Abstract
Context: The leaves of Eriobotrya japonica (Thunb.) Lindl. (Rosaceae) are used in traditional medicine to treat inflammatory diseases. However, information about the antigenotoxic and antioxidant properties of its leaves remains to be elucidated.
Objective: The objective of this work was to evaluate the mutagenic/antimutagenic, genotoxic/antigenotoxic, and antioxidant potentials of aqueous and total oligomers flavonoid (TOF) extracts from E. japonica.
Materials and methods: The mutagenic/antimutagenic and genotoxic/antigenotoxic potentials of extracts (50, 250, and 500 µg/plate) were evaluated, respectively, by the Ames test with 48 h incubation and the SOS chromotest test with 2 h incubation. The antioxidant capacity of these extracts (ranging from 50 to 700 µg/mL) was tested using xanthine/xanthine oxidase and the deoxyribose assays.
Results: Eriobotrya japonica extracts showed neither mutagenic nor genotoxic effect. The highest protective effect against methyl methanesulfonate and 2-aminoanthracene was obtained in the presence of aqueous extract, with IC50 values of 80 and 140 µg/plate, respectively, against S. typhimurium TA104. Moreover, this extract (500 µg/plate) was also able to reduce significantly the genotoxicity induced by nitrofurantoin and aflatoxin B1 with IC50 values of 140 and 240 µg/assay, respectively. Likewise, aqueous and TOF extracts inhibited xanthine oxidase and superoxide anion formation with IC50 values ranging from 45 to 95 and from 70 to 90 µg/mL, respectively. However, TOF extract is more efficient in inhibiting hydroxyl radical and chelating iron ion with IC50 values of 140 and 400 µg/mL, respectively, when compared with the aqueous extract.
Conclusion: Eriobotrya japonica prevents the genotoxicity of some carcinogenic substances probably thanks to its antioxidant capacities.
Introduction
Since ancient times, herbs and species have been added to different types of food to improve their flavor and organoleptic properties. Many natural compounds extracted from plants play important roles in multiple cell pathways and are involved in anticarcinogenic effects (Sripanidkulchai et al., Citation2002). In fact, antioxidant activities, iron-chelating activities as well as inhibition of bioactivating enzymes and induction of detoxifying enzymes may provide protection against cancer initiation (Juranek et al., Citation2010). As far as all cellular components, proteins, polyunsaturated fatty acids, nucleic acids, and carbohydrates are prominent biological targets of reactive oxygen species, giving rise to metabolic and cellular disturbances. Foods, rich in antioxidants, should play an essential role in the prevention of many pathologies such as cardiovascular diseases, cancer, and neurodegenerative diseases as well as cell inflammatory problems caused by cutaneous agents (Islam et al., Citation2013).
Eriobotrya japonica (Thunb.) Lindl. (Rosaceae), also known as “loquat,” is a small fruit tree with narrow leaves that are dark green on the upper surface and have a lighter color underneath. The leaves of E. japonica are traditionally used for preventing and treating chronic inflammatory diseases including asthma, phlegm, chronic bronchitis, cough, and regulating stomach diseases (Ito et al., Citation2000). In Tunisia, the decoction of leaves from E. japonica is used to treat aphthous ulcers and kidney stones (Boukef, Citation1986).
Many compounds including triterpenes, sesquiterpenes, flavonoids, tannins, and megastigmane glycosides isolated from the leaves of E. japonica are reported to have anti-inflammatory, hypoglycemic, antioxidant, antitumor, and antiviral activities (De Tommasi et al., Citation1991; Ito et al., Citation2000; Kim & Shin, Citation2009; Taniguchi et al., Citation2002).
Owing to the frequent use of E. japonica in traditional medicine, we investigated the protective effect of E. japonica extracts against DNA damage induced by 2-aminoanthracene (2-AA), aflatoxin B1 (AFB1), nitrofurantoin, and methyl methanesulfonate (MMS) as well as its antioxidant potentials.
Materials and methods
Plant material
The leaves of E. japonica were collected in the region of Monastir, located on the center coast of Tunisia, on June 2010. Identification was carried out by Prof. Harzallah Skhiri Fethia (Higher Biotechnology Institute of Monastir, University of Monastir, Tunisia) according to the Flora of Tunisia (Pottier-Alapetite, Citation1979). A voucher specimen has been kept in our laboratory for future reference. Leaves, dried at room temperature, were powdered and stored in a tightly closed container for further use.
Plant extracts
The fresh leaves of E. japonica were dried at room temperature and reduced to coarse powder. The powdered leaves (100 g) were extracted with boiling water (1 L) for 15–20 min. After filtration, the crude extract was frozen and lyophilized, leading to the aqueous extract which was dissolved in water.
Additionally, in order to obtain an extract enriched in total oligomer flavonoids (TOF), the powdered leaves were macerated in a water–acetone mixture (1:2), during 24 h with continuous stirring. The extract was filtered and the acetone was evaporated under low pressure in order to obtain an aqueous phase. Tannins were partially removed by precipitation with an excess of NaCl during 24 h at 5 °C and the supernatant was recovered. This latter was extracted with ethyl acetate, concentrated and precipitated with an excess of chloroform. The precipitate was then separated and the TOF extract was yielded (Ghedira et al., Citation1991).
Evaluation of metabolite content of E. japonica extracts
Phytochemical screening
Phytochemical screening of extracts from E. japonica leaves was carried out employing standard procedures, to reveal the presence of chemical constituents such as flavonoids, tannins, saponins, and coumarins (Khandelwal, Citation2004).
Determination of total polyphenol and flavonoid content
The total polyphenol content of E. japonica extracts was quantified by Folin–Ciocalteau’s reagent (Kumar & Chattopadhyay, Citation2006; Yuan et al., Citation2005). Aqueous extract (100 µL) was mixed with 2 mL of 2% Na2CO3 and incubated at room temperature for 2 min. After the addition of 100 µL Folin–Ciocalteau’s phenol reagents (50%), the reaction tube was further incubated for 30 min at room temperature, and finally the absorbance was read at 720 nm. Polyphenol content was expressed as mg of gallic acid equivalents per gram of dry weight through the calibration curve of gallic acid.
A known volume of leaf extract was placed in a 10 mL volumetric flask to estimate flavonoid content. After addition of 75 µL NaNO2 (5%) solution, 150 µL of freshly prepared AlCl3 (10%) and 500 µL of NaOH (1 N), the volume was adjusted with distilled water until 2.5 mL. The mixture was incubated 5 min at room temperature and the absorbance was measured at 510 nm wavelength using a Genesys 10 UV scanning spectrophotometer (Thermo Electron Scientific Instruments Corporation, Madison, WI). Quercetin was used as a standard for constructing a calibration curve. The flavonoid content was expressed as equivalent of quercetin (Bouhlel et al., Citation2011).
Determination of tannin content
According to Nwabueze (Citation2007), quantification of tannins in the samples was achieved by dissolving 5 g of the aqueous and TOF extracts in 50 mL of distilled water in a conical flask, allowing the mixture to stand 30 min with shaking the flask at 10 min intervals and then centrifuging it at 5000 × g to obtain a supernatant (tannin extract). These extracts were diluted to 100 mL in a standard flask using distilled water.
The diluted extract (5 mL) and 5 mL of standard tannic acid (0.01 g/L) were measured into different 50 mL volumetric flasks. Folin–Denis reagent (1 mL) was added to each flask, followed by 2.5 mL of saturated sodium carbonate solution. The solutions were made up to the 50 mL mark with distilled water and incubated at room temperature for 90 min. The absorption of each solution was measured against that of the reagent blank (containing 5 mL of distilled water instead of extract or standard tannic acid solution), at a 760 nm wavelength using a Genesys 10 UV scanning spectrophotometer (Thermo Electron Scientific Instruments Corporation, Madison, WI). Tannin content (mg/100 g) was calculated in triplicate, using the following formula:
Bacterial strains
Salmonella typhimurium strains TA102 and TA104 which are histidine-requiring mutants were kindly provided by Prof. I. Felzenswalb (Universidade do Estado do Rio de Janeiro [UERJ], Rio de Janeiro, Brazil) and maintained as described by Maron and Ames (Citation1983). The genotypes of the test strains were checked routinely for their histidine requirement, deep rough (rfa) character, UV sensitivity (uvrB mutation), and presence of the R factor. They were stored at −80 °C. Salmonella typhimurium TA104 and TA 102 strains are known to be more responsive to certain mutagens such as (2-AA) and (MMS) (Mortelmans et al., Citation2000; Nelson et al., Citation2001). Strains TA102 and TA104 contain AT base pairs at the G428 mutant site. The mutation is carried on the multi-copy plasmid pAQ1 in strain TA102 and on the chromosome in strain TA104. The plasmid confers tetracycline resistance which is a convenient marker to detect the presence of this plasmid. The G428 mutation is an ochre mutation, TAA, in the G428 gene which can be reverted by all six possible base-pair changes; both transitions and transversions. This mutation is also reverted by mutagens that cause oxidative damage (Mortelmans & Zeiger, Citation2000).
Escherichia coli PQ37 strain was kindly provided by Prof. M. Quillardet (Institut Pasteur, Paris, France). The construction details of this strain were described by Quillardet and Hofnung (Citation1985). Frozen permanent copies of the tested strain were prepared and stored at −80 °C.
Salmonella-microsome assay
The mutagenicity assay with S. typhimurium was performed as described by Maron and Ames (Citation1983). The test is based on the plate incorporation method, using S. typhimurium strains TA102 and TA104, with and without an exogenous metabolic system: S9 fraction in S9 mix. The S9 microsome fraction is prepared from the livers of rats treated with aroclor 1254 (Maron & Ames, Citation1983) and stored at −80 °C.
One hundred microliters of an overnight culture of bacteria were cultivated for 16 h at 37 °C, approximate cell density [(2–5) × 108 cells/mL] and 500 µL of sodium phosphate buffer (0.2 M, pH 7.4 for assay without S9) or 500 µL of S9 mix were added to 2 mL aliquots of top Agar (supplemented with 0.5 mM l-histidine and 0.5 mM d-biotin), containing different concentrations of each extract. The resulting complete mixture was poured on minimal agar plates prepared as described by Maron and Ames (Citation1983). The plates were incubated at 37 °C for 48 h and the revertant bacterial colonies of each plate were counted. Negative and positive control cultures gave numbers of revertants per plate that were within the normal limits found in the laboratory. An extract was considered mutagenic if the number of revertants per plate was at least doubled over the spontaneous revertant frequency (Marques et al., Citation2003). Data were collected with a mean ± standard deviation of three experiments (n = 3).
Antimutagenicity testing
A modified plate incorporation procedure (Lee et al., Citation2000) was employed to determine the effect of samples on 2-amino anthracene (2-AA) and methyl methanesulfonate (MMS) induced mutagenicity. In brief, 0.5 mL of S9 mixture for indirect mutagen (2-AA) was distributed in sterilized capped tubes in an ice bath, then 50, 250, and 500 µg/tube of tested compounds and/or 5 and 10 µg/tube of 2-AA for TA104 and TA102, respectively, and 100 µL of bacterial culture (prepared as described in the mutagenicity test) were added. After vortexing gently and preincubating at 37 °C for 45 min, 2 mL of top agar supplemented with 0.05 M l-histidine and d-biotine were added to each tube. The resulting mixture was vortexed and overlaid on the minimal agar plate. The plates were incubated at 37 °C for 48 h and the revertant bacterial colonies on each plate were counted. The inhibition rate of mutagenicity was calculated relative to those in the positive control group containing only the mutagen, by the following formula:
The SOS chromotest assay
The SOS chromotest employs the error-prone DNA repair pathway of E. coli PQ37, also known as the SOS response, a complex regulatory network that is induced by DNA-damaging substances (Walker, Citation1987). The test involves incubation of the bacteria with the sample under investigation and subsequent determination of β-galactosidase (β-gal) activity (i.e., the level of SOS induction). Alkaline phosphatase (AP) activity is also measured, as a toxicity control.
Antigenotoxicity assay
The SOS chromotest was employed to determine the effect of the E. japonica extracts on aflatoxin B1 (AFB1: indirect acting mutagen) and nitrofurantoin (direct acting mutagen) induced genotoxicity. Genotoxicity and antigenotoxicity assays were performed according to the procedure outlined by Quillardet and Hofnung (Citation1985). The extracts were dissolved in distilled water and tested in triplicate, with or without exogenous metabolic activation homogenate. A positive control was prepared by exposure of the bacteria to either nitrofurantoin (10 µg/assay) or AFB1 (0.1 µg/assay). After 2 h of incubation at 37 °C, with shaking, 300 µL samples were used for assay of β-gal assay and Ap assay. In fact, β-gal synthesis (lac Z gene) is dependent on the sfiA activation and is used as a measure of SOS repair system induction. The activity of the constitutive enzyme alkaline phosphatase was used as a measure of protein synthesis and toxicity. The SOS induction factor (IF) was calculated as the ratio of Rc/R0, where R0 and Rc are equal to β-galactosidase activity/alkaline phosphatase activity determined, respectively, in the absence and in the presence of the tested compound at a concentration c. The IF values obtained with the extract treated strains were compared with those obtained with vehicle-treated strains. The result was considered positive when the IF value was higher than 2 (Skandrani et al., Citation2009).
Antigenotoxicity was expressed as inhibition percentage of genotoxicity induced by either nitrofurantoin or AFB1 according to the formula:
where IF1 is the induction factor in the presence of both the test compound and the mutagen. IF2 is the induction factor in the absence of the tested compound and in the presence of mutagen and IF0 is the induction factor of the vehicle-treated strain.
Antioxidant activities
Inhibition of xanthine oxidase activity and superoxide radical scavenging effect
The inhibition of xanthine oxidase (XOD) was measured by following the decrease of uric acid absorbance at 290 nm as proposed by Bouhlel et al. (Citation2008). While the superoxide anion scavenging activity was detected spectrophotometrically with the nitrite method described by Russo et al. (Citation2005).
Hydroxyl radical assay
The hydroxyl radical scavenging activity was determined according to the method of Payà et al. (Citation1992). The hydroxyl radical scavenging activity of each extract was measured by the competition between the deoxyribose and the extract towards the hydroxyl radicals generated by the Fe3+/ascorbate/EDTA/H2O2 system (non-site-specific assay) or the Fe3+/ascorbate/H2O2 system (site-specific assay). The hydroxyl radical scavenging activity was calculated using the following formula:
where A0 is the absorbance of the control (without extract) and A1 is the absorbance in the presence of extract.
Statistical analysis
All experiments were performed in triplicate and the results were expressed as mean ± SD. Student’s t-test was used to analyze statistical significance between values obtained with the control and those obtained with treated assay, p values less than 0.05 were considered significant.
Results
Phytochemical study and metabolite content of E. japonica extracts
The phytochemical screening of aqueous and TOF extracts from E. japonica revealed the presence of flavonoids, tannins, saponins, and coumarins. TOF extract showed the presence of higher quantities of polphenols and flavonoids than aqueous extract. In fact, the flavonoidic and polyphenolic contents in 1 mg of TOF extract were respectively, equivalent to 500 µg of gallic acid and 633.3 µg of quercetin. It appears that the aqueous extract is richer in tannins than TOF extract. The tannin content in aqueous and TOF extracts was, respectively, 6.22 and 4.74 mg/100 g.
Mutagenic activity of E. japonica extracts
In a series of experiments preceding the antimutagenicity studies, it was ascertained, by the agar disk diffusion method, that the different concentrations (50, 250, and 500 µg/assay) of extracts added to both the Ames and SOS chromotest indicator bacteria do not influence their viability. The mutation frequencies obtained with the different tested concentrations of E. japonica did not change significantly when compared with spontaneous mutation frequencies in Ames strains. Indeed, none of the tested extracts induced a significant increase of TA102 and TA104 strains revertant number, even with or without the S9 metabolic system. It was inferred that neither E. japonica extracts nor their metabolites exhibit a mutagenic effect. The results of the Ames test, with and without metabolic activation, are reported in .
Table 1. Mutagenic study of E. japonica extracts by the S. typhimurium TA104 and TA102 assay systems in the presence and in the absence of the metabolic activation extract (S9).
Genotoxic activity of E. japonica extracts
Experiments carried out with the tested extract concentrations revealed no genotoxicity induction in so far as the induction factor is not higher than 1.5 (). In fact, according to Kevekordes et al. (1998), compounds are classified as non-genotoxic, if the IF value remains inferior to 1.5, as marginally genotoxic, if the IF value ranges between 1.5 and 2 and as genotoxic, if the IF value exceeds 2.
Table 2. Evaluation of genotoxicity of different extracts from E. japonica by the SOS chromotest with Escherichia coli PQ37 strain in the absence and in the presence of the exogenous metabolic activation extract (S9).
Antimutagenicity assay
Doses of 5 and 10 µg/plate of 2-AA with, respectively, S. typhimuruim TA104 and TA102 strains, and 162.5 µg/plate of MMS with both of them were chosen for the antimutagenicity study. Results reported in show a protective effect of E. japonica extracts against 2-AA and MMS induced mutagenicity. All extracts were effective in reducing point-mutations induced by both direct genotoxicant (MMS) and indirect genotoxicant (2-AA), in both TA102 and TA104 strains in a dose-dependent manner. The highest antimutagenicity rate, against MMS and 2-AA, was obtained with 500 µg/plate of aqueous extract. Inhibition percentages were 85% and 94% against MMS and 2-AA, respectively, in strain TA104 (), and IC50 values of 80 and 140 µg/plate, respectively. Whereas the highest antimutagenic effects of the same extract in the presence of strain TA102 were 45.1% and 78% against MMS and 2-AA, respectively. Likewise, the highest mutagenic inhibiting percentages obtained with the TOF extract were 65.3% against MMS, and 81.1% against 2-AA, in strain TA104 (at 500 µg/plate) with IC50 values of 300 and 200 µg/plate, respectively ().
Figure 1. Antimutagenicity and antigenotoxicity of E. japonica extracts. (A) Effect of E. japonica extracts on methyl methanesulfonate (MMS) induced mutagenicity in the S. typhimurium TA104 and TA102 assay system. (B) Effect of E. japonica extracts on 2-aminoanthracene (2-AA) induced mutagenicity in the S. typhimurium TA104 and TA102 assay system. (C) Effect of extracts on nitrofurantoin and aflatoxin B1 induced genotoxicity in the presence of E. coli PQ37. ***p < 0.001. TOF, total oligomers flavonoid extract.
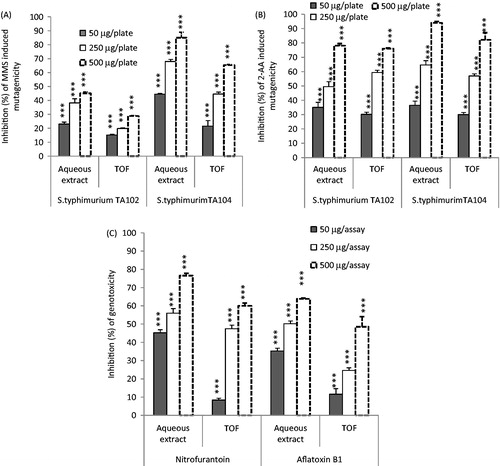
Antigenotoxicity assay
Doses of 10 µg/assay of nitrofurantoin (direct acting mutagen) and 0.1 µg/assay of AFB1 (indirect acting mutagen) were chosen for the antigenotoxicity studies, since these doses were not toxic to the tested strains, and since they induced the highest response of the SOS system for both nitrofurantoin and AFB1.
As shown in , all tested extracts were effective in reducing the IF induced by nitrofurantoin as well as the IF induced by AFB1. The aqueous extract was more effective inhibitor of nitrofurantoin and aflatoxin B1 induced genotoxicity than the TOF extract. In the presence of 500 µg/assay for each of aqueous and TOF extracts, the genotoxicity induced by nitrofurantoin decreased, respectively, by 76.56 and 67.63%. The IC50 values obtained with the above-mentioned extracts were, respectively, 140 and 280 µg/assay. Moreover, aqueous and TOF extracts, decreased by 63.82 and 48.53%, respectively, the genotoxicity induced by 0.1 µg/mL of AFB1 confirming the higher efficiency of aqueous extract, compared with the TOF extract, in lighting AFB 1 genotoxicity.
Xanthine oxidase inhibiting activity and superoxide radical scavenging capacity
The superoxide radical is a highly toxic species that is generated by numerous biological and photochemical reactions via the Haber–Weiss reaction. It can generate the hydroxyl radicals, which react with DNA bases, amino acids, proteins, and polyunsaturated fatty acids, and produces toxic effects. Toxicity of
should also be ascribed to the perhydroxyl intermediates
which react with polyunsaturated fatty acids. Finally, superoxide may react with nitric oxide to generate peroxynitrite, which is known to be toxic towards DNA, lipids, and proteins. The antioxidant activity of E. japonica extracts was evaluated by the X/XOD enzymatic system. The influence of the E. japonica leaf extracts on XOD activity, evaluated by uric acid formation as the final product, and its effect on the enzymatically generated
by this system, were evaluated in vitro. The IC50 (50% inhibitory concentration) values of the tested extracts as XOD inhibitors and as
scavengers are given in . Fifty percentage inhibition of uric acid production was obtained at concentrations of 95 and 90 µg/mL with, respectively, aqueous and TOF extracts, revealing that TOF extract is the best inhibitor of XOD. Whereas it appears from the IC50 values of
that aqueous extract is the most potent superoxide scavenger with an IC50 value of 45 µg/mL, when compared with aqueous extract (70 µg/mL). Comparison of the IC50 values of XOD activity and
. showed that aqueous and TOF extracts are better superoxide scavengers than inhibitors of XOD.
Table 3. Inhibition of XOD and scavenging of superoxide anions  by E. japonica extracts at the indicate concentrationsa.
by E. japonica extracts at the indicate concentrationsa.
Hydroxyl radical scavenging assay
Hydroxyl radicals are usually produced in vivo by Fenton-type reactions, in which transition metals (e.g., iron) reduce hydrogen peroxide. Reducing agents such as ascorbic acid can accelerate OH√ formation by reducing Fe3+ ions to Fe2+ (Thomas et al., Citation2009). In the deoxyribose assay, a mixture of Fe3+–EDTA, H2O2, and ascorbic acid generates hydroxyl radicals which can be detected by their ability to degrade the sugar deoxyribose into fragments (Li & Xie, Citation2000). If the resulting complex mixture of products is heated under acid conditions, malonaldehyde is formed and may be detected by reacting with thiobarbituric acid and forming a pink chromogen (Halliwell, Citation1991). Our results revealed a pronounced hydroxyl radical scavenging effect of extracts in the presence of EDTA, as they are capable of scavenging the free OH√ present in the solution and thus protect the transformation of deoxyribose (detector molecule) to thiobarbituric acid reactive material. shows the hydroxyl radical scavenging ability and chelating effect of different extracts of E. japonica, respectively, in non-site-specific and site-specific assays. By comparing both assays, it was observed that the TOF extract has better antioxidant activity than the aqueous extract with IC50 values of 140 and 400 µg/mL obtained with the non-site-specific and site-specific systems, respectively.
Table 4. Inhibitory effects of E. japonica extracts on hydroxyl radical mediated deoxyribose degradation. Hydroxyl radicals were generated by Fenton’s reaction using the deoxyribose assay system. The non-site-specific and site specific scavenging of hydroxyl radicals by E. japonica extracts were expressed as % inhibition.
Discussion
Mutations are important early steps in carcinogenesis; therefore, short-term genetic tests such as the Salmonella/reversion and the SOS chromotest assays have been successfully used for the detection of mutagens/carcinogens and of antimutagens/anticarcinogens (Rausher et al., Citation1998). Using the Ames test, it was shown that E. japonica extracts did not significantly change mutation frequencies of the tested S. typhimurium strains. In addition, the SOS chromotest assay revealed that the assayed tested concentrations of extracts did not significantly modify IF values of E. coli PQ37 strain, as well as with or without the metabolic activation system. In this current study, we demonstrated that DNA does not seem to be a relevant target for E. japonica-tested extracts and they do not produce DNA lesions which should block DNA synthesis and lead to the induction of the SOS system. These results were in agreement with our previous studies (Ben Sghaier et al., Citation2011; Boubaker et al., Citation2010; Kilani et al., Citation2005). In the present experimental conditions, the aqueous and TOF extracts were depicted as effective antimutagens against direct and indirect acting toxicants, suggesting that these extracts can act through various mechanisms. They reduced the number of revertants, induced by 2-AA in S. typhimurium strains, either by interfering with the metabolic activation of promutagens or by functioning as blocking agents (Ferguson et al., Citation2004). The P-450 enzyme system catalyzes the formation of N-hydroxy derivatives, such as N-hydroxy-2-aminoanthracene. Thus, an alteration in the function of the enzyme may result in altered reaction rates and differential pathways of the metabolism of mutagens and carcinogens. This modification provides protection against chemically induced mutagenesis. These extracts may also directly protect DNA from the electrophilic metabolites of the mutagen as far as flavonoids provide strong nucleophilic centers, which enable them to react with electrophilic mutagens and form adducts that may result in the prevention of genotoxic damages (Marnewick et al., Citation2000). The tested extracts showed significant antigenotoxicity towards nitrofurantoin as well as aflatoxin B1. This suggests that these extracts may interact and neutralize electrophiles such as nitrofurantoin, or may inhibit microsomal activation of aflatoxin B1 to electrophilic metabolites. The antigenotoxic and antimutagenic activities of the tested extracts may be ascribed to flavonoids (Bhouri et al., Citation2011), coumarins (Rezaee et al., 2014), and tannins (Buyukleyla et al., Citation2012) detected in the aqueous and TOF extracts. The observed protective effect of aqueous and TOF extracts towards mutagenic agents may correspond to a synergic participation of several of the compounds listed above. They may also inhibit several metabolic intermediates and reactive oxygen species (ROS) formed during the process of microsomal enzyme activation which are capable of breaking DNA strands. However, we cannot exclude the possibility that other compounds with anticarcinogenic properties, participate in the antigenotoxic effect of E. japonica leaf extracts. In order to investigate the mechanism by which the E. japonica extracts exert their antigenotoxic effect, the antioxidant activity of these extracts has been evaluated. The xanthine/xanthine oxidase assay demonstrated that the aqueous and TOF extracts were effective inhibitors of xanthine oxidase. These results were correlated with the chemical composition of each extract. In fact, the chemical study of the tested extracts revealed the presence of important quantities of flavonoids. The inhibition of xanthine oxidase by flavonoids has been previously demonstrated (Yan et al., Citation2013).
Hydroxyl radical is the most reactive radical known in chemistry, it can attack and damage almost every molecule found in living cells. Reactions of OH√ include its ability to interact with the purine and pyrimidine bases of DNA. It can also abstract hydrogen atoms from biologic molecules, including thiols, leading to the formation of sulfur radicals capable of combining with oxygen to generate oxysulfur radicals, a number of which may damage biologic macromolecules (Li et al., Citation2013). The present study showed that the TOF extract significantly exhibits a greater antioxidant activity in the non-site-specific assay than in the site-specific assay. This result suggests that TOF extract inhibits deoxyribose degradation by scavenging hydroxyl radicals directly better than by chelating iron ions. However, the aqueous extract exhibits a greater antioxidant activity in the site-specific assay than in the non-site-specific assay. This result suggests that the aqueous extract is a better chelating agent than scavenging hydroxyl radical aging. The protective effect of the tested extracts against hydroxyl radicals may be ascribed to several phenolic compounds present in these extracts. In fact, many phenolics were reported to act as reducing agents, hydrogen donors, and singlet oxygen quenchers (Özyürek et al., Citation2008; Saeed et al., Citation2012). The antigenotoxic effect of these extracts against the mutagen agents may be attributed to the antioxidant effect of these extracts against the hydroxyl radicals, superoxide anions, and/or their capacity to chelate (Othmana et al., Citation2007) or to stabilize transition metal ions, rendering them unable to participate in metal catalyzed initiation and radicals propagation (Gordon, Citation1990). Iron-mediated formation of ROS leading to DNA and lipid damage appears to result from the amplification of the iron normal function, which is to transport oxygen to tissues.
In conclusion, the results obtained in this study confirm that antimutagenic and antigenotoxic effects of all tested extracts are dependent on the biological system studied, such as S. typhimurium and E. coli PQ37, and on the type of the tested mutagens (2-AA, MMS, nitrofurantoin, and aflatoxin B1). This study confirms that the extracts of E. japonica are effective antioxidants which are achieved by the scavenging and chelating abilities observed against hydroxyl radicals or iron ions.
Acknowledgements
The authors thank Mr. Samir Boukattaya (Pr. of English at the faculty of Dental Medicine, Tunisia) for English editing. The authors acknowledge the “Ministry of Higher Education Scientific Research and Technology, Tunisia” the support of this study.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Ben Sghaier M, Boubaker J, Skandrani I, et al. (2011). Antimutagenic. antigenotoxic and antioxidant activities of phenolic-enriched extracts from Teucrium ramosissimum: Combination with their phytochemical composition. Environ Toxicol Pharmacol 31:220–32
- Bhouri W, Ben Sghaier M, Kilani S, et al. (2011). Evaluation of antioxidant and antigenotoxic activity of two flavonoids from Rhamnus alaternus L. (Rhamnaceae): Kaempferol 3-O-β-isorhamninoside and rhamnocitrin 3-O-β-isorhamninoside. Food Chem Toxicol 49:1167–73
- Boubaker J, Skandrani I, Bouhlel I, et al. (2010). Mutagenic, antimutagenic and antioxidant potency of leaf extracts from Nitraria retusa. Food Chem Toxicol 48:2283–90
- Bouhlel Chatti I, Boubaker J, Skandrani I, et al. (2011). Antioxidant and antigenotoxic activities in Acacia salicina extracts and its protective role against DNA strand scission induced by hydroxyl radical. Food Chem Toxicol 49:1753–8
- Bouhlel I, Kilani S, Skandrani I, et al. (2008). Acacia salicina extracts protect against DNA damage and mutagenesis in bacteria and human lymphoblast cell K562 cultures. Nutr Res 28:190–7
- Boukef MK. (1986). Les plantes dans la médecine traditionnelle tunisienne. Médecine traditionnelle et pharmacopée. Paris: Agence de Coopération Culturelle et Technique
- Buyukleyla M, Azirak S, Rencuzogullari E, et al. (2012). The genotoxic and antigenotoxic effects of tannic acid in human lymphocytes. Drug Chem Toxicol 35:11–19
- De Tommasi N, De Simone F, Cirino G, et al. (1991). Hypoglycemic effects of sesquiterpene glycosides and polyhydroxylated triterpenoids of Eriobotrya japonica. Planta Med 57:414–16
- Ferguson RL, Philpott M, Karunasinghe N. (2004). Dietary cancer and prevention using antimutagens. Toxicology 198:147–59
- Ghedira K, Chemli R, Richard B, et al. (1991). Contribution à l'étude de la pharmacopée traditionnelle de Tunisie, étude des parties aériennes d'Ajuga iva. Plantes Méd Phytothérapie 25:100–11
- Gordon MH. (1990). The mechanism of antioxidant action in vitro. In: Hudson BJF, ed. Food Antioxidants. London: Elsevier Applied Science, 1–18
- Halliwell B. (1991). Reactive oxygen species in living systems: Source, biochemistry and role in human disease. Am J Med 91:14–22
- Islam S, Nasrin S, Khan MA, et al. (2013). Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. growing in Rajshahi, Bangladesh. BMC Complement Altern Med 13:142
- Ito H, Kobayashi E, Takamatsu Y, et al. (2000). Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem Pharm Bull 48:687–93
- Juranek I, Horakova L, Rackova L, Stefek M. (2010). Antioxidants in treating pathologies involving oxidative damage: An update on medicinal chemistry and biological activity of stobadine and related pyridoindoles. Curr Med Chem 17:552–70
- Kevekordes S, Merch-Sundermann V, Burghaus CHM, et al. (1998). SOS induction of selected naturally occurring substances in Escherichia coli (SOS chromotest). Mutat Res 445:81–91
- Khandelwal KR. (2004). Practical Pharmacognosy. Pune, India: Nirali Prakashan
- Kilani S, Ben Ammar R, Bouhlel I, et al. (2005). Investigation of extracts from (Tunisian). Cyperus rotundus as antimutagens and radical scavengers. Environ Toxicol Pharmacol 20:478–84
- Kim SH, Shin TY. (2009). Anti-inflammatory effect of leaves of Eriobotrya japonica correlating with attenuation of p38 MAPK, ERK and NF-kappa B activation in mast cells. Toxicol In Vitro 23:1215–9
- Kumar A, Chattopadhyay S. (2006). DNA damage protecting activity and antioxidant potential of puina extract. Food Chem 100:1377–84
- Lee KT, Sohn IC, Park HJ, et al. (2000). Essential moiety of antimutagenic of Hedera geninmonodesmosides and bidemosides isolated from the stem bark of Kalapanax pictus. Planta Med 66:329–32
- Li C, Xie B. (2000). Evaluation of the antioxidant and pro-oxidant effects of tea catechin oxypolymers. J Agric Food Chem 48:6362–6
- Li X, Mai W, Wang L, Han W. (2013). A hydroxyl-scavenging assay based on DNA damage in vitro. Anal Biochem 438:29–31
- Marnewick LJ, Gelderblom CAW, Joubert E. (2000). An investigation on the antimutagenic properties of South African teas. Mutat Res 471:157–66
- Maron DM, Ames BN. (1983). Revised methods for the Salmonella mutagenicity test. Mutat Res 113:173–215
- Marques RCP, De Medeiros SRB, Da Silva Dias C, et al. (2003). Evaluation of the mutagenic potential of yangambin and of the hydroalcoholic extract of Ocotea duckei by the Ames test. J Mutat Res 536:117–20
- Mortelmans K, Zeiger E. (2000). The Ames Salmonella/microsome mutagenicity assay. Mutat Res 455:29–60
- Nelson GM, Swank AE, Brooks LR, et al. (2001). Metabolism, microflora effect and genotoxicity in haloacetic acid-treated cultures of rat cecal microbiota. J Toxicol Sci 60:232–41
- Nwabueze TU. (2007). Effect of process variables on trypsin inhibitor activity (TIA), phytic acid and tannin content of extruded African breadfruit-corn-soy mixtures: A response surface analysis. J Food Sci Technol 40:21–9
- Othmana A, Ismaila A, Ghania NA, Adenan I. (2007). Antioxidant capacity and phenolic content of cocoa beans. J Food Chem 100:1523–30
- Özyürek M, Bektasoglu B, Güçlü K, Apak R. (2008). Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal Chim Acta 616:196–206
- Payà M, Halliwell B, Hoult JRS. (1992). Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hydrochlorous acid and hydroxyl radicals. Biochem Pharmacol 44:205–14
- Pottier-Alapetite G. (1979). Flore de la Tunisie: Angiospermes, Dicotyledones. Apetales, Dialypetales. Tunisia: Ministère de L’Enseignement Supérieur et de la Recherche Scientifique et Ministère de l’Agriculture
- Quillardet PQ, Hofnung M. (1985). The SOS chromotest colorimetric bacterial assay for genotoxins: Procedures. Mutat Res 147:65–78
- Rausher R, Edenharder R, Platt KL. (1998). In vitro antimutagenic and in vivo anticlastogenic effects of carotenoids and solvent extracts from fruits and vegetables rich in carotenoids. Mutat Res 413:129–42
- Rezaee R, Behravan E, Behravan J, et al. (2014). Antigenotoxic activities of the natural dietary coumarins umbelliferone, herniarin and 7-isopentenyloxy coumarin on human lymphocytes exposed to oxidative stress. Drug Chem Toxicol 37:144–8
- Russo A, Cardile V, Lombardo L, et al. (2005). Antioxidant activity and antiproliferative action of methanolic extract of Geum quellyon Sweet roots in human tumor cell lines. J Ethnopharmacol 14:323–32
- Sripanidkulchai B, Tattawasart U, Laupatarkasem P, et al. (2002). Antimutagenic and anticarcinogenic and anticarcinogenic effects of Phyllanthus amarus. Phytomedecine 9:26–32
- Skandrani I, Bouhlel I, Limema I, et al. (2009). Moricandia arvensis extracts protect against DNA damage, mutagenesis in bacteria system and scavenge the superoxide anion. Toxicol In Vitro 23:166–75
- Saeed N, Khan M, Shabbir M. (2012). Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 12:221
- Taniguchi S, Imayoshi S, Kobayashi E, et al. (2002). Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry 59:315–23
- Thomas C, Mackey MM, Diaz AA, Cox DP. (2009). Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep 14:102–8
- Walker GC. (1987). The SOS response of Escherichia coli. In: Neidhardt FC, ed. Escherichia coli and Salmonella typhimurium. Cellular and Molecular Biology. Washington, DC: American Society for Microbiology, 1346–57
- Yan J, Zhang G, Hu Y, Ma Y. (2013). Effect of luteolin on xanthine oxidase: Inhibition kinetics and interaction mechanism merging with docking simulation. Food Chem 14:3766–73
- Yuan VY, Bone DE, Carrington F. (2005). Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem 91, 485–94
