Abstract
Context: The rising problem of resistance to present antimalarial drugs stresses the need to look for newer antiplasmodial components with effective modes of action. The roots of Berberis aristata DC. (Berberidaceae) are used in the traditional medicine for malaria in various parts of India.
Objective: The objective of this study was to evaluate antiplasmodial activity of B. aristata roots extract for the validation of its traditional medicinal use.
Material and methods: Aqueous root extract of Berberis aristata (AREBA) was screened for its in vitro as well as in vivo antiplasmodial activity against lethal rodent malaria parasite Plasmodium berghei NK65. In vitro activity was evaluated against schizont maturation of P. berghei using various concentrations ranging from 1 to 100 µg/mL. For in vivo studies, AREBA at the doses of 150, 250, 350, and 650 mg/kg/d was administered to P. berghei infected BALB/c mice orally for 4 consecutive days (D0–D3).
Results: AREBA showed in vitro antiplasmodial activity with an IC50 value of 40 µg/mL. In vivo studies demonstrated a variable dose-dependent chemosuppression with higher efficacy at lower doses. At a dose of 350 mg/kg/d, the suppressive and preventive activities were found to be 67.1% and 53.9%, respectively, followed by enhancing mean survival period up to 12.8 d for the curative assay versus 7.5 d for the untreated mice.
Discussion and conclusion: These results provide relevant scientific evidences for the traditional medicinal use of this plant as malaria remedy and further advocates the isolation and characterization of active antiplasmodial principle from this plant.
Introduction
Malaria is one of the major life-threatening health problems throughout tropical countries (Mueller et al., Citation2011). The situation is becoming adverse with the rising problem of resistance to the present antimalarial drugs (Kasturi et al., Citation2012). At present where there is no malaria vaccine, chemotherapy is the main remedy for the management of malaria. Thus, there is an urgent need to discover newer antimalarial components which can control this devastating disease. Natural products, mainly plants, are fundamental source of numerous drugs and therapeutics. Interestingly, the most important and effective drugs used in malaria treatment have been derived from the plants (Chandel et al., Citation2012). Their impact has widened the scope of antimalarial drugs search in traditional medicinal plants.
Berberis aristata DC. (Berberidaceae) (vern. Indian barberry, Daruhaldi and Daruharidra) is distributed throughout India, at an altitude of 1650–3400 m. The root extract of the plant is traditionally used in the treatment of skin diseases, eye diseases, wound healing, ulcers, malarial fever, diabetes, dysentery, uterine, and vaginal disorders (Kirtikar & Basu, Citation1933). This extract is also used as a traditional fabric dye in India which is scientifically validated (Semwal et al., Citation2012). Previous studies reported its use as an immunomodulatory as well as an anti-inflammatory agent (Gupta et al., Citation2008). The root extract of the plant, containing berberine (1), palmatine (2) and berbamine (3), exhibited antihyperglycemic and antioxidant activities (Singh & Kakkar, Citation2009). Chemical structures of the major bioactive alkaloids of B. aristata roots are depicted in .
Berberine (1), a major bioactive alkaloid of B. aristata roots, furnished potent antimicrobial and antioxidant activities (Dutta & Panse, Citation1962). Moreover, 1 was shown to have in vitro antiplasmodial activity against P. falciparum at an IC50 value of 0.968 µM (Wright et al., Citation2000). It exhibits antidepressant activity in various behavioral paradigms of despair by modulating brain biogenic amines such as norepinephrine, serotonin, and dopamine. Besides, 1 was also found effective against various other CNS disorders such as Alzheimer’s disease, schizophrenia, and anxiety (Potdar et al., Citation2012). Berberine (1) and palmatine (2) were tested for their antimalarial efficacy in vitro against mefloquine-resistant D-6 clone as well as against chloroquine, pyrimethamine, sulfadoxine, and quinine-resistant W-2 clone of P. falciparum. Compound 1 showed activity against both D-6 and W-2 clones with an IC50 value of 141 and 148 ng/mL, respectively, whereas 2 was found to be active with an IC50 value of 281 and 163 ng/mL, respectively. These alkaloids (1 and 2) were further screened for their in vivo activity in P. berghei parasitized mice, however, both the alkaloids were found to be inactive (Vennerstrom & Klayman, Citation1988). Berbamine (3), another alkaloid of the plant roots, exerts antimalarial activity against chloroquine-sensitive and -resistant strains of P. falciparum in vitro with the IC50 values of 603 and 359 nM, respectively (Ye et al., Citation1993). A further study by Angerhofer et al. (Citation1999) suggested that 3 shows in vitro activity with an IC50 value of 313 nM.
The aqueous extract from the roots of B. aristata is extensively used for the treatment of malaria in the various parts of India (Chauhan, Citation1999). However, there is no evidence as yet available which validated its traditional antimalarial use. Hence, the present work has been carried out in order to evaluate the antiplasmodial efficacy as well as safety of the roots of B. aristata for the first time.
Materials and methods
Plant material
Berberis aristata roots were collected from District Mandi, Himachal Pradesh (India), during September 2012. A voucher specimen (17919) was deposited in the herbarium of the Department of Botany (PAN), Panjab University, Chandigarh (India), where identification of the plant was confirmed by expert taxonomist Dr. M. L. Sharma.
Extraction
Shade-dried and powder roots (300 g) were extracted thrice with distilled water (500 mL) for 12 h at 45 °C. The solvent was evaporated up to dryness in a rota-evaporator with maintained temperature at 50 °C to yield black-brown residue (90 g). The resulting residue was stored in screw-capped vials at −4 °C temperature until tested.
Phytochemical screening
Various standard methods for phytochemical screening were adopted to determine the presence of major classes of secondary metabolites in the root extract of B. aristata (Harborne, Citation1973; Trease & Evans, Citation1989). Chemical tests adopted for preliminary phytochemical screening of the extract are shown in .
Table 1. Secondary metabolites in the root of Berberis aristata and chemical tests used for their detection.
Animals
BALB/c mice weighing 25–30 g were obtained from Central Animal House, Panjab University, Chandigarh (India). Animals were housed in standard plastic cages and acclimatized for a period of 30 d. Mice maintained at this facility were allowed ad libitum access to food and water. Experiments were carried out according to the guidelines of the committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA), India (registration no. 45/1999/CPCSEA) and approved by Institutional Animal Ethics Committee (IAEC) of the University (approval no. 1334-50/CAH).
Maintenance of parasitic strain
Plasmodium berghei (NK-65), a chloroquine sensitive strain, was maintained in vivo in BALB/c mice by weekly inoculation of 1 × 107 P. berghei infected erythrocytes in naive mice. On day 0, all experimental mice were inoculated intraperitoneally with 0.2 mL of infected blood in citrate saline containing 1 × 107 P. berghei infected red blood cells.
Schizont maturation inhibition assay
The antiplasmodial activity of AREBA was assessed according to the standard method based on the assessment of the inhibition of schizont maturation (WHO, Citation2001). Short term in vitro culture of blood stages of P. berghei was maintained using the candle jar method (Trager & Jensen, Citation1976). RPMI–1640 (Gibco, Waltham, MA) supplemented with 0.06% (w/v) HEPES, 5% (w/v) sodium bicarbonate; antibiotics – gentamycin (50 µg/mL), penicillin (100 Ul/mL), and streptomycin (100 µg/mL) along with 10% (v/v) inactivated fetal calf serum (FCS) were used as the culture medium. Normal and P. berghei parasitized erythrocytes were mixed in a proportion to have 2–4% parasitemia at 0 h. Stock solution of AREBA was prepared by dissolving 20 mg of the extract in 1 mL of double-distilled water which was further diluted to achieve the required concentration (i.e., 10, 20, 40, 80, and 100 µg/mL) before being tested in 24-well plate. Each concentration was run in triplicate wells along with 10 µM chloroquine as a positive control and distilled water (0.2 mL) as a negative control. After 21 h of incubation, thin smears from each well were prepared, stained with Giemsa’s stain and erythrocytic blood stages of parasite were counted under light microscope. The IC50 value was determined using Probit analysis by plotting a graph of extract concentration against percentage of schizont maturation inhibition corresponding to that concentration after 0 and 21 h of incubation. The percentage of schizont maturation inhibition was calculated by 100[(A − B)/A], where A is the average schizont maturation in the untreated control well and B is the average schizont maturation in the extract or drug-treated wells.
Acute toxicity
The acute toxicity of AREBA was determined by following the standard procedure as described by Lorke (Citation1983). Briefly, three groups of five female mice each were weighed and administered orally with 1, 2, and 5 g/kg bw of mouse of the extract after 4 h of fasting to determine the LD50 value (median lethal dose). Test animals were observed for 14 d for various signs of toxicity including weight loss and mortality.
Evaluation of suppressive antiplasmodial activity (4-d test)
Knight and Peters’ (Citation1980) 4-d test was employed to evaluate schizontocidal activity. Four groups of mice, containing six mice in each group, were orally administered 150, 250, 350, and 650 mg/kg/d of AREBA. Two groups of control mice were administered chloroquine (5 mg/kg/d) as a positive control to one group and equivalent volume of distilled water (0.2 mL/mouse/d) as a negative control to another group for 4 consecutive days. Thin blood films were made from the tail of each mouse on the fifth day. Thin blood smears were fixed in methanol and stained with Giemsa (Citation1904) stain. The percentage of parasitaemia suppression was determined by counting the number of parasitized erythrocytes out of 500 red blood cells in random fields under a light microscope. Average percentage of parasitaemia suppression was calculated as 100[(A − B)/A], where A is the average percentage parasitaemia in the negative control group and B is the average parasitaemia in the test group.
Evaluation of preventive activity
A method described by Peters (Citation1965) was followed to assess the preventive activity. Five groups of mice (six mice in each group) were administered orally with 150, 250, and 350 mg/kg/d doses of AREBA and 1.2 mg/kg/d pyrimethamine (positive control) and 0.2 mL/mouse/d distilled water (negative control) for 4 consecutive days (D0–D3), respectively. On day 5, mice were inoculated with 1 × 107 P. berghei infected red blood cells. After 72 h, the parasitaemia level was assessed by studying Giemsa stained blood smears.
Evaluation of curative activity
The standard method of Ryley and Peter (Citation1970) was followed to evaluate curative activity. Standard inoculum of 1 × 107 P. berghei parasitized red blood cells was injected intraperitoneally into mice on the first day. After 72 h, mice were divided into five groups of six mice in each group. Different doses of AREBA (150, 250, and 350 mg/kg/d) were administered orally to these groups. Chloroquine (5 mg/kg/d) was given to the positive control group and an equal volume of distilled water to the negative control group. The extract and drug were given once daily for 5 d. Thin smears were prepared with blood from the tail of each mouse for 5 d, to monitor the parasitaemia level in each group. Mean survival time for each group was determined arithmetically by finding the average survival time (days) of mice (post-inoculation) over a period of 30 d (D0–D29).
Statistical analysis
Data were expressed as mean ± SD for six mice in each group. Statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Tukey’s honesty significant different test. The results with p ≤ 0.05 were considered as statistically significant.
Results
Phytochemical screening
The phytochemical screening studies revealed that alkaloids are the major constituents of the root of B. aristata whereas flavonoids and saponins are present in moderate concentration. The results of phytochemical screening and the chemical tests adopted for the detection of secondary metabolites are depicted in .
In vitro antiplasmodial activity
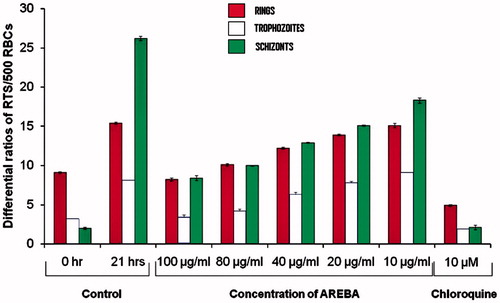
In vitro screening of AREBA showed almost three-fold increase in the parasitaemia in control well after 21 h of incubation of culture as compared with 0 h. AREBA was found to inhibit P. berghei schizont maturation in a dose-dependent manner, with an IC50 value of 40 µg/mL after 21 h. The maximum 67.9% inhibition was observed with 100 µg/mL AREBA, while 80, 40, 20, and 10 µg/mL concentrations showed 61.8%, 50.7%, 42.3%, and 30.1% schizonts maturation inhibition, respectively, whereas standard drug chloroquine (10 µM) exhibited 92.3% schizont maturation inhibition when compared with the distilled water (0.2 mL) used as a negative control ().
Acute toxicity
The LD50 value of AREBA was found to be more than 5 g/kg body weight of female mice fasted for 4 h. No reduction in the weight of experimental animals was observed after 2 weeks. Behavioral changes, like sluggishness, writhing, and palpitation, were not observed after administration of the different doses. No hepatomegaly and splenomegaly were observed in mice treated at the end of 14 d. The morphology of all organs (liver, spleen, and kidney) was found to be same as that of normal tissue, which proves its clinical safety.
Suppressive antiplasmodial activity
Oral administration of 150, 250, 350, and 650 mg/kg body weight of AREBA exhibited chemosuppression by 25.1%, 51.3%, 67.1%, and 5.8%, respectively, while standard drug chloroquine (5 mg/kg/d) caused 84.7% chemosuppression, which was statistically more significant (p < 0.001) than that of the extract-treated groups. All mice of the control group died due to higher infection by day 7 (). At higher dose (650 mg/kg, bw), AREBA was found to exhibit only 5.6% suppressive antiplasmodial activity. Therefore, lower doses of the extract (150, 250, and 350 mg/kg body weight) were selected for the evaluation of preventive and curative activities.
Table 2. Suppressive and preventive activities with mean survival time of AREBA treated BALB/c mice infected with P. berghei red blood cells.
Preventive activity
AREBA exerted a dose-dependent preventive activity with 150, 250, and 350 mg/kg doses exhibited 24.3, 38.5, and 53.9% chemosuppression, respectively. However, the standard drug pyrimethamine (1.2 mg/kg/d) caused a considerably higher chemosuppression (86.8%) than the extract-treated groups, in which a higher dose of 350 mg/kg produced 53.9% chemosuppression ().
Curative activity
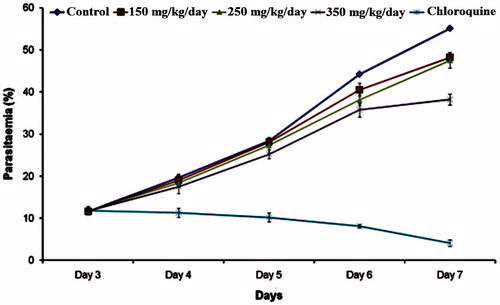
The parasitaemia of AREBA-treated groups on day 7 was 48.2%, 47.5%, and 38.2% for 150, 250, and 350 mg/kg of doses, respectively (). Infection in control- and chloroquine-treated groups was 55.1% and 7.5%, respectively. Oral administration of 150 mg/kg/d and 250 mg/kg/d of AREBA exerted 12.5 and 13.7% chemosuppression, respectively, with significant p (<0.01) value, while 350 mg/kg/d of AREBA and 5 mg/kg of chloroquine was found to exert 30.6 and 92.5% chemosuppression, respectively, with significant p (<0.001) value, as compared with the negative control group ().
Discussion
The discovery of a novel herbal remedy, with effective antiplasmodial efficacy, could be a highly important achievement in the field of malaria chemotherapy. AREBA was found to exhibit considerable in vitro antiplasmodial activity with an IC50 value of 40 µg/mL. An in vitro study by Chung et al. (Citation2010) revealed that a pure compound, 2-isopropenyl-6-acetyl-8-methoxy-1,3-benzodioxin-4-one, isolated from a traditional antimalarial plant Carpesium divaricatum, showed activity against P. falciparum strain D10 with an IC50 value of 2.3 μM. Herein, the in vitro antimalarial activity of AREBA (IC50 value = 40 µg/mL) can be comparatively considered to be a potent activity for a crude extract. Therefore, on the basis of in vitro results, AREBA was further studied for in vivo antiplasmodial activity in order to assure its safety and efficacy in various doses.
Rodent malaria parasites have been widely used as an experimental model due to their adaptability to the laboratory conditions. Plasmodium berghei (NK-65) is a lethal rodent malaria parasite. Mice infected with parasite die within 7–10 d of infection in normal course of infection (Chandel & Bagai, Citation2010). In the present study, in vitro results showed that AREBA is effective at high dose, while in vivo results showed that it is effective only when administered at lower concentration. High dose of AREBA (650 mg/kg body weight) was found to exert only 5.6% chemosuppressive activity. Since all plants work synergistically with the immune system, high concentration of extract may down-modulate the natural immune resistance of host to malaria. AREBA has also been found to exert preventive activity. A dose of 350 mg/kg/body weight showed 53.9% chemosuppression. A similar in vivo study revealed that various crude extracts obtained from Psidium guajava, Ocimum sanctum, and Murraya koenigii showed antiplasmodial activity against P. berghei infected albino mice with various higher doses namely 350, 750, and 1000 mg/kg, p.o., bw for 7 d (Rajendran et al., Citation2014). Herein, the in vivo activity of AREBA was recommended to be strong as previous studies, as the lower dose, i.e., 350 mg/kg bw exerted suppressive activity by 53.9% ().
Various secondary metabolites, such as berberine (1), palmatine (2), and berbamine (3) from the genus Berberis, have been found to exert in vitro antiplasmodial activity. The activity shown by these secondary metabolites was found due to their ability of intercalation or insertion between the planar bases of DNA (Phillipson & O’Neill, Citation1987; Schwikkard & Van Heerden, Citation2002). Hence, activity shown by AREBA is perhaps due to these constituents. However, the pure compounds (1 and 2) did not show any activity when studied in vivo (Vennerstrom & Klayman, Citation1988). In our case, the extract is highly effective in both in vitro and in vivo studies. Hence, it could be suggested that the activity of AREBA is due to the synergistic effect of these alkaloids together with other secondary metabolites. The standard drug pyrimethamine was used as the reference in order to compare the preventative activity with AREBA. Pyrimethamine interferes with folic acid mechanism which is necessary for the DNA synthesis of parasite, thus leading to the death of parasite (Gregson & Plowe, Citation2005). This principle may be further followed-up, in order to establish the mechanism of action of AREBA and its active constituents.
One of the biggest problems in dealing with Plasmodium parasite is recrudescence. This problem is observed during the long-term treatment of malaria (Lacrue et al., Citation2011). Moreover, similar antiplasmodial studies suggested that follow-up of mice for at least 1 month becomes necessary in order to monitor the efficacy of any antimalarial drug (Rajendran et al., Citation2014). Thus, all mice were observed for their mean survival time period in the present study. The mean survival time (MST) of the mice in AREBA-treated groups was found to be 8.9 (150 mg/kg), 11.6 (250 mg/kg), and 12.8 (350 mg/kg) d, whereas 29.8 d was recorded for chloroquine (5 mg/kg)-treated group (). The dose of 350 mg/kg bw in mice was found to be effective in all the parameters studied, and enhances the mean survival time of mice effectively.
Conclusions
In the present study, AREBA exhibited both in vitro and in vivo antiplasmodial activities. AREBA was found to be more active in lower doses which produced potent suppressive and preventive effects on P. berghei-infected mice by increasing the mean survival period. This study also revealed that the results of in vivo experiments are more significant than those of in vitro experiments. Since the major alkaloids such as berberine and berbamine of the plant have been shown to have antimalarial property, herein, it could be suggested that antiplasmodial activity of AREBA is mainly due to these compounds. The present study validated the traditional use of B. aristata roots in the treatment of malaria. However, further studies are required to determine the exact mode of action and characterization of its active antiplasmodial components.
Declaration of interest
This work was financially supported by University Grants Commission, New Delhi. The authors would like to pay their sincere gratitude to the Editor and Reviewers of this manuscript for their valuable suggestions to improve the quality of this work.
References
- Angerhofer CK, Guinaudeau H, Wongpanich V, et al. (1999). Antiplasmodial and cytotoxic activity of natural bisbenzylisoquinoline alkaloids. J Nat Prod 62:59–66
- Chandel S, Bagai U, Vashishat N. (2012). Antiplasmodial activity of Xanthium strumarium against Plasmodium berghei-infected BALB/c mice. Parasitol Res 110:1179–83
- Chandel S, Bagai, U. (2010). Antiplasmodial activity of Ajuga bracteosa against Plasmodium berghei infected BALB/c mice. Indian J Med Res, 131:440–44
- Chauhan NS. (1999). Medicinal and Aromatic Plants of Himachal Pradesh. New Delhi, India: Indus Publishing Company, 114–15
- Chung IM, Seo SH, Kang EY, et al. (2010). Antiplasmodial activity of isolated compounds from Carpesium divaricatum. Phytother Res 24:451–3
- Dutta NK, Panse MV. (1962). Usefulness of berberine (an alkaloid from Berberis aristata) in the treatment of cholera (experimental). Indian J Med Res 50:732–6
- Giemsa G. (1904). Eine Vereinfachung und Vervollkommnung meiner Methylenblau–Eosin–Färbemethode zur Erzielung der Romanowsky-Nocht'schen Chromatinfärbung. Centralblatt für Bakteriologie I Abteilung 32:307–13
- Gregson A, Plowe CV. (2005). Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev 57:117–45
- Gupta SK, Agarwal R, Srivastava S, et al. (2008). The anti-inflammatory effects of Curcuma longa and Berberis aristata in endotoxin-induced uveitis in rabbits. Invest Ophthalmol Vis Sci 49:4036–40
- Harborne JB. (1973). Phytochemical Methods, A Guide to Modern Technique of Plant Analysis. New York, USA: Chapman and Hall
- Kasturi K, Mallika DS, Amos SJ, et al. (2012). Current opinion on an emergence of drug resistant strains of Plasmodium falciparum through genetic alterations. Bioinformation 8:1114–18
- Kirtikar KR, Basu BD. (1933). Indian Medicinal Plants, 2nd ed., vol. 1. Allahabad, India: LM Basu and Co., 340–3
- Knight DJ, Peters W. (1980). The antimalarial action of N-benzyloxydihydrotriazines. The action of Clociguanil (BRL50216) against rodent malaria and studies on its mode of action. Ann Trop Med Parasitol 84:393–404
- LaCrue AN, Scheel M, Kennedy K, et al. (2011). Effects of artesunate on parasite recrudescence and dormancy in the rodent malaria model Plasmodium vinckei. PLoS One 6:e26689
- Lorke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol 54:275–87
- Mueller I, Slutsker L, Tanner M. (2011). Estimating the burden of malaria: The need for improved surveillance. PLoS Med 8:e1001144
- Peters W. (1965). Drug resistance in Plasmodium berghei. Exp Parasitol 17:80–9
- Phillipson JD, O’Neill MG. (1987). New leads to the treatment of protozoal infection based on natural product molecules. Acta Pharm Nord 1:131–44
- Potdar D, Hirwani RR, Dhulap S. (2012). Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia 83:817–30
- Rajendran C, Begam M, Kumar D, et al. (2014). Antiplasmodial activity of certain medicinal plants against chloroquine resistant Plasmodium berghei infected white albino BALB/c mice. J Parasit Dis 38:148–52
- Ryley JF, Peters W. (1970). The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol 64:209–22
- Schwikkard S, Van Heerden FR. (2002). Antimalarial activity of plant metabolites. Nat Prod Rep 19:675–92
- Semwal RB, Semwal DK, Kapoor P. (2012). Dyeing properties of Berberis aristata DC with natural and synthetic mordants. Trends Appl Sci Res 7:392–9
- Singh J, Kakkar P. (2009). Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J Ethnopharmacol 123:22–6
- Trager W, Jensen JB. (1976). Human malaria culture parasite in continuous culture. Science 193:673–5
- Trease A, Evans WC. (1989). Pharmacognosy. London, UK: Bailiere Tindall
- Vennerstrom JL, Klayman DL. (1988). Protoberberine alkaloids as antimalarials. J Med Chem 31:1084–7
- WHO (World Health Organisation) (2001). In vitro micro test (mark III) for the assessment of response of Plasmodium falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine pyrimethamine and artemisinin. Geneva: WHO, CTD/MAL/97, 20
- Wright CW, Marshall SJ, Russell PF, et al. (2000). In vitro antiplasmodial, antiamoebic, and cytotoxic activities of some monomeric isoquinoline alkaloids. J Nat Prod 63:1638–40
- Ye Z, Dyke KV, Yang B. (1993). Interaction of berbamine and chloroquine or artemisinin against chloroquine-sensitive and -resistant plasmodium falciparum in vitro. Drug Dev Res 30:229–37