepatocarcinogenesis in Wistar rats. Afr J Biotechnol 8:4618–22
- Jain N, Mishra RN. (2011). Antioxidant activity of trikatu mega ext. Int J Pharm Biomed Res 2:624–8
- Jaskula BW. (2010). Beryllium. In: Mineral Commodity Summaries. U.S. Geological Survey. Available from: http://minerals.usgs.gov/minerals/pubs/commodity/beryllium/mcs-2010-beryl.pdf
- Kasolo JN, Bimenya GS, Ojok L, Ogwal-okeng JW. (2011). Phytochemicals and acute toxicity of Moringa oleifera roots in mice. J Pharm Phytother 3:38–42
- Kooltheat N, Sranujit RP, Chumark P, et al. (2014). An ethyl acetate fraction of Moringa oleifera Lam. inhibits human macrophage cytokine production induced by cigarette smoke. Nutrients 6:697–710
- Li Y, Wicha MS, Schwartz SJ, Sun D. (2011). Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. J Nutr Biochem 22:799–806
- Manaheji H, Jafari S, Zharinghalam J, et al. (2011). Analgesic effects of methanolic extracts of the leaf or roots of Moringa oleifera on complete Freund’s adjuvant induced arthritis in rats. J Chin Int Med 9:216–22
- Mao QQ, Xian YF, Ip SP, Che CT. (2011). Involvement of serotonergic system in the antidepressant-like effect of piperine. Prog Neuropsychopharmacol Biol Psychiatry 35:1144–7
- Mathur R, Asthana K, Sharma S, Prakash AO. (1985). Measurement of lethal dose of some beryllium compounds. IRCS Med Sci 13:163
- Mishra D, Gupta R, Pant S, et al. (2009). Co-administration of monoisoamyl simercaptosuccinic acid and Moringa oleifera seed powder protects arsenic induced oxidative stress and metal distribution in mice. Toxicol Mech Methods 19:169–82
- Misra HP, Fridovich I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–5
- National Toxicology Program (NTP). (2011). Beryllium and beryllium compounds. Rep Carcinog 12:67–70
- Nikiforova VI, Voronin SA. (1989). The mutagenic effect of beryllium on animals. Tsitol Genet 23:27–30
- Nilsen AM, Vik R, Behrens C, et al. (2010). Beryllium sensitivity among workers at a Norwegian aluminum smelter. Am J Ind Med 53:724–32
- Nirala SK, Bhadauria M, Mathur R, Mathur A. (2007). Amelioration of beryllium induced alterations in hepatorenal biochemistry and ultramorphology by co-administration of tiferron and adjuvants. J Biomed Sci 14:331–45
- Nirala SK, Bhadauria M, Mathur R, Mathur A. (2008). Influence of α-tocopherol, propolis and piperine on therapeutic potential of tiferron against beryllium induced toxic manifestations. J Appl Toxicol 28:44–54
- Nirala SK, Bhadauria M, Upadhyay AK, et al. (2009). Reversal of effects of intraperitoneally administered beryllium nitrate by tiron and CaNa3DTPA alone or in combination with α-tocopherol. Ind J Exp Biol 47:955–63
- Panda S, Kar A, Sharma P, Sharma A. (2013). Cardioprotective potential of N, α-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorg Med Chem Lett 23:959–62
- Perry ST, Kulkarni SB, Lee KL, Kenney FT. (1982). Selective effect of the metallocarcinogen beryllium on hormonal regulation of gene expression in cultured cells. Cancer Res 42:473–6
- Rajesh P, Balasubramaniam V, Ramesh N, et al. (2010). A biochemical approach on Curcuma longa Linn. (turmeric) against alcoholic liver diseases by using Swiss albino mice and SDS-PAGE analysis. Int J Med Res 1:6–17
- Rastogi T, Bhutda V, Moon K, et al. (2009). Comparative studies on anthelmintic activity of Moringa oleifera and Vitex negundo. Asian J Res Chem 2:181–2
- Sahu P, Bhatt A, Chaurasia A, Gajbhiye V. (2012). Enhanced hepatoprotective activity of piperine loaded chitosan microspheres. Int J Drug Dev Res 4:229–33
- Sawyer RT, Dobis DR, Goldstein M, et al. (2005). Beryllium-stimulated reactive oxygen species and macrophage apoptosis. Free Radic Biol Med 38:928–37
- Sehgal A, Kumar M, Jain M, Dhawan DK. (2012). Piperine as an adjuvant increases the efficacy of curcumin in mitigating benzo(a)pyrene toxicity. Hum Exp Toxicol 31:473–82
- Sharma SK, Krishnamurthy CR. (1968). Production of lipid peroxides of brain. J Neurochem 15:147–9
- Singh GP, Sharma SK. (2012). Antimicrobial evaluation of leaf extract of Moringa oleifera Lam. Int Res J Pharm 3:212–15
- Singh NP, McCoy MT, Tice RR, Schneir EL. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:188–91
- Singh RP, Padmanathi B, Rao AR. (2000). Modulatory influence of Adhatoda vesica (Justicia adhatoda) leaf extract on the enzymes of xenobiotic metabolism antioxidant status and lipidperoxidation in mice. Mol Cell Biochem 213:99–109
- Snedecor GW, Cochran WG. (1994). Statistical Method, 8th ed. Ames: Lowa State University Press
- Srinivasan K. (2007). Black pepper and its pungent principle piperine: A review of diverse physiological effects. Crit Rev Food Sci Nutr 47:735–48
- Strupp C. (2011). Beryllium metal I. Experimental results on acute oral toxicity, local skin and eye effects, and genotoxicity. Ann Occup Hyg 55:30–42
- Sultana B, Anwar F, Ashraf M. (2009). Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 14:2167–80
- Taiwo OA, Slade MD, Cantley LF, et al. (2010). Prevalence of beryllium sensitization among aluminium smelter workers. Occup Med 60:569–71
- Tarasub N, Tarasub C, Ayutthaya WDN. (2011). Protective role of curcumin on cadmium-induced nephrotoxicity in rats. J Environ Chem Ecotoxicol 3:17–24
- Tayarani I, Cloez I, Clement M, Bourre JM. (1989). Antioxidant enzymes and related free elements in aging brain capillaries and chloroid plexus. J Neurochem 53:817–24
- Vijayakumar RS, Nalini N. (2006). Piperine, an active principle from Piper nigrum, modulates hormonal and apolipoprotein profiles in hyperlipidemic rats. J Basic Clin Physiol Pharmacol 17:71–86
- Ying X, Yu K, Chen X, et al. (2013). Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell Immunol 285:49–54