Abstract
Context: During diabetes mellitus, non-enzymatic reaction between amino groups of protein and carbonyl of reducing sugars (Millard reaction) is responsible for the major diabetic complications. Various efforts have been made to influence the process of protein glycation.
Objectives: This review article provides an extensive survey of various studies published in scientific literature to understand the process of protein glycation and its measurement. Moreover, evaluation and identification of potential inhibitors (antiglycation agents) of protein glycation from natural and synthetic sources and their mechanism of action in vitro and in vivo are also addressed.
Method: In this review article, the mechanism involved in the formation of advanced glycation end products (AGEs) is discussed, while in second and third parts, promising antiglycation agents of natural and synthetic sources have been reviewed, respectively. Finally, in vivo studies have been addressed. This review is mainly compiled from important databases such as Science, Direct, Chemical Abstracts, SciFinder, and PubMed.
Results: During the last two decades, various attempts have been made to inhibit the process of protein glycation. New potent inhibitors of protein glycation belonging to different classes such as flavonoids, alkaloids, terpenes, benzenediol Schiff bases, substituted indol, and thio compounds have been identified.
Conclusion: Antiglycation therapy will be an effective strategy in future to prevent the formation of AGEs for the management of late diabetic complications Current review article highlighted various compounds of natural and synthetic origins identified previously to inhibit the protein glycation and formation of AGEs in vitro and in vivo.
Introduction
In this review, we summarized the outcome of various studies conducted in last two decades to discover inhibitors of protein glycation in vitro and in vivo. We also discussed underlying mechanism of action of various potential inhibitors. This review article has been divided into four main parts. In the first part, we have discussed the mechanism involved in the formation of advanced glycation end products (AGEs), their structures and characteristics, site specificity, and various techniques used to measure AGEs. In the second part, in vitro discovery of antiglycation agents of natural origin has been reviewed, while in the third part, promising antiglycation agents of synthetic origin have been mentioned. Finally, in vivo studies on different inhibitors of protein glycation have been addressed.
Formation of AGEs
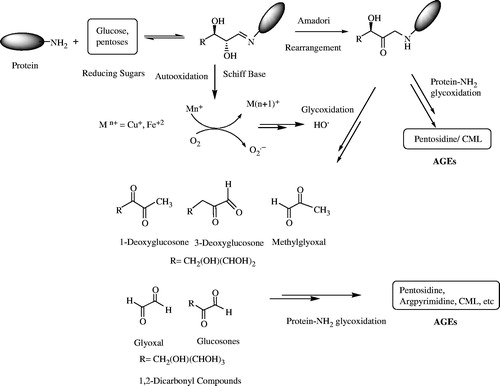
The non-enzymatic protein glycation and oxidative stress are directly related to the on-set and the progression of diabetes mellitus (Xi et al., Citation2008). During protein glycation, amino groups of protein react with aldehydic or keto groups of reducing sugars to form Schiff's bases which are then converted into relatively stable compounds called Amadori products through a rearrangement. The glycation process, before this conversion, is known as the early-stage glycation. Amadori compounds are then converted into relatively more reactive substances, such as dicarbonyl compounds and 3-deoxyglucosone (3DG), which result in the formation of AGEs through dehydration, and rearrangement as shown in . Many reducing sugars, such as glucose, fructose, galactose, lactose, xylose, and deoxyribose, are involved in protein glycation with glucose being the most common (Sakai et al., Citation2002). Glycation of proteins and other biomolecules is time- and concentration-dependent process. During diabetes, the rate of formation of AGEs increases spontaneously due to increased glucose levels in the blood. AGEs contribute significantly to many complications of diabetes (Graves et al., Citation2006). The rapid protein glycation and formation of AGEs lead to the failure of various organs due to serious damage and dysfunction, which included the heart, eyes, kidneys, nerves, and the blood vessels (Mosihuzzman et al., Citation2013). The possible mechanism involved in the AGEs formation is shown in .
AGEs are complex and heterogeneous group of compounds having a vital role in diabetes-related complications (Singh et al., Citation2001). Under the physiological conditions, the formation of AGEs is comparatively a slow process, and thus AGEs are accumulated prominently in long-lived proteins including lens crystallins and tissue collagens. However, glycation of catalytic proteins (enzymes) and insulin causes key functional impairments. The quantity of AGEs increases with an increase in time and glucose concentration, and thus their formation increases in case of poor glycemic control and with aging (Bendayan, Citation1998). The formation of AGEs also involved the oxidation reactions, which are accelerated in the presence of oxygen and slow down in anaerobic environment (Graves et al., Citation2006).
AGE-modified proteins are created over long periods of time to promote protein cross linking and aggregation which is a source of intracellular reactive oxygen intermediates. It has provided a molecular mechanism link between oxidant stress and the pathogenesis of Alzheimer's disease (Yan et al., Citation1994).
The glycoxidation process is actually the autoxidation of Amadori products to AGEs. Amadori products are also converted to superoxide radicals in the presence of molecular oxygen and transition metals via protein dicarbonyl and protein enediol (Kern & Engerman, Citation2001). The superoxide radicals generated in this reaction can be transformed via the Fenton reaction into the hydroxyl radical (OH) which is a very reactive species (Ahmed, Citation2005).
Site specificity of protein glycation
Glycation of protein is not an arbitrary reaction. There is variation in rate and extent of glycation depending on amino groups of various amino acids in protein. The protein structures and endogenous ligands actually determine the specificity of glycation. The specificity of protein glycation is affected due to both acidic and basic neighboring groups, either through effects on the pKa of the amino groups, which enhances their nucleophilicity, or through the catalysis of the Amadori rearrangement, which is the rate-limiting step in protein glycation (Zhang et al., Citation2009). Amino acids having free amino groups on the N-terminus such as lysine and arginine residues undergo protein glycation. However, if lysine residues are present on the molecular surface, histidine residue in close vicinity facilitates glycation process (Obayashi et al., Citation1996).
Techniques for the estimation of protein glycation
Spectrofluorimeteric methods: Due to inter- or intra-molecular cross-links, AGEs are formed and converted into more complicated structures due to polymerization and insolubilization. AGEs are complex materials which emit fluorescence, and finally become brown pigments. AGEs' concentration is, therefore, conveniently measured by using the intensity of fluorescence. Spectrofluorimeter is used to measure these fluorescent AGEs (Sakai et al., Citation2002). Some of the AGEs are colored compounds with characteristic fluorescence (an excitation at 330 nm and an emission at 400 nm (Matsuda et al., Citation2003).
Mass spectrometry techniques: Electrospray ionization (ESI) and matrix-assisted laser desorption ionization (MALDI): Both MALDI and ESI-MS have been utilized for the quantitative measurements of the glycated proteins and peptides (Harms et al., Citation2004; Makoto et al., Citation2002). ESI-MS is, therefore, a tool to examine the rate of glycation of peptides and to identify various reaction intermediates (Li et al., Citation2010). In order to measure the extent of protein glycatin, mass spectrometry, i.e., ESI and MALDI, techniques have been used in many studies (Janani et al., Citation2001). The MALDITOF-MS with outstanding sensitivity is especially a useful tool in detecting the AGEs and other post-translational modification products and its site of glycation (Faustinus et al., Citation2004). The AGEs can be identified with respect to the relative mass changes. The mass difference due to various modifications can be used to estimate the average glycation rate of the protein (Glenis et al., Citation2003).
SDS-PAGE analysis: Gel electrophoresis (SDS-PAGE) was employed to investigate the chelating activity of feruloyl oligosaccharides (FOs) and their inhibition of protein glycation in a bovine serum albumin (BSA)/glucose system. Methacrylamido phenylboronate acrylamide gel electrophoresis (mP-AGE) was used to distinguish between glycated and unglycated proteins, and as a tool for the detection, identification, and separation of protein glycation (Kislinger et al., Citation2005). By employing (SDS-PAGE), it was shown that FOs significantly decreased the formation of AGEs and it might be beneficial as glycation inhibitors under specified conditions (Morais et al., Citation2010).
Mechanism of inhibition
Since mechanism of AGEs formation involved various steps, therefore, different compounds have been identified which can inhibit this process at different steps. Type A inhibitors, are called “sugar competitors”, act to transform free amino groups of proteins and peptides in order to prevent sugar attachment (Wang et al., Citation2009). Inhibitors of Type B react with aldose or ketose sugars and divert them from binding to proteins. Type B inhibitors react with more than one step of the protein glycation and AGE formation (Khalifa et al., Citation1999). Aminoguanidine is an example of Type B inhibitors, which reacts with the carbonyl group of sugars as well as trapping methyl glyoxal. Inhibitors of Type D actually trap MG, while inhibitors of Type E are the Amadori product. Type E inhibitors inhibit the cross-linking of AGE-BSA (Lehman & Ortwerth, Citation2001).
Inhibitors of protein glycation
Due to increased understanding of the damaging effects of glycation process, it is highly desirable to manage this process effectively either by prevention or by managing the consequences of glycation which could be useful for millions of affected people (Lehman & Ortwerth, Citation2001; Wang et al., Citation2009).
Discovery of new potential antiglycation agents of natural and synthetic origins with enhanced inhibitory activity and reduced toxicity is the effective approach to control the development and prevention of diabetic complications.
Antiglycation agents of natural origin in vitro
Natural compounds isolated from medicinally important plants have shown promising antiglycation activity in vitro and in vivo. Rutin (flavonoid), found in fruits and vegetables, forms metabolites after ingestion that include 3,4-dihydroxyphenylacetic acid (3,4-DHPAA), 3,4-dihydroxytoluene (3,4-DHT), m-hydroxyphenyl acetic acid (m-HPAA), 3-methoxy-4-hydroxyphenylacetic acid (homovanillic acid, HVA), and pentahydroxyflavonol (quercetin) (Rahbar et al., Citation2000). All five metabolites of rutin effectively inhibit the N-carboxymethylysine (CML) formation. It is observed that rutin and its vicinal dihydroxyl group containing metabolites inhibit autoxidation, glycation, and hyperglycemia-induced collagen-linked fluorescent adduct formation (Nagasawa et al., Citation2003; Namiki et al., Citation2003). Thus, it is likely that metal chelation and/or free radical scavenging properties of rutin metabolites contribute to the inhibition of glucose autoxidation due to vicinal dihydroxyl groups. In fact, early glycation stage, especially ketoamine formation, does not involve reactive oxygen species or metal-catalyzed oxidation. It is, therefore, suggested that the inhibition exhibited by rutin and its metabolites may involve mechanisms such as the trapping of reactive amino groups, making them unable to react with glucose (Type A) (Duraisamy et al., Citation2003). Thus, rutin metabolites were found to be promising candidates for non-toxic inhibition of glycation-induced protein–AGE adduct formation (Cervantes-Laurean et al., Citation2006).
A series of natural flavonoids were investigated for their potential as an inhibitor of protein glycation. It was observed that there are some structural features of flavonoids which are considered for the inhibition of AGEs formation, such as the antiglycation activities which became stronger with an increase in hydroxyl groups at the C-30-, C-40-, C-5-, and C-7 positions. Flavones are more active than corresponding flavanones, isoflavones, and flavonols. Methylation or glucosylation of the hydroxyl group also alters the activity of flavones, flavonols, and flavanones. In addition to AGEs' inhibitory activity, various flavonoids also exhibit strong scavenging activity for 1,1-diphenyl-2-picrylhydrazyl and superoxide anion radicals (Pashikanti et al., Citation2010). Kaempferol-7-β-D-glucopyranoside and glycine-N-(1H-benzimidazol-2-yl)-methyl ester also exhibited a significant antiglycation activity in vitro with non-toxic effects on mouse 3T3 fibroblast cells (Matsuda et al., Citation2003).
Free radical formation is involved in the glycation process and radical scavengers can be effective for glycation inhibition. Garcinol exhibited moderate antioxidative activity and antichelating activity, along with potent glycation inhibition activity (Atta-ur-Rahman et al., Citation2007). In another study, garcinol exhibited a potent glycation suppressing activity against a phosphate-buffered d-fructose and BSA reaction. It suppressed fluorescence and protein cross-link formation. The mechanism of the activity of garcinol was inferred to be the chelation with a trace metal ion which catalyzed the glycation (Cervantes-Laurean et al., Citation2006). Natural compounds belonging to different classes have shown significant inhibitory potential against protein glycation in vitro (Atta-ur-Rahman et al., Citation2007; Cervantes-Laurean et al., Citation2006; Matsuda et al., Citation2003; Vinson & Howard, Citation1996). Some of them are presented in .
Table 1. Natural compounds as promising inhibitors of protein glycation.
Pyridoxamine (PM) (4-aminomethyl)-5-(hydroxymethyl)-2-methylpyridin-3-ol) in particular inhibits the generation of hydroxyl radical from protein–Amadori. Pyridoxamine (PM) strongly inhibits the increase in hydroxyl radicals in a concentration-dependent manner as compared with its two PM structural analogs, 3-hydroxypyridine (3-HP) and 4- aminomethylpyridine (4-AMP). Pyridoxamine (PM) inhibits oxidative propagation of protein damage via two mechanisms: (a) scavenging of hydroxyl radicals and (b) inhibition of metal-catalyzed oxidative degradation of either glucose or various intermediates of glycated protein (Yamaguchi et al., Citation2000).
Irbesartan (2-butyl-3-({4-[2-(2H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1,3-diazaspiro[4,4]non-1-en-4-one) inhibits the AGE-induced TGF-α overproduction by tubular cells. TGF-α is a major etiologic agent in tubulointerstitial fibrosis in diabetic nephropathy (Chetyrkin et al., Citation2008). In a study, feruloyl oligosaccharides (FOs) significantly decreased the formation of AGEs in the tested system by using SDS-PAGE. These results indicate that under specified conditions, FOs might be beneficial inhibitors of protein glycation (Kislinger et al., Citation2005).
In vitro inhibition of protein glycation (synthetic compounds)
Various studies have been conducted to synthesize lead molecules which can significantly prevent the formation of AGEs. In a study, aminoguanidine (AG), P-resorcylidene aminoguanidine (RAG), dl-penicillamine (PNCA), and captopril were investigated for their effects on early and advanced glycation of human serum albumin (HSA). The results demonstrated that AG, RAG, PNCA, and captopril are effective in preventing the formation of AGEs. The protective effects might be due to the reduction of oxidative stress. These results clearly indicate the possible therapeutic use of AG and RAG as glycooxidation inhibitors (Matsui et al., Citation2010). Aminoguanidine (AG), a prototype therapeutic dicarbonyl scavenger, was found to be the most effective drug against the formation of AGEs and to reverse the glycation-mediated damage in normal aging (Jakug et al., Citation1999). In another study, pioglitazone, metformin, and pentoxifylline were tested against protein glycation and results are shown in .
Figure 2. Dose–response curves and inhibitory effects of pioglitazone, pentoxyfylline, and metformin as compared with that of minoguanidine (s). Data are average from three separate experiments.
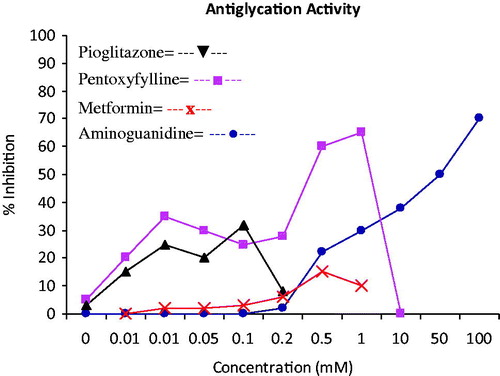
Metformin (glucophage) is a biguanide derivative which is used in the management of type 2 diabetes. The mechanism of reduction of circulating methyl glyoxal (MG) by metformin involved the trapping of MG and other dicarbonyl compounds produced during the glycation process. Furthermore, metformin inhibits the cross-linking of AGE-BSA in glucose-BSA type assays, while pentoxyfylline and pioglitazone showed reasonable inhibition of cross-linking at a lower concentration as shown in . An analog of pentoxyfyllin having hydroxyl group at C-5 position instead of an oxygen, also known as lisofylline (1-(5-R-hydroxyhexyl)-3,7-dimethylxanthine), is a better potent inhibitor of glycation than the pentoxyfylline which causes the reaction between alcohol and glucose to form hemiacetal thus scavenging glucose. Aminoguanidine exhibited a high activity at higher concentrations (Lehman & Ortwerth, Citation2001).
Aspirin is a promising inhibitor of post-Amadori Maillard reactions, even though its exact mechanism of action is unclear. However, it was suggested that aspirin may act as a free radical scavenger and/or chelator and thus inhibits the oxidative pathways of the Maillard reaction at post-Amadori stage. This supports the theory that aspirin functions as a free radical scavenger (Suji & Sivakami, Citation2006).
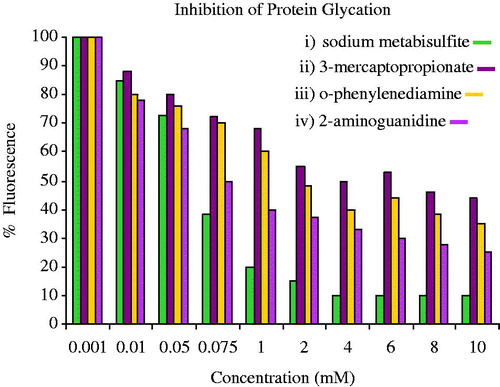
The dicarbonyl-reactive compounds, 2-aminoguanidine (2-AG), semicarbazide, and o-phenyl-enediamine (OPD), were able to inhibit process of protein glycation at relatively low concentrations (1–2 mM) as compared with previously reported inhibitors (; Khalifa et al., Citation1999).
The activity of inhibitor which increased considerably by sulfating of the OH group on C-4, as this carbon, is involved in AGE cross-linking. This is perhaps the reason that bisulfates are more effective inhibitors due to its enhanced ability as dicarbonyl-trapping agents (Hadley et al., Citation2001).
Aminosalicylic acid with a single free amino group inhibits the protein glycation at various steps of AGE formation, such as blocking free carbonyl groups on reducing sugars, Amadori stage, and dicarbonyl intermediates stage (Ahmed, Citation2005). Moreover, aminosalicylic acid and aminoguanidine protect the endothelial cells against glucose-mediated toxicity, thus having great therapeutic potential (Namiki et al., Citation2003). In another study, aminosalicylic acid was found to be more effective than aminoguanidine in reducing the anti-proliferative effects of both high glucose and BSA-AGE. This could be because aminosalicylic acid is a more potent antioxidant than aminoguanidine (Courderot-Masuyer et al., Citation1999).
AGEs are produced when brain aminophospholipids react with acetoacetate. Urea actually significantly decreased the glycating effect of acetoacetate, suggesting its protective physiological role in the body (Kim et al., Citation2007; Lin et al., Citation2003).
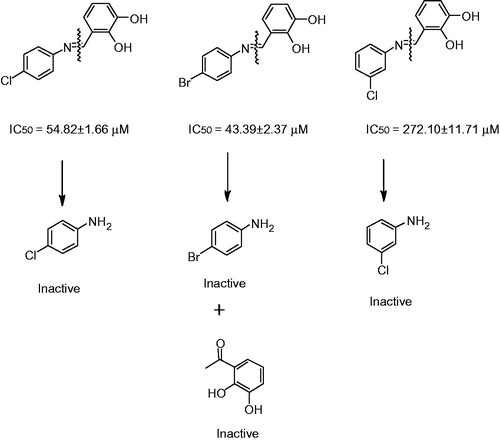
Recently, a series of substituted benzenediol Schiff bases has been synthesized which significantly inhibited the AGEs formation, as compared with their corresponding starting material. It was confirmed that although Schiff bases are converted to starting materials, less concentration in physiological condition but these were Schiff bases which actually exhibit the inhibition in vitro (; Aguilar-Hernández & Mendez, Citation2007).
In vivo inhibition of protein glycation
Pyridoxamine (PM) is a therapeutic agent for the inhibition of AGEs formation and delaying the development of long-term complications in age-related chronic diseases. PM not only inhibits the formation of both AGEs and advanced lipid end products (ALEs) in vitro but also exhibits inhibition in both STZ-diabetic and Zucker model rats. It mainly inhibits protein modifications and slows down the progression of nephropathy in diabetes and dyslipidemia with low toxicity (Choudhary et al., Citation2011). PM is also effective against the development of retinopathy in the STZ-diabetic rat (Onorato et al., Citation2000; Stitt et al., Citation2001).
Administration of aminoguanidine, 50 mg/kg daily intra peritoneal injection, significantly reduced the development of albuminuria in diabetic rats, without any effect on glycation of hemoglobin or collagen (Degenhardt et al., Citation1999). In a study, it was hypothesized that the Schiff base adduct formed from aminoguanidine and pyridoxal might be a better compound than aminoguanidine (Taguchi et al., Citation1999). In another study, aminoguanidine, a scavenger of free methylglyoxal, considerably increased the wound healing in the young and old mice which is due to reduced migration of methylglyoxal and proliferation of fibroblasts (Fleming et al., Citation2013).
Vitamin E and stobadine (ST), a pyridoindole antioxidant, separately exhibited a reduction in diabetes-induced hyperglycemia. In STZ-diabetic rats, vitamin E treatment was more effective as compared with ST treatment. In normal control rats, treatment with each antioxidant, as well as their combination, resulted in significant reduction of cardiac protein glycation. However, the exact mechanism(s) of the effects of the both antioxidants on abnormal intracellular calcium regulation of diabetic heart and liver are not fully known. It was also observed that by the using both antioxidants together, hyperglycemia-induced oxidative stress can be controlled effectively (Pekiner et al., Citation2002).
The inhibitory potential of l-arginine and spermidine on hemoglobin glycation in serum of normal and diabetic rats was examined (Gugliucci, Citation2003). In this study, it was observed that l-arginine and polyamines prevent diabetic complications by forming a Schiff-base linkage with the carbonyl fragments, Amadori products, or with post-Amadori intermediates (Méndez & Balderas, Citation2006).
A new compound 1-(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)-6-methyl-1,3-dihydro-furo[3,4-c]pyridine-7-ol (TM2002) was synthesized that inhibited the glycation process and oxidative stress in vitro as well as in vivo without affecting the blood pressure. Actually, TM2002 exhibited inhibition of AGEs formation through a reduction of oxidative stress by hydroxyl radicals scavenging and inhibition of the Fenton reaction which generates hydroxyl radicals (Thomas & Thomas, Citation2001).
Oral administration of protocatechualdehyde (PCA) (3,4-dihydroxy benzaldehyde) (25 mg/kg body weight), for 8 weeks, significantly improved the development of lens opacity (cataract) with positive effect on glycemic control in streptozotocin-induced diabetic rats. PCA was found to be 80-fold more effective than aminoguanidine in inhibiting the AGEs formation and the prevention of diabetic complications. This has increased therapeutic interest in PCA. Protocatechualdehyde inhibited the lens opacity in streptozotocin-induced diabetic cataract in rats which can be explained by the inhibitory action of PCA on AGEs formation and its reductive effect on receptors of AGEs (RAGE) and TGF-β expression (Shunya et al., Citation2007).
A new compound XLF-III-43 (conjugate between coumarin and aspirin) has shown significant inhibition against AGE formation and cross-link in vitro and the prevention of diabetic nephropathy in the streptozotocin-model rat of diabetes. The exact biochemical mechanism(s) that how XLF-III-43 mediates the inhibition of AGE formation is under investigation. The compound XLFIII-43 may be a potential candidate for the prevention of diabetic nephropathy (Harms et al., Citation2004).
Various studies have shown N-(2-acetamidoethyl) hydrazinecarboximid-amide hydrochloride (ALT-946) as a potent inhibitor of AGE-derived protein modification and its limiting effects on NO production. It was shown that ALT-946 and aminoguanidine reduce severe glomerulosclerosis and cortical tubular degeneration in the context of reduced renal AGE accumulation (Young et al., Citation2007).
Ellagic acid (EA), a flavonoid, also exhibited inhibition against eye lens haziness through inhibition of AGEs in the lens organ culture system. The possible inhibition mechanism of ellagic acid against different proteins is through scavenging of the carbonyl compounds (Muthenna et al., Citation2012).
AGE-breakers
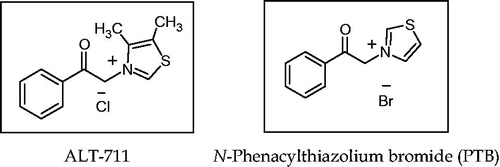
Currently, two successful strategies, targeting AGEs formations (one inhibiting the formation of AGEs) and the other breaking already established AGEs linkages, have been vigorously pursued, such therapeutic approaches can be very effective against diabetic complications and in normal aging process (Muthenna et al., Citation2012). Non-reversible covalent cross-links between tissue proteins and other organic compounds form advanced glycation products. Novel therapeutics such as AGE-breakers N-phenacylthiazolium bromide (PTB) or its the chloride form which was developed by Alteon, commonly known as alagebrium (3-phenacyl-4, 5-dimethylthiazolium chloride) ALT-711, can easily break this α-dicarbonyl bond, and thus can remove the AGEs cross-links (Asif et al., Citation2000; ). Various studies in vitro and in vivo have established the ability of PTB to actually diminish AGE-derived cross-links; therefore, future research is likely to be focused on finding different mechanisms by which PTB breaks the AGE cross-links. Wolffenbuttel et al. (Citation1998) first conducted an in vivo study in rats, which confirmed that AGE-breaker re-established large artery properties during chronic treatment in experimental diabetes (Sourris et al., Citation2009).
Receptor for AGEs (RAGE) is associated with increased oxidative stress, cell growth, and inflammation. The curcumin obtained from natural source inhibited the activation of hepatic stellate cells (HSCs), the major effectors during hepatic fibrogenesis. The underlying mechanism of action revealed that curcumin actually eliminated the effects of AGEs by suppressing gene expression of RAGE (Wolffenbuttel et al., Citation1998). A novel therapeutic strategy for diabetic complications has been the blockade of AGE-RAGE-oxidative stress (Jianguo et al., Citation2012).
In a study, chronic administration of anti-RAGE antibodies to mice with diabetes mellitus inhibited nephropathy without any adverse effects (Yamagishi et al., Citation2008). Further studies have shown that blockade of RAGE by neutralizing antibodies reduced atherosclerosis in uremic mice (Tan et al., Citation2006).
Conclusion
It is clear that the process of protein glycation alters the biological activity of proteins and initiates their degradation process, while inhibition of glycation reaction could be effective to avoid major diabetic complications. Various compounds of natural and synthetic origins such as flavonoid (flavones, flavanones, isoflavones, and flavonols), phenols derivatives, imidazol, Schiff bases, thiazolidine, and sulfonate identified previously have shown significant inhibition against the protein glycation and formation of AGEs. The mechanisms may involve the trapping of reactive amino groups, making them unable to react with glucose or scavenging of the carbonyl compounds, the chelation with the trace metal ion which catalyzed the glycation, scavenging of hydroxyl radicals, and inhibition of metal catalyzed oxidative degradation of either glucose or various intermediates of glycated protein. Thus, antiglycation therapy will be an effective strategy in future to prevent the formation of AGEs for the management of late diabetic complications.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ahmed N. (2005). Advanced glycation endproducts – Role in pathology of diabetic complications. Diabetes Res Clin Pract 67:3–21
- Aguilar-Hernández M, Méndez JD. (2007). In vitro glycation of brain aminophospholipids by acetoacetate and its inhibition by urea. Biomed Pharmacother 61:693–7
- Atta-ur-Rahman, Choudhary MI, Basha FZ, et al. (2007). Science at the interface of chemistry and biology: Discoveries of alpha-glucosidase inhibitors and antiglycation agents. Pure Appl Chem 79:2263–8
- Asif M, Egan J, Vasan S, et al. (2000). An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci USA 97:2809–13
- Bendayan M. (1998). Immunocytochemical detection of advanced glycation end products in rat renal tissue as a function of age and diabetes. Kidney Int 54:438–47
- Cervantes-Laurean D, Schramm DD, Jacobson EL, et al. (2006). Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites. J Nutr Biochem 17:531–40
- Chetyrkin SV, Mathis ME, Ham AJ, et al. (2008). Propagation of protein glycation damage involves modification of tryptophan residues via reactive oxygen species: Inhibition by pyridoxamine. Free Radical Biol Med 44:1276–85
- Choudhary MI, Abbas G, Ali S, et al. (2011). Substituted benzenediol Schiff bases as promising new anti-glycation agents. J Enzym Inhbi Med Chem 26:98–103
- Courderot-Masuyer C, Dalloz F, Maupoil V, Rochette L. (1999). Antioxidant properties of aminoguanidine. Fundam Clin Pharmacol 13:535–40
- Degenhardt TP, Fu MX, Voss E, et al. (1999). Aminoguanidine inhibits albuminuria, but not the formation of advanced glycation end-products in skin collagen of diabetic rats. Diabetes Res Clin Pract 43:81–9
- Duraisamy Y, Gaffney J, Slevin M, et al. (2003). Aminosalicylic acid reduces the antiproliferative effect of hyperglycaemia, advanced glycation endproducts and glycated basic fibroblast growth factor in cultured bovine aortic endothelial cells: Comparison with aminoguanidine. Mol Cell Biochem 246:143–53
- Faustinus KY, Inteaz A, Varoujan AY, et al. (2004). Effect of limited solid-state glycation on the conformation of lysozyme by ESI-MSMS peptide mapping and molecular modeling. Bioconjugate Chem 15:27–34
- Fleming TH, Theilen TM, Masania J, et al. (2013). Aging-dependent reduction in glyoxalase delays wound healing. Gerontology 59:427–37
- Graves DT, Liu R, Alikhani M, et al. (2006). Diabetes-enhanced inflammation and apoptosis – Impact on periodontal pathology. J Dent Res 85:15–21
- Glenis JW, Rashmi P, Helaman E, et al. (2003). DNA sequencing research group (DSRG) 2003 – A general survey of core dna sequencing facilities. J Biomol Tech 14:231–7
- Gugliucci A. (2003). A practical method to study functional impairment of proteins by glycation and effects of inhibitors using current coagulation/fibrinolysis reagent kits. Clin Biochem 36:155–8
- Hadley J, Malik N, Meek K. (2001). Collagen as a model system to investigate the use of aspirin as an inhibitor of protein glycation and crosslinking. Micron 32:307–15
- Harms MJ, Wilmarth PA, Kapfer DM, et al. (2004). Laser light-scattering evidence for an altered association of βB1-crystallin deamidated in the connecting peptide. Protein Sci 13:678–86
- Jakug V, HrnEiarova M, Crasky J, et al. (1999). Inhibition of nonenzymatic protein glycation and lipid peroxidation by drugs with antioxidant activity. Life Sci 65:1991–3
- Janani V, Kamna A, Balaram P. (2001). Helical peptide models for protein glycation: Proximity ejects in catalysis of the Amadori rearrangement. Chem Biol 8:611–25
- Jianguo L, Youcai T, Qiaohua K, et al. (2012). Curcumin inhibits gene expression of receptor for advanced glycation endproducts (RAGE) in hepatic stellate cells in vitro by elevating PPARg activity and attenuating oxidative stress. Br J Pharmacol 166:2212–27
- Kern TS, Engerman RL. (2001). Pharmacological inhibition of diabetic retinopathy: Aminoguanidine and aspirin. Diabetes 50:1636–42
- Khalifa RG, Baynes JW, Hudson BG. (1999). Breakthroughs and view: Amadorins: Novel post-Amadori inhibitors of advanced glycation end product. Biochem Biophys Res Commun 257:251–8
- Kim YS, Kim NH, Lee SW, et al. (2007). Effect of protocatechualdehyde on receptor for advanced glycation end products and TGF-beta1 expression in human lens epithelial cells cultured under diabetic conditions and on lens opacity in streptozotocin-diabetic rats. Eur J Pharmacol 569:171–9
- Kislinger T, Humeny A, Peich CC, et al. (2005). Analysis of protein glycation products by MALDI-TOF/MS. Ann NY Acad Sci 1043:249–59
- Lehman TD, Ortwerth BJ. (2001). Inhibitors of advanced glycation end product-associated protein cross-linking. Biochim Biophys Acta 1535:110–19
- Li H, Zheng X, Wang H, et al. (2010). XLF-III-43, a novel coumarin–aspirin compound, prevents diabetic nephropathy in rats via inhibiting advanced glycation end products. Eur J Pharmacol 627:340–7
- Lin RY, Choudhury RP, Cai W, et al. (2003). Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 168:213–20
- Makoto S, Munetada O, Masato K. (2002). Experimental studies on the role of fructose in the development of diabetic complications. Kobe J Med Sci 48:125–36
- Matsuda H, Wang T, Managi H, Yoshikawa M. (2003). Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorgan Med Chem 11:5317–23
- Matsui T, Yamagishi S, Takeuchi M, et al. (2010). Irbesartan inhibits advanced glycation end product (AGE)-induced proximal tubular cell injury in vitro by suppressing receptor for AGEs (RAGE) expression. Pharmacol Res 61:34–9
- Méndez JD, Balderas FL. (2006). Inhibition by l-arginine and spermidine of hemoglobin glycation and lipid peroxidation in rats with induced diabetes. Biomed Pharmacother 60:26–31
- Morais MP, Mackay JD, Bhamra SK, et al. (2010). Analysis of protein glycation using phenylboronate acrylamide gel electrophoresis. Proteomics 10:48–58
- Mosihuzzman M, Naheed S, Hareem S, et al. (2013). Studies on α-glucosidase inhibition and anti-glycation potential of Iris loczyi and Iris unguicularis. Life Sci 92:187–92
- Muthenna P, Akileshwari C, Reddy GB. (2012). Ellagic acid, a new antiglycation agent: Its inhibition of N-(carboxymethyl) lysine. Biochem J 442:9221–30
- Nagasawa T, Tabata N, Ito Y, et al. (2003). Inhibition of glycation reaction in tissue protein incubations by water soluble rutin derivative. Mol Cell Biochem 249:3–10
- Namiki M. (2003). Advances in the Maillard reaction and glycation researches mainly on the Namiki pathway. Seikagaku 34:37–42
- Obayashi H, Nakano K, Shigeta H, et al. (1996). Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem Biophys Res Commun 226:37–41
- Onorato JM, Jenkins AJ, Thorpe SR, Baynes JW. (2000). Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. J Biol Chem 275:21177–84
- Pashikanti S, de Alba DR, Boissonneault GA, Cervantes-Laurean D. (2010). Rutin metabolites: Novel inhibitors of nonoxidative advanced glycation end products. Free Radical Biol Med 48:656–63
- Pekiner B, Ulusu NN, Das-Evcimen N, et al. (2002). In vivo treatment with stobadine prevents lipid peroxidation, protein glycation and calcium overload but does not ameliorate Ca2+ -ATPase activity in heart and liver of streptozotocin-diabetic rats: Comparison with vitamin E. Biochim Biophys Acta 1588:71–8
- Rahbar S, Natarajan R, Yerneni K, et al. (2000). Evidence that pioglitazone, metformin and pentoxifylline are inhibitors of glycation. Clin Chim Acta 301:65–77
- Sakai M, Oimomi M, Kasuga M. (2002). Experimental studies on the role of fructose in the development of diabetic complications. Kobe J Med Sci 48:125–36
- Shunya T, Yuko I, Yasuko K, et al. (2007). A novel inhibitor of advanced glycation and endoplasmic reticulum stress reduces infarct volume in rat focal cerebral ischemia. Brain Res 1183:124–37
- Singh R, Barden A, Mori T, Beilin L. (2001). Advanced glycation end-products: A review. Diabetologia 44:129–46
- Sourris KC, Harcourt BE, Forbes JM. (2009). A new perspective on therapeutic inhibition of advanced glycation in diabetic microvascular complications: Common downstream endpoints achieved through disparate therapeutic approaches. Am J Nephrol 30:323–35
- Stitt AW, Gardiner TA, Duffy N, et al. (2001). Inhibition of advanced glycation/lipoxidation endproducts (AGE/ALEs) prevents retinopathy in experimental diabetes. Diabetologia 44:A290
- Suji G, Sivakami S. (2006). DNA damage by free radical production by aminoguanidine. Ann NY Acad Sci 1067:191–9
- Taguchi T, Sugiura M, Hamada Y, Miwa I. (1999). Inhibition of advanced protein glycation by a Schiff base between aminoguanidine and pyridoxal. Eur J Pharmacol 378:283–9
- Tan KC, Shiu SW, Chow WS, et al. (2006). Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia 49:2756–62
- Thomas T, Thomas TJ. (2001). Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell Mol Life Sci 58:244–58
- Vinson JA, Howard TB. (1996). Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J Nutr Biochem 7:659–63
- Wang J, Sun B, Cao YP, Tian Y. (2009). Protein glycation inhibitory activity of wheat bran feruloyl oligosaccharides. Food Chem 112:350–3
- Wolffenbuttel BHR, Boulanger CM, Crijns FRL, et al. (1998). Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA 95:4630–4
- Xi M, Hai C, Tang H, et al. (2008). Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res 22:228–37
- Yamagishi S, Nakamura K, Matsui T, et al. (2008). Agents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: A novel therapeutic strategy for diabetic vascular complications. Expert Opin Investig Drugs 17:98–996
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H. (2000). Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem 48:180–5
- Yan SD, Chens X, Schmid AM, et al. (1994). Glycated tau protein in Alzheimer disease: A mechanism for induction of oxidant stress. Proc Natl Acad Sci USA 91:7787–91
- Young SK, Nan HK, Sang WL, et al. (2007). Effect of protocatechualdehyde on receptor for advanced glycation end products and TGF-β1 expression in human lens epithelial cells cultured under diabetic conditions and on lens opacity in streptozotocin-diabetic rats. Eur J Pharmacol 569:171–9
- Zhang Q, Ames JM, Smith RD, et al. (2009). A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J Proteome Res 8:754–69