Abstract
Context: Pereskia aculeata Miller (Cactaceae) is a cactus distributed from south to northeast of Brazil, where its leaves are commonly used as a vegetable, in skin wound healing, and to treat inflammation.
Objectives: The objective of this study was to perform the chemical characterization and to evaluate the antinociceptive activity of the hydromethanolic fraction obtained from the methanol extract of P. aculeata leaves.
Materials and methods: Chemical characterization was performed by UPLC–MS analysis. The antinociceptive activity was evaluated by the acetic acid-induced writhing, formalin, and tail-flick tests in mice, administering the single oral doses of 100, 200, and 300 mg/kg 1 h before each test.
Results: Tryptamine, abrine, mescaline, hordenine, petunidin, di-tert-butylphenol isomers, and quercetin were identified. The antinociceptive activity was inversely proportional to the administered doses in the acetic acid test, as the dose of 100 mg/kg reduced by 78% the number of writhings, while the doses of 200 and 300 mg/kg reduced by 64% and 41%, respectively. In the formalin test, the dose of 300 mg/kg inhibited by 50% and 86% the licking paw time in the first and second phases, respectively, while the doses of 200 mg/kg (45% and 62%, respectively) and 100 mg/kg (15% and 48%, respectively) were less effective. The sample did not respond to the tail-flick test. Those results suggested a peripheral and central antinociception devoid of an opioid effect.
Conclusion: Pereskia aculeata not only is a plant food with high nutritional value but also presents analgesic potential. It is the first time that this bioactivity is reported for this species.
Introduction
Pereskia aculeata Miller (Cactaceae), known as Barbados gooseberry, is a climbing plant native to South America and adapted only at low altitudes and naturally distributed from south to northeast of Brazil, where its succulent leaves are commonly used by the natives as a vegetable in traditional cuisine (Peterson et al., Citation2009; Pinto & Scio, Citation2014). The nutritional value of the leaves is related mainly to the high content of proteins (25.5% w/w), which is much higher when compared with other vegetables frequently used as foods, including bean (18–20% w/w), common corn (7.6–10.0% w/w), lettuce (1.3% w/w), or kale (1.6% w/w) (Mercê et al., Citation2001). In many low-income communities, the leaves of P. aculeata are considered as the main proteins source, so this vegetable is also known as “meat of the poor”. Tryptophan is the most abundant amino acid in these proteins. The leaves also contain high levels of total dietary fiber, vitamins A, C, and folic acid, in addition to minerals, such as calcium, magnesium, manganese, and zinc (Pinto & Scio, Citation2014; Takeiti et al., Citation2009).
Besides used in food, the leaves of P. aculeata are also used in Brazilian traditional medicine as emollients due to their high mucilaginous content, in skin wound healing, and to treat inflammation. Cytotoxicity against the tumor cell lines HL60 and MCF-7, and antioxidant effects were reported (Pinto et al., Citation2012). In addition, some species of Pereskia genus are reported to be used as natural remedies in cancer-related diseases, rheumatism, inflammation, headache, gastric pain, ulcers, hemorrhoids, atopic dermatitis, for pain relief and as tonics to revitalize the body (Sim et al., Citation2010a,Citationb).
Nevertheless, there are only few studies about chemical composition of P. aculeata leaves and their therapeutic potential, although natural products, especially those used in traditional medicine, are still a major source of new bioactive compounds (Newman & Cragg, Citation2012). Thus, this study aimed to perform the chemical characterization and to evaluate the antinociceptive activity of the hydromethanolic fraction obtained from methanol crude extract of P. aculeata leaves.
Materials and methods
Plant material and preparation of hydromethanolic fraction
Leaves of P. aculeata were collected in Juiz de Fora (MG, Brazil) in August 2010, in the morning. The plant was identified and a voucher specimen (No. 57539) was deposited in the Herbarium Leopoldo Krieger of Federal University of Juiz de Fora for future evidence. The leaves were air dried in a well-ventilated place at room temperature (25 °C) for 15 d. Once dried, the material (approximately 1 kg) was powdered using a knife mill (Marconi MA048, Piracicaba, SP, Brazil) and then extracted by maceration with methanol until exhaustion. The extract was concentrated on a rotary evaporator (R-3 BUCHI, Flawil, Switzerland) under monitored temperature (50 ± 2 °C) to obtain the crude methanol extract (140 g), which was dissolved in water/methanol (8:2 v/v) and then fractioned with different solvents in the order of increasing polarity: hexane, dichloromethane, ethyl acetate, and butanol. This process resulted in a remaining hydromethanolic fraction (HMF – 12 g) which was concentrated on a rotary evaporator, as described above, and stored in a refrigerator at 4 °C.
Evaluation of antinociceptive activity of hydromethanolic fraction
Animals
Male Swiss mice (20–30 g) bred in Center of Reproductive Biology (Federal University of Juiz de Fora, Brazil) were used. The animals were kept under standard temperature (22 °C), 12/12 h light/dark cycle, with food (Nuvital, Colombo, PR, Brazil) and water ad libitum. Before performing each test, the animals were kept without food for a period of 12 h. The groups consisted of 6–8 mice. All experimental procedures are in accordance with the Ethical Principles of Animal Research adopted by Brazilian College of Animal Experimentation (COBEA – Protocol no. 009/2009).
Acetic acid-induced writhing test
Groups of mice received different doses of HMF (100, 200, or 300 mg/kg), vehicle (negative control), or indomethacin (Henrifarma, Cambuci, SP, Brazil) 10 mg/kg, used as the reference drug. All solutions were prepared in saline with Tween 80 (Sigma-Aldrich, St. Louis, MO) 12% (v/v) and administered orally at 10 mL/kg. One hour after administration of respective treatments, mice were ip injected with acetic acid 0.6% 10 mL/kg. For 30 min, the number of writhings (constriction of abdominal wall with extension of hind paws) was counted (Koster et al., Citation1959).
Formalin test
One hour after oral administration of vehicle, indomethacin, HMF at different doses (100, 200, or 300 mg/kg), or 30 min after ip administration of morphine (Cristália, Itapira, SP, Brazil) 7.5 mg/kg (10 mL/kg); 20 μL of 2% formalin (v/v) was injected into the sup-plantar tissue of the right hind paw. The licking paw time was determined during 5 min (first phase) and between 15 and 30 min (second phase) after formalin injection (Hunskaar et al., Citation1985).
Tail-flick test
Each mouse was immobilized inside a small plastic tube with ventilation, so that the tail remained outside the tube. One-third of the tail was immersed in a 55 °C ± 1 water bath and the baseline reaction time was measured twice at 20 min intervals. Then, 1 h after oral administration of vehicle, HMF in different doses (100, 200, or 300 mg/kg), or 30 min after ip injection of morphine 7.5 mg/kg (10 mL/kg), the reaction time was measured again at 20 min intervals up to 2 h. A 15 s cut-off time was used to prevent tissue damage (D’Amour & Smith, Citation1941).
Statistical analysis
The results were expressed as mean ± SEM and one-way ANOVA followed by the Newman–Keuls test was used for statistical analysis. Two-way ANOVA followed by the Bonferroni test was used for the tail-flick test. p < 0.05 was considered significant. The percentage of antinociception was calculated compared with vehicle as follows:
Ultra-performance liquid chromatography–mass spectrometry (UPLC–MS) analysis
Sample preparation
HMF (1 mg) was dissolved in methanol (10 mL). The solution was filtered using a 0.22 μm filter and then diluted with HPLC grade methanol (Tedia, Fairfield, OH) to 100 ppm.
Electrospray ionization–mass spectrometry fingerprint
The sample HMF was diluted in a solution containing 50% (v/v) HPLC grade methanol and 50% (v/v) deionized water and 0.5% of ammonium hydroxide. Electrospray ionization–mass spectrometry fingerprint (ESI-MS) fingerprints in the positive ion mode were acquired and accumulated over 60 s, and spectra were scanned in a range between m/z 100 and 1000, using a Micromass-Waters Q-TOF mass spectrometer (Waters, Manchester, UK). Capillary and cone voltages were set at 3.00 kV and 30.00 V, respectively, with a source temperature of 150 °C and a desolvation temperature of 350 °C. The collision energy was 30 eV. ESI-MS was performed by direct infusion with typical flow rate of 10 μL/min using a syringe pump (Harvard Apparatus, Holliston, MA). The collision gas pressure was optimized to produce extensive fragmentation of the ion under investigation. The compounds were identified by comparison of their MS/MS fragmentation spectra with literature data.
Ultra-performance liquid chromatography analysis
Ultra-performance liquid chromatography (UPLC) was performed using a Waters UPLC system (Waters, Manchester, UK). Chromatographic separation was performed on an ACQUITY TQD (1.7 μm, 50 mm × 2.1 mm i.d.) (Waters Corporation, Milford, MA). Mobile phase A was water containing 0.1% formic acid. Mobile phase B was acetonitrile. The column temperature was ambient. The UPLC flow rate was 0.25 mL/min. A HMF solution of 5 μL was injected into the UPLC system. A mobile phase gradient was used with the percentage of B in A varying as follows: initial concentration, 35% B; 7 min, 55% B; 10 min, 55% B; 13 min, 35% B.
Search for indole compounds
Since P. aculeata leaves are abundant in proteins rich in tryptophan, the precursor of indole alkaloids (Dewick, Citation2009; Takeiti et al., Citation2009), Hopkins and Cole reaction (Hopkins & Cole, Citation1901) was used to verify the presence of indole compounds. Briefly, a small amount of HMF was solubilized in a glyoxylic acid solution, and then concentrated sulfuric acid was added slowly. A violet ring appears when sample contains indole compounds.
Results
Evaluation of antinociceptive activity of HMF
Acetic acid-induced writhing test
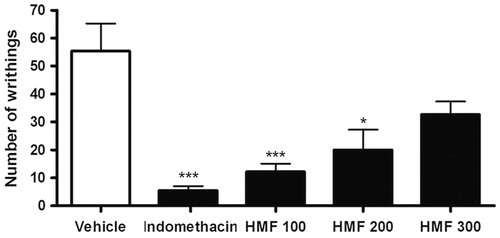
The acetic acid-induced writhing test showed that the antinociceptive activity of HMF was inversely proportional to the administered doses, since the dose of 100 mg/kg was the most active, reducing by 78% the number of writhings, while the doses of 200 and 300 mg/kg inhibited the painful stimulus by 64% and 41%, respectively, as shown in .
Figure 1. Effect of different doses of oral HMF on acetic acid-induced writhing test in mice. Vehicle, indomethacin 10 mg/kg, and HMF 100, 200, and 300 mg/kg were administered orally 60 min before acetic acid 0.6% injection. The number of writhings was counted for 30 min. The values of each column represent the mean ± SEM. ANOVA followed by the Newman–Keuls test, used as post hoc. Significant values: ***p < 0.001 and *p < 0.05 compared with vehicle.
Formalin test
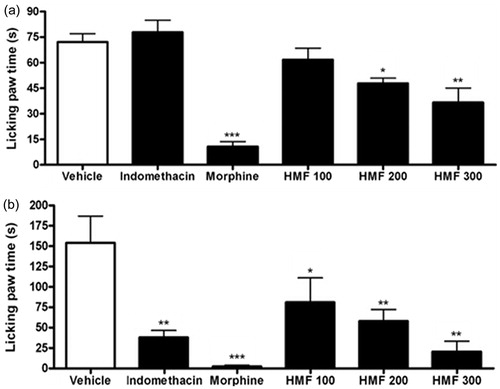
The results showed that the antinociceptive activity of HMF was dose dependent in the formalin test, unlike occurred in the acetic acid-induced writhing test. In the first phase, the dose of 300 mg/kg was the most effective, reducing about 50% the licking paw time compared with the negative control, while the doses of 200 and 100 mg/kg inhibited the nociception by 45% and 15%, respectively (). The dose of 300 mg/kg was also the most active in the second phase, inhibiting the nociception by 86%. The doses of 200 and 100 mg/kg reduced the painful stimulus by 62% and 48%, respectively ().
Figure 2. Effect of different doses of oral HMF on first (a) and second (b) phases of the formalin test in mice. Vehicle, indomethacin 10 mg/kg, and HMF 100, 200, and 300 mg/kg were administered orally while morphine 7.5 mg/kg was administered intraperitoneally 60 and 30 min, respectively, before formalin injection. The licking paw time was counted for 5 min after formalin injection. The values of each column represent the mean ± SEM. ANOVA followed by the Newman–Keuls test, used as post hoc. Significant values: ***p < 0.001, **p < 0.01, and *p < 0.05 compared with vehicle.
Tail-flick test
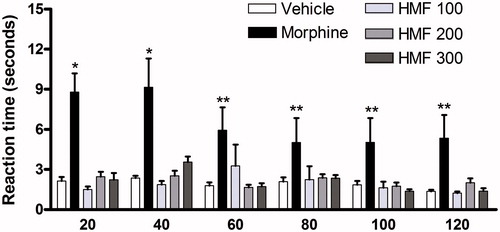
No activity was observed for HMF in the tail-flick test in none of the tested doses ().
Figure 3. Effect of different doses of oral HMF on tail flick test in mice. Vehicle, indomethacin 10 mg/kg and HMF 100, 200, and 300 mg/kg were administered orally while morphine 7.5 mg/kg (10 mL/kg) was administered intraperitoneally 60 and 30 min, respectively, before one-third of the tail was immersed in a 55 °C ± 1 water bath. The reaction time was measured at 20 min intervals up to 2 h. A 15 s cut-off time was used to prevent tissue damage. The values of each column represent the mean ± SEM. Two-way ANOVA followed by the Bonferroni test, used as post hoc. Significant values: **p < 0.01 and *p < 0.05 compared with vehicle.
Ultra-performance liquid chromatography–mass spectrometry (UPLC–MS) analysis and search for indole compounds
The compounds identified in HMF by UPLC–MS/MS are detailed in , and the chemical structures are shown in . In addition, a violet ring appeared in the Hopkins and Cole reaction, which suggested the presence of indole compounds.
Figure 4. Chemical structures of the compounds identified in hydromethanolic fraction of methanol crude extract of Pereskia aculeata leaves (HMF) by UPLC/MS/MS in the positive ion mode.
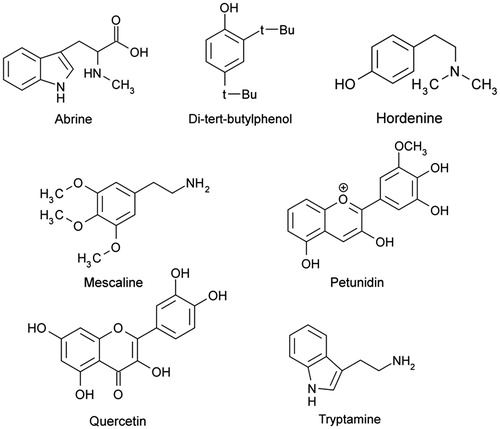
Table 1. Compounds identified in the hydromethanolic fraction from methanolic extract of Pereskia aculeata leaves by UPLC–MS/MS analysis in the positive ion mode.
Discussion
In spite of being a more appropriate assay for opioids, this acetic acid-induced writhing test is widely used as a model of induction of inflammatory origin pain, as the acetic acid solution induces the synthesis of arachidonic acid metabolites and other inflammation mediators (Ha et al., Citation2013). Thus, the antinociceptive effects of non-steroidal anti-inflammatories and peripheral analgesics may also be observed from this model. Moreover, there is an important involvement of serotonergic and noradrenergic neurons at pain modulation induced by acetic acid, thus drugs that act in noradrenergic receptors, tricyclic antidepressants, and selective serotonin reuptake inhibitors also respond to this test (Dickenson & Ghandehari, Citation2007; Galceran et al., Citation2011).
The higher doses of HMF were less effective, which is not surprising, as similar saturated dose–response curves after oral administrations of plant extracts with central activities have been reported, such as the extract of Allium macrostemon (Alliaceae) and Heteropterys brachiata (Malpighiaceae) (Huerta-Reyes et al., Citation2013; Lee et al., Citation2010). As plant extracts or fractions contain many compounds, a competitive or non-competitive antagonism may occur. In non-competitive antagonism, the threshold dose of an agonist may be not considerably high, but the maximum response is decreased by an antagonist, which may be active beyond the receptor, such as effects on intracellular second messengers (Williamson et al., Citation1996). To better understand if the mechanisms of the antinociceptive activity of HMF are central, peripheral or both, the formalin and tail-flick tests were accomplished.
The formalin test is a chemical model of pain induction that may determine if the mechanism of action of an analgesic drug occurs centrally or peripherally. The subplantar injection of formalin causes a biphasic behavioral response characterized by paw licking during an initial phase that occurs until 5 min after the injection, which is related to the direct activation of nociceptors in C and Aδ afferent fibers (neurogenic pain); and during a second phase that occurs between 15 and 30 min after the injection, associated to the release of inflammatory mediators, including prostaglandins, histamine, bradykinin, and serotonin (inflammatory pain). Opioid analgesics, such as morphine, inhibit the nociception caused by formalin in both phases, while anti-inflammatories, including indomethacin, are capable of suppress only the second phase (Monteiro et al., Citation2014). Non-opioid analgesics that act centrally and peripherally are active in both phases of the formalin test; however, they are more effective in the second phase, in which, generally, lower doses are sufficient to cause the antinociceptive effect (Shibata et al., Citation1989). Thus, the results suggested that the mechanism of action of HMF did not involve opioid receptors activation, but it was probably associated to a central and peripheral activity. The differences between the effectiveness of the different doses tested in formalin and acetic acid-induced writhing tests may occur as these are distinct models of pain induction, with distinct pain-induced agents and mechanisms.
The tail-flick test consists in evaluating the response of a drug in consequence of a thermogenic stimulus. The mechanisms associated to this response are not totally clear; however, it is recognized that this test is effective only to determine the analgesic activity of opioid agonists (Le Bars et al., Citation2001). The response of HMF in the tail-flick test reinforced the results found in the formalin test, as both tests suggested that HMF did not demonstrated opioid action in both cases.
Pinto et al. (Citation2012) reported the presence of phenols, tannins, coumarins, anthrones, flavonoids, and alkaloids in HMF. In this study, some of these chemical constituents were identified, including tryptamine, abrine, mescaline, hordenine, petunidin, di-tert-butylphenol isomers, and quercetin. According to the literature, they were also detected in other species of Cactaceae and Pereskia (Doetsch et al., Citation1980; Kobayashi et al., Citation2000; Neal et al., Citation1971; Sim et al., Citation2010a,Citationb; Stintzing & Carle, Citation2005).
Pereskia aculeata leaves are abundant in protein rich in tryptophan, an indole amino acid (Takeiti et al., Citation2009). In plants, tryptophan is the precursor of indole alkaloids, compounds with structural similarities to bioactive amines. Most of these alkaloids, when biologically active, affect the adrenergic, serotonergic, dopaminergic or cholinergic systems, including tryptamine, abrine, harmine, strychnine, psilocybin, reserpine, among many others (Dewick, Citation2009). These systems are directly related to the spinal cord pain control (García-Ramírez et al., Citation2014). In addition, phenylethylamine alkaloids, including hordenine and mescaline, which were identified in HMF, are very similar to norepinephrine in terms of chemical structure and may act like adrenergic drugs (Casado et al., Citation2008; Dewick, Citation2009). Furthermore, it is well documented the inhibition of nociceptive response of quercetin in several animal models (Filho et al., Citation2008). Thus, it is possible that the alkaloids identified and quercetin might be involved in the antinociceptive activity of HMF.
Conclusion
This study showed that the leaves of P. aculeata are not only a vegetable of high nutritional value but are also endowed with chemical constituents with analgesic potential, which effects are likely to occur at peripheral and central level, although they are probably not related to opioid receptors activation. It is possible that the presence of alkaloids and quercetin in HMF might be involved in the antinociceptive activity. As far as we know, this study is the first report on the analgesic potential of this plant food.
Acknowledgements
The authors are grateful to Dr. Daniela Zappi for the botanical identification of the species, to Delfino Antônio Campos for technical assistance and to the Reproduction Biology Center of the Federal University of Juiz de Fora for providing the mice.
Declaration of interest
The authors report that they have no conflicts of interest. This work was supported by the grant from FAPEMIG CEX APQ 01137-09, FAPESP, CNPq, and CAPES.
References
- Casado R, Uriarte I, Cavero RY, Calvo MI. (2008). LC-PAD determination of mescaline in cactus “peyote” (Lophophora williamsii). Chromatographia 67:665–7
- D’Amour FE, Smith DL. (1941). A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–9
- Dewick PM. (2009). Medicinal Natural Products: A Biosynthetic Approach, 3rd ed. Chichester, UK: John Wiley & Sons Ltd
- Dickenson AH, Ghandehari J. (2007). Anti-convulsants and anti-depressants. In: Stein C, ed. Handbook of Experimental Pharmacology: Analgesia. Berlin: Springer, 147–77
- Doetsch PW, Cassady JM, McLaughlin JL. (1980). Cactus alkaloids: XL. Identification of mescaline and other β-phenethylamines in Pereskia, Pereskiopsis and Islaya by use of fluorescamine conjugates. J Chromatogr A 189:79–85
- Filho AW, Filho VC, Olinger L, Souza MM. (2008). Quercetin: Further investigation of its antinociceptive properties and mechanism of action. Arch Pharmacal Res 31:713–21
- Galceran CB, Sertie JAA, Lima CS, Carvalho JCT. (2011). Anti-inflammatory and analgesic effects of 6α,7β-dihydroxy-vouacapan-17β-oic acid isolated from Pterodon emarginatus Vog. fruits. Inflammopharmacology 19:139–43
- García-Ramírez DL, Calvo JR, Hochman S, Quevedo JN. (2014). Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord. PLoS One 9:e89999
- Ha LM, Que DTN, Huyen DTT, et al. (2013). Toxicity, analgesic and anti-inflammatory activities of tectorigenin. Immunopharmacol Immunotoxicol 3:336–40
- Hopkins FG, Cole SW. (1901). On the proteid reaction of Adamkiewicz, with contributions to the chemistry of glyoxylic acid. Proc R Soc Lond 68:21–33
- Huerta-Reyes M, Herrera-Ruiz M, González Cortazar M, et al. (2013). Neuropharmacological in vivo effects and phytochemical profile of the extract from the aerial parts of Heteropterys brachiata (L.) DC. (Malpighiaceae). J Ethnopharmacol 146:311–17
- Hunskaar S, Fasmer OB, Hole K. (1985). The formalin test in mice, a useful technique for evaluating mild analgesia. J Neurosci Methods 14:69–76
- Kobayashi N, Schmidt J, Nimtz M, et al. (2000). Betalains from Christmas cactus. Phytochemistry 54:419–26
- Koster R, Anderson M, De Beer EJ. (1959). Acetic acid for analgesic screening. Fed Proc 18:412–18
- Le Bars D, Gozariu M, Cadden SW. (2001). Animal models of nociception. Pharmacol Rev 53:597–652
- Lee S, Kim DH, Lee C, et al. (2010). Antidepressant-like activity of the aqueous extract of Allium macrostemon in mice. J Ethnopharmacol 131:386–95
- Mercê ANR, Landaluze JS, Mangrich AS, et al. (2001). Complexes of arabinogalactan of Pereskia aculeata and Co2+, Cu2+, Mn2+, and Ni2+11. Bioresource Technol 76:29–37
- Monteiro EMH, Chibli LA, Yamamoto CH, et al. (2014). Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients 6:1931–44
- Neal JM, Sato PT, Johnson CL, McLaughlin JL. (1971). Cactus alkaloids X: Isolation of hordenine and N-methyltyramine from Ariocarpus kotschoubeyanus. J Pharm Sci 60:477–8
- Newman DJ, Cragg GM. (2012). Natural products as source of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–35
- Peterson ID, Downie DA, Hill MP. (2009). Using molecular methods to determine the origin of weed populations of Pereskia aculeata in South Africa and its relevance to biological control. Biol Control 48:84–91
- Pinto NCC, Santos RC, Machado DC, et al. (2012). Cytotoxic and antioxidant activity of Pereskia aculeata Miller. Pharmacologyonline 3:63–9
- Pinto NCC, Scio E. (2014). The biological activities and chemical composition of Pereskia species (Cactaceae) – A review. Plant Foods Hum Nutr 69:189–95
- Shibata M, Ohkubo T, Takahashi H, Inoki R. (1989). Modified formalin test: Characteristic biphasic pain response. Pain 38:347–52
- Sim KS, Nurestri AMS, Norhanom AW. (2010a). Phenolic content and antioxidant activity of crude and fractioned extracts of Pereskia bleo (Kunth) DC. (Cactaceae). Afr J Pharm Pharmacol 4:193–201
- Sim KS, Nurestri AMS, Sinniah SK, et al. (2010b). Acute oral toxicity of Pereskia bleo and Pereskia grandifolia in mice. Pharmacogn Mag 6:67–70
- Stintzing FC, Carle R. (2005). Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol Nutr Food Res 49:175–94
- Takeiti CY, Antonio GC, Motta EMP, et al. (2009). Nutritive evaluation of a non-conventional leafy vegetable (Pereskia aculeata Miller). Int J Food Sci Nutr 60:148–60
- Williamson E, Okpako D, Evans FJ. (1996). Selection, Preparation and Pharmacological Evaluation of Plant Material. Chichester, UK: John Wiley & Sons Ltd
