Abstract
Context: Coccinia indica Naud (Cucurbitaceae) has been traditionally used for the treatment of depression but these claims have not been validated.
Objectives: The objective of this study is to investigate antidepressant activity of various extracts and fractions of C. indica aerial parts, and to estimate content of quercetin in the plant using TLC densitometry.
Materials and methods: Coccinia indica aerial parts were successively extracted using solvents in increasing order of polarity, namely n-hexane, chloroform, methanol, and water. Various extracts were evaluated for antidepressant activity at doses of 200 or 400 mg/kg, p.o., upon acute administration in mice using the forced swim test (FST). The bioactive extract was partitioned successively using solvents in increasing order of polarity, namely n-hexane, ethyl acetate, and n-butanol. All fractions were also screened for antidepressant activity at doses of 25 or 50 mg/kg, p.o., upon acute administration in mice.
Results: The methanol extract significantly reduced the duration of immobility in FST at dose of 400 mg/kg without affecting locomotor activity in open field test, thus, confirmed its antidepressant activity, which was statistically equivalent to the standard drug (imipramine, 15 mg/kg, i.p.). Ethyl acetate fraction (EAF) exhibited antidepressant activity at 50 mg/kg. Comparative TLC fingerprint studies confirmed the presence of quercetin in methanol extract and EAF. Quercetin was used as a chemical marker to standardize C. indica aerial parts using the validated TLC densitometric method, and the content of quercetin was found to be 0.00172% w/w.
Conclusions: The present studies scientifically validated traditional claims of C. indica for antidepressant activity.
Introduction
Depression typically presents as lowered mood, difficulty in thinking, loss of interest, and physical complaints such as headache, disturbed sleep, loss of energy, and change in sex drive (Mann, Citation2005; Tierney et al., Citation2006). It incurs substantial personal, economic, and social costs for both the individuals afflicted and those close to them (Hansson, Citation2002). Depression is one of the top five most prevalent diseases worldwide (Kruijshaar et al., Citation2005). By 2020, it is expected to be the second leading cause of disability globally (Smith et al., Citation2008). According to the World Health Organization (WHO), depression is a medical and social problem affecting 350 million people worldwide (WHO, Citation2013). The lifetime prevalence of depression is as high as 20% in the general population worldwide with a female-to-male ratio of about 5:2 (Kendler et al., Citation2001).
Although synthetic drugs are easily available in the market such as tricyclic antidepressants (clomipramine), monoamine oxidase inhibitors (tranilcipromine), and selective serotonin reuptake inhibitors (fluoxetine) for the treatment of depression (Baldessarini, 2001; Majeroni & Hess, Citation1998). But these drugs are associated with severe side effects such as dry mouth, mydriasis, constipation, sleeplessness, temporary fatigue, restlessness, and headaches (Dziukas & Vohra, Citation1991). In the light of drawbacks associated with these synthetic drugs, researchers throughout the globe are searching for newer, safer, and more efficacious alternatives (Arnold & Gulumian, Citation1984; Dwyer et al., Citation2010; Zolla, Citation1980). Recently, natural product researchers are investigating plants based on their use in traditional systems of medicine to develop newer and safer drugs (Dhawan, Citation1995; Oliver-Bever, Citation1983). Coccinia indica Naud (Cucurbitaceae), known as Ivy Gourd, has a long tradition of use in the treatment of mental disorders such as depression and stress (Ahmed & Azam, Citation2014; Khare, Citation2007; Rahmatullah et al., Citation2012). The plant is also used as an antidiabetic, anti-inflammatory, antipyretic, and analgesic (Anonymous, Citation2005; Chopra et al., Citation1956). Preliminary phytochemical screening has shown the presence of flavonoids, alkaloids, and triterpenoids in C. indica aerial parts (Chandira et al., Citation2010).
A survey of literature revealed a startling fact that the plant has not been investigated to validate its traditional claims for antidepressant activity. Thus, it was considered worthwhile to evaluate antidepressant activity of various extracts and fractions of C. indica aerial parts, and also to estimate content of quercetin in the plant.
Materials and methods
Collection and identification of plant material
Coccinia indica aerial parts were collected form Punjabi University, Patiala, India, in August, 2011. The plant identity was confirmed by Dr. Veena Chandra, Scientist D and Director, Botany Division, Forest Research Institute, Dehra Dun (Reference no. – 341/2011-Bot-15-1, dated 12 September 2011) and also confirmed by Dr. S. K. Srivastava, Scientist D and Director, Botanical Survey of India, Dehra Dun (Reference no. – BSI/NRC/Tech(ident.)/2011–12/585, dated 22 September 2011).
Solvents
Methanol (S.D. Fine Chemicals, Mumbai, India), chloroform, n-hexane, n-butanol, and ethyl acetate (E Merck, Delhi, India) of LR grade were used for the preparation of various crude extracts and fractions of C. indica aerial parts. Toluene, ethyl acetate (E Merck, Delhi, India), and glacial acetic acid (Ranbaxy Laboratory Chemicals, Mumbai, India) of AR grade were used for thin-layer chromatographic studies.
Preparation of various extracts and fractions
Coccinia indica aerial parts were dried under sunlight and powdered in a grinder. Dried powdered plant material (2 kg) was extracted in a Soxhlet apparatus successively using solvents in increasing order of polarity, namely n-hexane, chloroform, and methanol. The water extract was prepared by boiling the marc of plant material with distilled water for 2 h on a hot plate. The solvents from crude extracts were recovered under reduced pressure using rotary vacuum evaporator (BUCHI, Uster, Switzerland).
The bioactive extract (25 g) of plant material was suspended uniformly in water, placed in a round bottom flask and partitioned successively using solvents in increasing order of polarity namely n-hexane, ethyl acetate, and n-butanol by heating at 50 °C for 30 min along with continuous stirring. This procedure of partitioning with each solvent was repeated nine times. All the separated layers of each solvent were pooled and concentrated under reduced pressure to get n-hexane fraction (HF), ethyl acetate fraction (EAF), n-butanol fraction (BF), and remaining bioactive extract (RBE). Various extracts and fractions were subjected to phytochemical screening to ascertain various classes of phytoconstituents present therein (Farnsworth, Citation1966).
Pharmacological studies
Animals
Laca mice (either sex) of body weight 20–25 g purchased from the Central Research Institute, Kasauli, India, were used for pharmacological studies. The animals were fed with normal laboratory pellet diet and water ad libitum. The approval was taken from Institutional Animal Ethics Committee of Punjabi University, Patiala before carrying out animal studies (107/99/CPCSEA/2013-22, dated 14 September 2013). The animals were acclimatized to laboratory conditions daily for 1 h for continuous 7 d before the start of experiment. All the experiments were carried from 9 AM to 12 PM as per the guidelines of Committee for the Purpose of Control and Supervision on Experiments on Animals. Groups of six animals were used in all sets of experiments. The animals were overnight fasted before use. The doses were administered orally with the help of an oral cannula fitted on a tuberculin syringe.
Vehicle and standard drug
Distilled water + Tween 80 (2%) was used as a vehicle for preparing various test doses of crude extracts, fractions, and standard drug in such a concentration as to administer a volume ranging 0.2–0.25 ml to the mice. Imipramine, received as a gift sample from Triko Pharmaceuticals, Rohtak, India, was used as a standard antidepressant drug at the dose of 15 mg/kg, i.p.; quercetin (Sigma-Aldrich, St. Louis, MO) was used as the standard chemical marker for TLC densitometric studies.
Experimental protocol
Two sets of experimental protocols were designed. Each set comprising ten groups of animals.
Set I, comprising groups I–X, was used to study antidepressant activity of various crude extracts of C. indica aerial parts.
Group I – control group received vehicle (0.25 ml, p.o.).
Group II – standard group received imipramine (15 mg/kg, i.p.).
Groups III and IV – test groups received 200 and 400 mg/kg doses of n-hexane extract, respectively.
Groups V and VI – test groups received 200 and 400 mg/kg doses of chloroform extract, respectively.
Groups VII and VIII – test groups received 200 and 400 mg/kg doses of methanol extract, respectively.
Groups IX and X – Test groups received 200 and 400 mg/kg doses of water extract, respectively.
Set II, comprising groups XI–XX, was used to study antidepressant activity of various fractions obtained from bioactive extract of C. indica aerial parts.
Group XI – control group received vehicle (0.25 ml, p.o.).
Group XII – standard group received imipramine (15 mg/kg, i.p.).
Groups XIII and XIV – test groups received 25 and 50 mg/kg doses of HF, respectively.
Groups XV and XVI – test groups received 25 and 50 mg/kg doses of EAF, respectively.
Groups XVII and XVIII – test groups received 25 and 50 mg/kg doses of BF, respectively.
Groups XIX and XX – test groups received 25 and 50 mg/kg doses of RBE, respectively.
Evaluation of antidepressant activity using despair swim test
Mice were forced to swim, after 1 h of administration of test substances, in a Plexiglas cylinder (height 40 cm; diameter 18 cm) containing water up to the level of 15 cm, and maintained at 25 ± 2 °C (Kumar et al., Citation2008). Mice were allowed to swim for 6 min. During this test period, the total duration of immobility (floating in the water in a slightly hunched but upright position, its nose above the surface) was noted.
Evaluation of locomotor activity using open field behavior test
The apparatus was composed of a square wooden arena (40 cm × 40 cm × 40 cm) surrounded by a grey PVC wall (Campos et al., Citation2005). The floor was divided into 25 squares marked by black lines. At the beginning of the test, the mice were placed individually into the central part of the open field apparatus. The number of crossings and rearings was recorded for 5 min.
Statistics
The results have been expressed as mean ± standard deviation (SD). The test drugs were compared with standard drug and control by one way analysis of variance (ANOVA) followed by Student–Newman–Keul’s test (Scheffer, Citation1980).
TLC densitometric method development studies
Preparation of standard solution
A standard solution of the marker compound was prepared by dissolving accurately weighed 5 mg of compound in 5 ml of methanol.
Preparation of test samples
The coarsely powered plant material, 10 g of aerial parts, was exhaustively extracted with methanol in a Soxhlet apparatus. The extraction was continued until 2 ml of solvent was collected; during siphoning process of Soxhlation, in a watch glass, it did not leave any residue after evaporation. The methanol extract was filtered, concentrated under reduced pressure, and the volume was adjusted to 10 ml with methanol.
Following the procedure mentioned above, 10 g of aerial parts was exhaustively extracted with methanol in a Soxhlet apparatus. The methanol extract of C. indica aerial parts was suspended uniformly in water, taken in a round bottom flask and partitioned with ethyl acetate by refluxing for 30 min at 50 °C along with continuous stirring. This procedure of partitioning with ethyl acetate was repeated for nine times. All the separated layers of ethyl acetate were pooled and concentrated under reduced pressure to get EAF.
TLC fingerprint profiles
A fixed volume, 5 µl of methanol extract, EAF, and quercetin was applied on pre-coated TLC plate (E Merck, Mumbai, India; 0.2 mm; aluminum base) using CAMAG LINOMAT 5 (CAMAG Scientific Inc., Wilmington, NC). The plate was developed using solvent system toulene:ethyl acetate:glacial acetic acid (15:11:2) in a chamber, saturated for 10 min, to a distance of 8 cm. The plate was dried and visualized under ultraviolet light (254 nm).
Preparation of standard plot
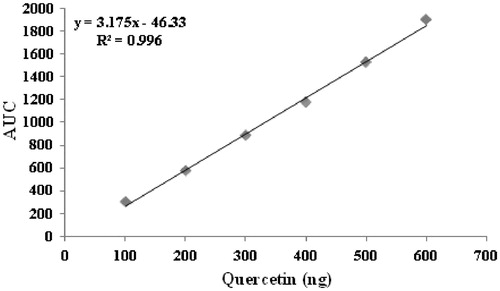
The stock solution of marker was diluted with methanol to get six dilutions of different concentrations (10, 20, 30, 40, 50, and 60 µg/ml). A volume of 10 µl from each dilution was applied in triplicate on pre-coated TLC plate. The plate was developed in solvent system toulene:ethyl acetate:glacial acetic acid (15:11:2) in a chamber saturated for 10 min, to a distance of 8 cm. The developed plate was dried in a current of hot air and then scanned in TLC scanner at 254 nm. The area under the curve (AUC) of the peak corresponding to marker compound was noted in each track.
Estimation of marker in methanol extract
Test solutions (10 µl) of methanol extract was applied in triplicate on pre-coated TLC plate (5 × 10 cm). The plate was developed and scanned following the same procedure as used for the preparation of standard plot. The average AUC of the peak corresponding to the marker compound was noted at 254 nm in the test sample. The content of marker compound was calculated from the regression equation of the standard plot.
TLC densitometric method validation studies
Instrument precision
Instrumental precision was checked by repeated scanning (n = 7) of the same spot of marker (500 ng/spot) and expressed as percent coefficient of variance (% CV).
Repeatability
The repeatability of the method was confirmed by analyzing 500 ng/spot of marker individually on a TLC plate (n = 5) and expressed as % CV.
Intra-day and inter-day variation
The variability of the method was studied by analyzing aliquots of standard solution containing 500 ng/spot of marker on same day (intra-day precision) and on different days (inter-day precision).
Limit of detection (LOD) and limit of quantification (LOQ)
LOD and LOQ were determined by applying different concentrations of standard solutions of marker along with methanol as the blank and determined on the basis of signal-to-noise ratio. The LOD was determined at a S/N of 3:1 and the LOQ at S/N of 10:1.
Recovery studies
The accuracy of the method was assessed by performing recovery studies at three different levels (50, 100, and 150% addition of marker). The recovery and the average percent were calculated.
Specificity
This was ascertained by analyzing the standard compound and the sample. The band for marker from sample solutions was confirmed by comparing the Rf and spectra of the bands to those of the standard. The peak purity of quercetin was analyzed by comparing the spectra at three different levels, i.e., start, middle, and end positions of the bands.
Results
Various extracts of C. indica were prepared successively using solvents in an increasing order of polarity. shows yields of various extracts. All extracts were screened for the presence of different classes of phytoconstituents. The results of phytochemical screening showed the presence of lipids in n-hexane extract; alkaloids, and steroids in chloroform extract; alkaloids, flavonoids, triterpenoids, steroids, tannins, and carbohydrates in methanol extract; and tannins, saponins, carbohydrates, and proteins in water extract.
Table 1. Percentage yields of various extracts of C. indica aerial parts.
All extracts of C. indica aerial parts were subjected to antidepressant activity in mice using despair swim test. shows the mean immobility time of the mice after administration of 200 or 400 mg/kg, p.o., doses of crude extracts, imipramine (15 mg/kg, i.p.), and vehicle, p.o. Among the various extracts, only methanol extract exhibited significant antidepressant activity with respect to control and equivalent to the standard drug at the dose of 400 mg/kg. Water and n-hexane extracts were found to be devoid of antidepressant activity whereas chloroform extract exhibited mild activity at the doses of 200 or 400 mg/kg. Although the chloroform extract of plant significantly reduced mean time spent by mice in immobile state at the doses of 200 or 400 mg/kg with respect to control, but not equivalent to the standard drug. These observations infer that methanol extract is the most bioactive extract of C. indica aerial parts. The effect of the methanol extract on locomotor activity was also evaluated by the open field test. The methanol extract did not exhibit any changes on the number of crossings and rearings in the open field test at 200 or 400 mg/kg ().
Table 2. Antidepressant activity of various extracts of C. indica aerial parts using forced swim test.
Table 3. Effect of methanol extract of C. indica aerial parts on the locomotor activity using open field test.
The bioactive methanol extract was further fractionated using solvents in an increasing order of polarity, namely n-hexane, ethyl acetate, and n-butanol. Yields of various fractions are shown in . Phytochemical screening of various fractions showed the presence of steroids in HF; flavonoids, alkaloids, triterpenoids, and tannins in EAF; and alkaloids in BF. Various fractions of bioactive extract were subjected to antidepressant activity in mice using despair swim test. shows the mean immobility time of the mice after administration of 25 or 50 mg/kg, p.o., doses of fractions, imipramine, and vehicle. Among the various fractions, only EAF exhibited significant antidepressant activity at the dose of 50 mg/kg with respect to control and the activity was equivalent to the standard drug. HF and RBE could not able to reduce immobility time in mice with respect to control at both doses, inferring devoid of antidepressant activity. Although BF did not achieve therapeutic level as it could not able to reduce immobility time in mice when compared with standard drug, but reduction in immobility time was significant with respect to control. This observation confirms mild antidepressant activity of BF.
Table 4. Percentage yields of various fractions obtained from methanol extract.
Table 5. Antidepressant activity of various fractions obtained from methanol extract using forced swim test.
Comparative TLC fingerprint studies confirmed the presence of quercetin in methanol extract and EAF. Thus, quercetin was taken as the chemical marker to standardize C. indica using the validated TLC densitometric method. shows comparative fingerprint profile of methanol extract, EAF, and quercetin. A standard plot was prepared between different concentrations of quercetin and their peak areas after scanning at 254 nm (). The content of quercetin in C. indica aerial parts was found to be 0.00172% w/w (). TLC densitometric method was validated as per ICH guidelines. The instrumental precision, repeatability, linearity range, correlation coefficient, intra-day precision, inter-day precision, LOD, LOQ, and accuracy were found to be 1.32% CV, 0.78% CV, 100–600 ng, 0.996, 1.61% CV, 1.22% CV, 13 ng/spot, 35 ng/spot, and 98.82%, respectively ( and ). It is clearly evident from ultraviolet spectra and thin layer chromatogram overlay that there is no interference in quantitative analysis ( and ), thus, confirming specificity of the developed TLC densitometric method.
Figure 1. Comparative TLC fingerprint profile of quercetin, methanol extract and EAF of C. indica aerial parts visualized under ultraviolet light at 254 nm. (1) Quercetin; (2) methanol extract; (3) EAF.
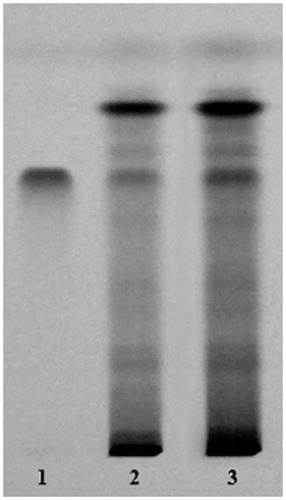
Figure 3. TLC densitometric chromatogram of quercetin and methanol extract of C. indica aerial parts.
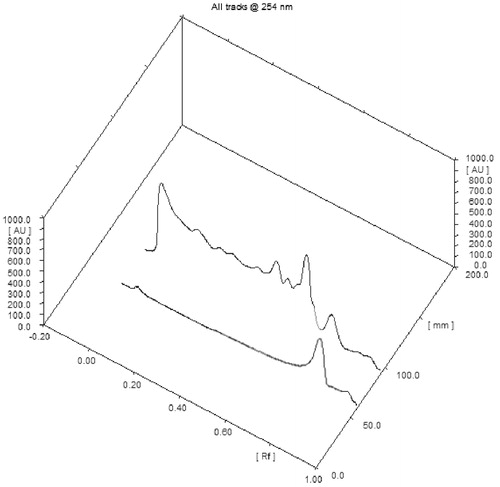
Figure 4. Spectra overlay of quercetin with corresponding peak in methanol extract of C. indica aerial parts.
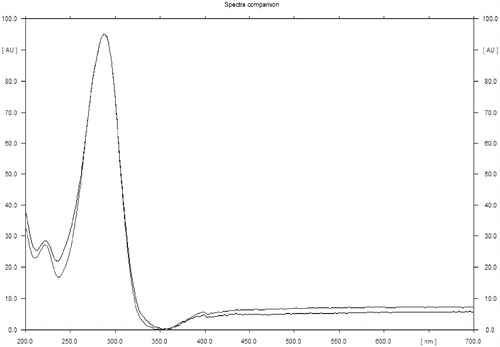
Table 6. Percentage yield of quercetin in C. indica aerial parts.
Table 7. Method validation parameters in TLC densitometric analysis of quercetin in C. indica aerial parts.
Table 8. Recovery studies of quercetin.
Discussion
The antidepressant activity of C. indica aerial parts was assessed by employing a well-established experimental model, i.e., the Despair swim test. The model was chosen since it is effective, cheap, simple, less time consuming, and requires no preliminary training of the mice and does not cause much discomfort to the animals while handling. The model is principally based on the observations that mice forced to swim in a restricted space from which they cannot escape induce to characteristic behavior of immobility. This behavior reflects a state of despair which can be reduced by several agents which are therapeutically effective in human depression. The ultimate manifestation of depression in the animals is exhibited by a decrease in motor activity, which is measured by the time spent by the animal in immobile state (Weiss et al., Citation1981).
Various extracts of C. indica aerial parts were evaluated for antidepressant activity. Among the various extracts, only methanol extract exhibited significant antidepressant activity at the dose of 400 mg/kg, p.o. It did not show any effect on locomotor activity of mice in open field test, thus, suggested that the decrease in the immobility as elicited by methanol extract in the forced swim test (FST) was unrelated to the psychostimulant effect. These observations confirmed antidepressant activity of methanol extract of C. indica aerial parts. Therefore, bioactive methanol extract was fractionated using standardized procedure to get HF, EAF, and BF. The dose selection of different fractions and RBE, for assessing antidepressant activity, was made on the basis of their yields with respect to methanol extract. The doses, 20, 10, 50, and 350 mg/kg, p.o., were used for the evaluation of antidepressant activity of HF, EAF, BF, and RBE, respectively. HF and RBE did not show antidepressant activity, whereas EAF and BF exhibited mild activity (data not presented). The doses of various fractions and RBE were then rescheduled considering results obtained from initial studies. Finally, two dose levels, i.e., 25 or 50 mg/kg, p.o., were selected for all fractions and RBE to assess antidepressant activity. Among the various fractions, EAF significantly decreased the duration of immobility in mice, thus, confirming its significant antidepressant activity at the dose of 50 mg/kg, p.o. BF also exhibited mild antidepressant activity at the dose of 50 mg/kg. It is interesting to note here that the yield of EAF is about 2% of crude methanol extract but it exhibited antidepressant activity at very high dose, i.e., 50 mg/kg in comparison with the methanol extract, which showed activity at 400 mg/kg. It is suggested that few constituents of crude methanol extract may act synergistically to show antidepressant activity. While fractionating methanol extract, these bioactive constituents have been distributed in EAF and BF.
It is suggested that bioactive extract and fraction of C. indica aerial parts may act as antidepressant by monoamine oxidase inhibition (Dixon Clarke & Ramsay, Citation2011; Sandler et al., Citation1988), and increasing availability of serotonin and noradrenaline in the synaptic cleft (Machado et al., Citation2008). Preliminary phytochemical studies showed the presence of flavonoids, alkaloids, and triterpenoids in bioactive EAF of C. indica aerial parts.
The available literature reveal that flavonoids play a pivotal role in treating nervous disorders. A number of reports on plants are available in which flavonoids have shown CNS activities (Butterweck et al., Citation2000; Jagar & Saaby, Citation2011). Therefore, an attempt was made to characterize the crude methanol extract and EAF of C. indica aerial parts by comparing TLC profiles of reference flavonoids with the crude extract and fraction. Comparative TLC profiling confirmed the presence of quercetin in the methanol extract and EAF. Quercetin is being reported for the first time in this plant. A survey of literature revealed that quercetin possesses antidepressant activity. Quercetin isolated from Opuntia ficus-indica (L.) Mill. (Cactaceae) has been reported to exhibit antidepressant activity at the dose of 50 mg/kg, p.o., using FST (Park et al., Citation2010). Dixon Clarke and Ramsay (Citation2011) reported antidepressant activity of Hypericum perforatum L. (Hypericaceae) due to quercetin at the dose of 100 mg/kg, p.o. Ginkgo biloba L. (Ginkoaceae) leaf extracts have been reported to exhibit significant antidepressant activity at the doses of 10 or 50 mg/kg, p.o. (Sakakibara et al., Citation2006). The activity was attributed to its major bioactive principle, quercetin. These reports suggest that quercetin may be one of the constituents of C. indica aerial parts responsible for antidepressant effects of plant. Thus, the content of quercetin was determined in C. indica aerial parts by a validated TLC densitometric method. TLC densitometry is a sophisticated instrumental technique with merits of easy method development and validation, automation, scanning, full optimization, selective detection principle, minimum sample preparation, etc., enable it to be a powerful analytical tool for quantitatively determination of particular compound(s) in complex mixtures of inorganic, organic, and biomolecules (Srivastava, Citation2011). The content of quercetin in C. indica aerial parts was found to be 0.00172% w/w. The developed method for the estimation of quercetin in C. indica was validated as per the guidelines of ICH. Percentage coefficient of variance observed in validation parameters of developed method such as instrumentation precision, inter and intra-day precision and repeatability complied with in the prescribed limit. Recovery of quercetin in accuracy studies was more than 98%. Further, no deviation was observed in ultraviolet spectra and thin-layer chromatograms of sample and standard. These observations infer that the developed method for the estimation of quercetin in C. indica aerial parts is precise, accurate, reproducible, and specific.
Bioactivity-guided fractionation studies of EAF are in progress with a view to isolate quercetin and other bioactive constituent(s) from C. indica responsible for antidepressant activity.
Acknowledgements
The authors duly acknowledge Prof R. C. Gupta, co-ordinator of DBT-IPLS project for providing access to instrumentation facilities at Sophisticated Instrumentation Laboratory, Punjabi University, Patiala.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Ahmed MN, Azam MNK. (2014). Traditional knowledge and formulations of medicinal plants used by the traditional medical practitioners of Bangladesh to treat schizophrenia like psychosis. Schizophr Res Treat 2014:1–10
- Anonymous. (2005). Database on Medicinal Plants Used in Ayurveda. New Delhi: Central Council for Research in Ayurveda and Siddha
- Arnold AH, Gulumian M. (1984). Pharmacopoeia of traditional medicine in Venda. J Ethnopharmacol 12:35–74
- Baldessarini RJ. (2001). Drugs and the treatment of psychiatric disorders. In: Hardman JG, Limbird LE, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill, 387–445
- Butterweck V, Jurgenliemk G, Nahrstedt A, Winterhoff H. (2000). Flavonoids of Hypericum perforatum show antidepressant activity in forced swim test. Planta Med 66:3–6
- Campos AR, Barros AI, Albuquerque FA, et al. (2005). Acute effects of guarana (Paullinia cupana Mart.) on mouse behavior in forced swimming and open field tests. Phytother Res 19:441–3
- Chandira M, Vankateswarlu BS, Gangwar RK, et al. (2010). Studies on anti-stress and free radical scavenging activity of whole plant of Coccinia indica Linn. Int J Pharm Sci 1:50–4
- Chopra RN, Nayar SL, Chopra IC. (1956). Glossary of Indian Medicinal Plants. New Delhi, India: Council of Scientific and Industrial Research
- Dhawan BN. (1995). Centrally acting agents from Indian plants. In: Koslovo SH, Srinivasa MR, Coelho GV, eds. Decade of the Brain: India/USA Research in Mental Health and Neurosciences. Rockville, MD: National Institute of Mental Health, 203–14
- Dixon Clarke SE, Ramsay RR. (2011). Dietary inhibitors of monoamine oxidase A. J Neural Transm 118:1031–41
- Dwyer AV, Whitten DL, Hawrelak JA. (2010). Herbal medicines, other than St. John’s Wort, in the treatment of depression: A systematic review. Altern Med Rev 16:40–9
- Dziukas LJ, Vohra J. (1991). Tricyclic antidepressant poisoning. Med J Australia 154:344–50
- Farnsworth NR. (1966). Biological and chemical screening of plants. J Pharm Sci 55:255–76
- Hansson L. (2002). Quality of life in depression and anxiety. Int Rev Psychiatry 14:185–9
- Jagar AK, Saaby L. (2011). Flavonoids and CNS. Molecules 16:1471–85
- Kendler KS, Gardner CO, Neale MC, Prescott CA. (2001). Genetic risk factors for major depression in men and women: Similar or different heritabilities and same or partly distinct genes? Psychol Med 31:605–16
- Khare CP. (2007). Indian Medicinal Plants: An Illustrated Dictionary. New York: Springer Science and Business Media
- Kruijshaar ME, Barendregt J, Vos T, et al. (2005). Lifetime prevalence estimates of major depression: An indirect estimation method and quantification of recall bias. Eur J Epidemiol 20:103–11
- Kumar S, Madaan R, Sharma A. (2008). Pharmacological evaluation of bioactive principle of Turnera aphrodisiaca. Indian J Pharm Sci 70:740–4
- Machado DG, Bettio LEB, Cunha MP, et al. (2008). Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur J Pharmacol 587:163–8
- Majeroni BA, Hess AD. (1998). The pharmacologic treatment of depression. J Am Board Fam Pract 11:127–39
- Mann JJ. (2005). The medical management of depression. N Engl J Med 353:1819–34
- Oliver-Bever B. (1983). Medicinal plants in tropical West Africa II: Plants acting on the nervous system. J Ethnopharmacol 7:11–93
- Park SH, Sim YB, Suh HW. (2010). Antidepressant like effect of kaempferol and quercitirin, isolated from Opuntia ficus-indica var. Saboten. Exp Neurobiol 19:30–8
- Rahmatullah M, Hasan A, Parvin W, et al. (2012). Medicinal plants and formulations used by the Soren clan of the Santal tribe in Rajshahi District, Bangladesh for treatment of various ailments. Afr J Tradit Complement Altern Med 9:350–9
- Sakakibara H, Ishida K, Grundmann O, et al. (2006). Antidepressant effect of extracts from Ginkgo biloba leaves in behavioral models. Biol Pharm Bull 29:1767–70
- Sandler M, Clow A, Watkins PJ, Glover V. (1988). Tribulin – An endocoid marker for anxiety in man. Stress Med 4:215–19
- Scheffer WC. (1980). Statistics for the Biological Sciences. Philippines: Addison-Wesley Publishing Company
- Smith AJ, Sketris I, Cooke C, et al. (2008). A comparison of antidepressant use in Nova Scotia, Canada and Australia. Pharmacoepidemiol Drug Saf 17:697–706
- Srivastava MM. (2011). An Overview of HPTLC: A Modern Analytical Technique with Excellent Potential for Automation, Optimization, Hyphenation and Multidimensional Applications. Berlin: Springer-Verlag
- Tierney LM, McPhee SJ, Papadakis MA. (2006). Current Medical Diagnosis and Treatment. San Francisco: McGraw-Hill
- Weiss JM, Goodman PA, Losito GO, et al. (1981). Behavioral depression produced by uncontrollable stressor: Relationship to norepinephrine, dopamine and serotonin levels in various regions of rat brain. Brain Res Rev 3:167–205
- WHO. (2013). Investigating in Mental Health: Evidence for Action. Geneva: World Health Organization Press
- Zolla C. (1980). Traditional medicine in Latin American, with particular reference to Mexico. J Ethnopharmacol 2:37–51