Abstract
Context: Juncus effusus L. var. decipiens BUCHEN. f. leschenaultii GAY has been used in traditional medicine for the treatment of anxiety and insomnia.
Objective: The objective of this study was to evaluate the effects of ethanol extract from the pith of Juncus effusus (JEE) on anti-inflammatory activities in RAW 264.7 cells.
Materials and methods: The production of inflammatory mediators and the underlying mechanisms using 3.1, 6.3, and 12.5 μg/mL concentrations of JEE were investigated. In addition, the topical anti-inflammatory effects of JEE (0.5, 1, and 2 mg/mL) on 12-O-tetradecanoylphorobol-13 acetate (TPA)-induced ear edema and oral administration of JEE (50, 100, and 200 mg/kg) on carrageenan-induced paw-edema were studied in mice.
Results: JEE reduced the release of nitric oxide (NO, IC50 value = 1.98 μg/mL), prostaglandin E2 (IC50 value = 5.5 μg/mL), and pro-inflammatory cytokines, IL-1β (IC50 value = 4.74 μg/mL) and IL-6 (IC50 value = 20.48 μg/mL). JEE also suppressed the protein expression of inducible NO synthase and cyclooxygenase-2 in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. Mechanism studies showed attenuation of LPS-induced activation of NF-κB by JEE via abrogation of IκBα degradation and a subsequent decrease in nuclear p65 level. Phosphorylation of all three MAP kinases (ERK, JNK, and p38) in LPS-stimulated RAW 264.7 cells was also suppressed in a dose-dependent manner. In acute inflammation models of mice, topical application (1 and 2 mg) and oral administration (50, 100, and 200 mg/kg) of JEE ameliorated TPA-induced ear edema and carrageenan-induced paw edema, respectively, in dose-dependent manners.
Discussion and conclusion: These results indicate that JEE exhibited anti-inflammatory activities by suppressing the production of inflammatory mediators in LPS-stimulated RAW 264.7 cells and by attenuating edema in mice.
Introduction
Inflammation is a host response to infection and injury resulting in the production of a variety of inflammatory mediators. Activation of macrophages by stimuli led to production of a variety of inflammatory mediators such as prostaglandin E2 (PGE2), nitric oxide (NO), and pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6. However, if left uncontrolled, the inflammatory mediators become involved in the pathogenesis of inflammatory disorders (Ritchlin et al., Citation2003).
Expression of these inflammatory mediators is regulated by the activation of nuclear factor-kappaB (NF-κB) through the inhibitor κB kinase (IKK)/IκB/NF-κB pathway. Under normal conditions, NF-κB is located in the cytoplasm as an inactive complex bound to its inhibitory protein, IκB (Ghosh & Hayden, Citation2008). Upon cell stimulation, a series of TLR-mediated signal pathways occur through TIRAP/IRAK family/TRAF6/TAK1 to activate downstream of IKK signaling pathways, which induce degradation of IκBα (Lu et al., Citation2008). The free NF-κB translocates to the nucleus and activates the expression of inflammatory mediators (Lappas et al., Citation2002; Surh et al., Citation2001).
The mitogen-activated protein (MAP) kinase families, including extracellular signal regulated kinase (ERK), c-jun N terminal kinase (JNK), and p38, regulate inflammatory responses. Upon stimulation by lipopolysaccharide (LPS), the activation of MAP kinases mediates the signaling pathways leading to activation of NF-κB (Guha & Mackman, Citation2001; Zhou et al., Citation2008). Therefore, NF-κB and MAP kinases are important targets for anti-inflammatory molecules.
Because natural plants have proven to be a valuable source for new therapeutic agents, we have searched anti-inflammatory plants and selected Juncus effusus L. var. decipiens BUCHEN. f. leschenaultii GAY. The dried pith of J. effuses has been used in China for the treatment of anxiety and insomnia (Liao et al., Citation2011). It contains several classes of compounds, including phenanthrene derivatives, triterpenes, flavonoids, and phenolic acid derivatives, as well as essential oil components (Singhuber et al., Citation2012). A study of the pharmacological activity of J. effusus focused mainly on the phenanthrene derivatives (Behery et al., Citation2007; Hanawa et al., Citation2002). Recently, an isolated phenanthrene compound exhibited anxiolytic and sedative activities in in vivo and in vitro studies (Liao et al., Citation2011; Singhuber et al., Citation2012). However, the effects of J. effusus extract (JEE) on anti-inflammatory activity have not been previously explored.
In this study, we investigated the inhibitory effects of JEE on the production of inflammatory mediators (NO, PGE2, IL-1, and IL-6) and elucidated anti-inflammatory mechanisms in LPS-stimulated RAW 264.7 cells. The anti-inflammatory activity of JEE was also evaluated in 12-O-tetradecanoyphorbol-13 acetate (TPA)-induced ear edema and carrageenan-induced paw edema of mice. JEE was found to attenuate the release of inflammatory mediators that was correlated with the inactivation of NF-κB and MAP kinases and remarkably inhibited both ear and paw edema models. This finding suggests the potential of JEE as an anti-inflammatory agent.
Materials and methods
Preparation of J. effusus extract
Dried stems of J. effusus var. decipiens collected in the Jiangsu province of China in the year 2012 were purchased from Ominherb (Youngchun, Korea) and authenticated by Dr. H. Lee, a herbalist, in the Korea Promotion Institute for Traditional Medicine Industry. A voucher specimen has been deposited in Korea Promotion Institute for Traditional Medicine Industry (KOTMIN-IN4). Juncus effusus was extracted with ethanol at a ratio of 1:10 (w/v) and then refluxed for 24 h at 60 °C. The extracted solution was filtered, and the solvents were evaporated under vacuum at 40 °C (Eyela, Tokyo, Japan), after which they were freeze dried to obtain the concentrated extract (yield 7.2%, w/w).
Animals
BALB/c mice were obtained from Koatek (Seoul, Korea) and fed with laboratory feed (Purina, Seoul, Korea) and water ad libitum. Mice were acclimatized in a specific pathogen-free animal facility under conditions of 20–22 °C, 40–60% relative humidity, and a 12 h/12 h (light/dark) cycle for at least for 7 d. All animal-related experimental procedures were approved by the animal care committee of Korea Promotion Institute for Traditional Medicine Industry (approval no. KOTMIN-2014-009).
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and antibiotics (penicillin/streptomycin) were purchased from Hyclone (Logan, UT). LPS, SP600125, SB203580, PD98059, pyrrolidine dithiocarbamate (PDTC), and Griess reagent were obtained from Sigma Chemical Co. (St. Louis, MO). Antibodies against iNOS and COX-2 were purchased from BD Biosciences (San Jose, CA) and Cayman Chemical (Ann Arbor, MI), respectively. Anti-phospho or total antibodies to JNK, ERK, p38, IκBα, and NF-κB p65 were obtained from Cell Signaling (Beverly, MA). Secondary antibodies, goat anti-rabbit, goat anti-mouse, and rabbit anti-goat were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The 1 × RIPA buffer, NE-PER Nuclear Protein Extraction kit, phosphatase/protease inhibitor cocktail, and enhanced chemiluminescence (ECL) detection reagent were from Pierce (Rockford, IL).
Cell culture and viability assay
RAW 264.7 cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and then cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM). The cell viability was assessed using the CellTiter 96 Aqueous One kit (Promega, Madison, WI). Briefly, RAW 264.7 cells (5 × 104 cells) were seeded onto each well of a 96-well plate and incubated at 37 °C for overnight. Cells were incubated with different concentrations of JEE for 24 h and 20 μL of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium (MTS) was added to each well and incubated for 2 h. The optical densities were measured at 490 nm using a microplate reader (Tecan System, San Jose, CA).
Measurement of nitrite, PGE2, and pro-inflammatory cytokines
RAW 264.7 cells were seeded onto a 24-well culture plate at 37 °C for overnight in medium. The cells were pre-incubated with different concentrations of JEE for 1 h and then incubated for 18 h with or without LPS. The nitrite in culture medium was measured as an indicator of NO production based on the Griess reagent. The levels of PGE2 and pro-inflammatory cytokines (IL-1β and IL-6) in the culture supernatant were quantified to determine the inhibitory activities of JEE using an EIA and ELISA kits (Cayman Chemical, Ann Arbor, MI) according to the instructions of the manufacturer.
RNA extraction and real-time-PCR
Total cellular RNAs were extracted from LPS-stimulated macrophage Raw 264.7 cells using TRI Solution™ according to the instructions of the manufacturer (BSK Bioscience, Gyeongbuk, Korea). One microgram of total RNA was converted to cDNA using OligodT15 and Goscript™ Reverse transcription system kit (Promega, Madison, WI). The RT-PCR reaction was carried out on the StepOne Plus™ (Applied Biosystems, Foster City, CA) using HotStart® SYBR® Green qPCR Master Mix according to the instructions of the manufacturer (USB, Cleveland, OH). The amplification conditions and primer sequences were the same as previously described (Park et al., Citation2013). The results of real-time (RT)-PCR were presented as pro-inflammatory cytokine gene (IL-1β and IL-6) induction fold, and these were calculated using β-actin, which was amplified under the same conditions, as an internal control.
Western blot analysis
Whole cell protein lysates were prepared in RIPA lysis buffer in the presence of protease inhibitors. For nuclear and cytosolic extraction, cells were washed with PBS and prepared by the NE-PER nuclear protein extract kit according to the instructions of the manufacturer. Protein samples (10–30 μg for each) were loaded on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes in 20% methanol/25 mM Tris/192 mM glycine. After transfer, the membranes were washed with TTBS (25 mM Tris-HCl, 150 mM NaCl, and 0.2% Tween-20), blocked with 5% non-fat dry milk in TTBS, and then probed with primary antibodies overnight. After overnight at 4 °C of incubation followed by three washes, the membranes were incubated with appropriate secondary HRP-conjugated antibodies for 1 h at room temperature. The protein bands were then visualized using an ECL system. The densities of the bands were measured with the ImageQuant LAS 4000 luminescent image analyzer and ImageQuant TL software system (GE Healthcare, Little Chalfont, UK).
NF-κB DNA-binding activity
The binding activity of NF-κB to DNA was performed using Trans-AM ELISA kit according to the instructions of the manufacturer (Active Motif, Carlsbad, CA). This assay consists of a 96-well plate each coated with an oligonucleotide containing the consensus region (5′-GGGACTTTCC-3′) that the active form of NF-κB from cell lysates binds. The primary antibody binds to the epitope on p65 only when NF-κB is activated and bound to its target. A secondary HRP-conjugated antibody binds to the primary complex which then produces a signal quantifiable by spectrophotometery (measurable by optical density at a wavelength of 450 nm).
TPA-induced ear edema
Ear edema was induced according to a previously described method with a minor modification (Carlson et al., Citation1985; De Young et al., Citation1989). Mice were divided into five groups (n = 6). The right ear of each mouse received a topical application of TPA (Sigma, St. Louis, MO) as 1.25 μg/10 μL acetone solution (each side of the ear). JEE (0.5, 1, or 2 mg/ear) or Indomethacin (0.25 mg/ear, Sigma) was dissolved in acetone and applied topically immediately after TPA treatment. The thickness of ears was measured before and at 1.5, 3, 4.5, and 6 h after TPA treatment using a micrometer (CD-15APX, Mitutoyo Co., Tokyo, Japan). Six hours after TPA treatment, mice were sacrificed and both ears were removed. The swelling was measured as the difference in weight between the punches from right and left ears.
Carrageenan-induced paw edema
Paw edema was performed according to a previously described method with a minor modification (Handy & Moore, Citation1998). Mice were divided into five groups (n = 6). JEE or Indomethacin (10 mg/kg) was dissolved in PBS and administered orally (50, 100, or 200 mg/kg) 30 min before carrageenan (Sigma, St. Louis, MO) injection. Paw swelling was induced by injection of 30 μL of 1% v/v carrageenan solution into the plantar surface of left hind paw. The paw volume was measured by caliper immediately prior to the carrageenan injections and at 3 min, 0.5, 1, 2, 2.5, and 3 h. Paw thickness was determined as the difference between the final and the initial thickness.
Statistical analysis
The data are expressed as the mean ± SEM and were analyzed using one-way ANOVA. Statistical significance was determined by one-way ANOVA followed by Tukey's post hoc test. A p value of less than 0.05 was considered statistically significant.
Results
Effect of JEE on production of NO/PGE2 and expression of iNOS/COX-2
The cytotoxicity of JEE in RAW 264.7 cells was examined using the MTS assay and the cell viability was not affected by treatment with JEE up to a concentration of 50 μg/mL for 24 h (data not shown). Accumulation of nitrite in the culture media was measured for the investigation of the inhibitory effect of JEE on NO production. Stimulation of cells with LPS resulted in a significant increase in nitrite levels compared with non-stimulated cells (). The increase in nitrite levels was dose dependently inhibited by JEE treatment (an IC50 value of 1.98 μg/mL). To determine whether the suppression of NO production by JEE was due to decreased iNOS protein expression, cells were stimulated with LPS in the presence or absence of JEE. The expression of iNOS protein was almost undetectable in unstimulated RAW 264.7 cells. However, iNOS expression was markedly increased with the exposure to LPS and JEE inhibited iNOS expression in LPS-stimulated cells.
Figure 1. Effect of JEE on LPS-stimulated NO/PGE2 production and iNOS/COX-2 expression in RAW 264.7. (A) The levels of nitrite and protein expression of iNOS were determined by Griess reagents and Western blotting, respectively. (B) PGE2 production and COX-2 protein expression were determined by EIA and Western blotting, respectively. The data presented are means ± SEM of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 were used to indicate significance compared with the LPS-stimulated value.
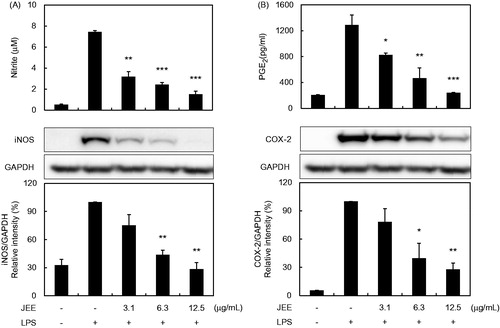
The effects of JEE on PGE2 production and COX-2 protein expression following LPS stimulation in RAW 264.7 cells were also analyzed. As shown in , the concentration of PGE2 in the culture supernatant showed a marked increase with LPS stimulation, and this increase was reduced by treatment with JEE (an IC50 value of 5.5 μg/mL). Because PGE2 is the resultant product of COX-2 enzyme activity, we performed Western blot analysis for the evaluation of COX-2 protein levels. The undetected levels of COX-2 in the absence of LPS were significantly up-regulated after LPS treatment and JEE caused a reduction in the LPS-stimulated expression of COX-2 protein.
Effect of JEE on production of pro-inflammatory cytokine
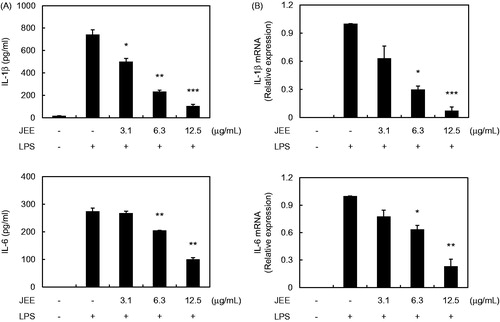
For further analysis of the anti-inflammatory effect of JEE, we measured the production and mRNA levels of IL-1β and IL-6 from LPS-stimulated RAW 264.7 cells using ELISA and real-time RT-PCR. As shown in , treatment of RAW 264.7 cells with LPS resulted in a significant increase in the production of IL-1 and IL-6 and pre-treatment with JEE resulted in dramatically reduced production of IL-1β (an IC50 value of 4.74 μg/mL) and IL-6 (an IC50 value of 20.48 μg/mL) in a dose-dependent manner compared with the supernatant of LPS-stimulated cells. The inhibitory effects of JEE on mRNA expression showed a pattern similar to those on pro-inflammatory cytokine production (). The mRNA levels of IL-1β and IL-6 were up-regulated in LPS-stimulated cells; however, pre-treatment with JEE inhibited the increased mRNA levels, indicating that JEE prevented pro-inflammatory cytokine production through suppression of gene expression in LPS-stimulated cells.
Figure 2. Effect of JEE on LPS-stimulated production and mRNA expression of IL-1β and IL-6 in RAW 264.7 cells. (A) The production of IL-1β and IL-6 was determined by ELISA. (B) Total mRNA was prepared and transcription levels in the cells were detected by a real-time (RT)-PCR analysis. The data presented are means ± SEM of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 were used to indicate significance compared with the LPS with the LPS-stimulated value.
Inhibition of JEE on activation and translocation of NF-κB
The nuclear translocation of NF-κB transcription factor is preceded by the degradation of IκBα translocation bound to NF-κB, thus preventing the nuclear localization by inhibiting IκBα degradation. Therefore, we examined the effects of JEE on LPS-stimulated degradation of IκB. As shown in , the level of IκB protein in the cytoplasm decreased by LPS treatment indicating IκBα degradation. However, the decreased level of IκB protein in the cytoplasm was markedly increased by JEE treatment in a dose-dependent manner, implying the JEE prevents NF-κB activation.
Figure 3. Effect of JEE on LPS-stimulated phosphorylation of IκBα and NF-κB in RAW 264.7 cells. (A) Cell lysates were analyzed by Western blotting analysis using specific antibodies against p-IκBα, IκBα, and NF-κB. (B) To confirm the effect of JEE on NF-κB activation, the binding activity of NF-κB to DNA was determined in nuclear extract by Trans-AM p65 assay. *p < 0.05, **p < 0.01, and ***p < 0.001 were used to indicate significance compared with the LPS with the LPS-stimulated value.
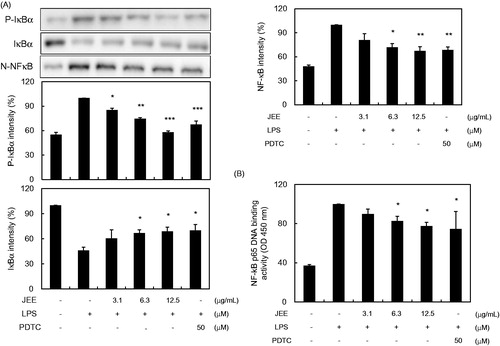
We further examined the effect of JEE on NF-κB activation and translocation of NF-κB p65 from the cytosol to the nucleus using Western blot analysis. As shown in , LPS stimulation caused translocation of p65 from the cytosol to the nucleus, while treatment with JEE or PDTC (a NF-κB pathway inhibitor) reduced this translocation (). A similar inhibition of nuclear translocation of NF-κB by JEE or PDTC was further confirmed by Trans-AM assay (), suggesting that JEE inhibits NF-κB activity.
Suppression of JEE on MAP kinase activation
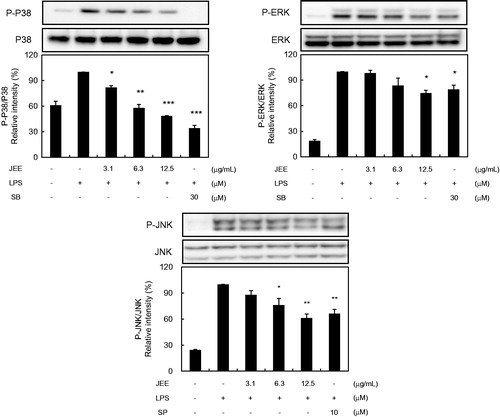
To determine whether the inhibitions of inflammatory responses by JEE are mediated through the MAP kinase pathway, we examined the effect of JEE on LPS-induced phosphorylations of MAP kinases in RAW 264.7 cells by Western blotting. LPS induced significant phosphorylations of p38, JNK, and ERK MAP kinases, and pre-treatment with JEE resulted in the suppression of LPS-induced phosphorylation of all three MAP kinases (). The application of SB203580 (a p38 inhibitor), SP600125 (a JNK inhibitor), and PD98059 (an ERK inhibitor) inhibited the phosphorylations of MAP kinases.
Figure 4. Effect of JEE on LPS-stimulated activation of MAP kinases in RAW 264.7 Cells. Cell lysates were prepared and analyzed by Western blotting using antibodies against p-ERK, p-JNK p-38, ERK, JNK, and p38. The data presented are means ± SEM of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 were used to indicate significance compared with the LPS-stimulated value.
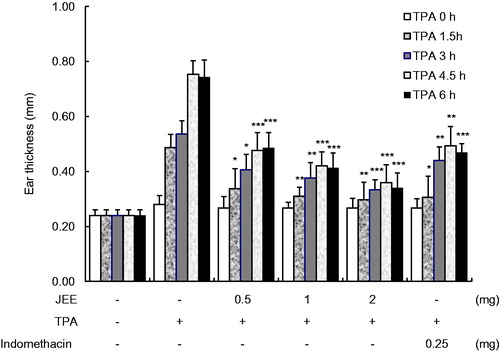
Effect of JEE on TPA-induced ear edema
To evaluate the anti-inflammatory activity of JEE in in vivo, a TPA-induced mouse ear model was used. Each side of the right ear was stimulated with TPA to induce inflammation and JEE was applied to examine its effect on acute inflammation in mice. As shown in , ear edema was maximum at 4.5 h after TPA application and JEE significantly reduced TPA-induced ear edema at a dose- and time-dependent manner. When compared with indomethacine, a reference drug, JEE, showed a stronger effect in a dose of 1 and 2 mg in attenuating ear edema of mice. These results confirmed the anti-inflammatory effect of JEE on a topical inflammation in vivo.
Figure 5. Effect of JEE on TPA-induced ear edema of mice. JEE and indomethacin were applied topically immediately after TPA treatment, and ear thickness measured at before and 1.5, 3, 4.5, and 6 h intervals. Each value represents as means ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 were used to indicate significance compared with control.
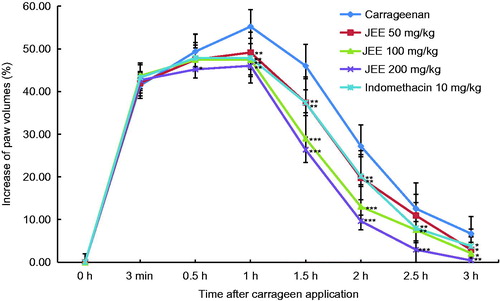
Effect of JEE on carrageenan-induced paw edema
We examined the effect of JEE on acute inflammation in an animal model using carrageenan-induced mouse paw edema. As shown in , paw edema was obvious at 3 min right after carrageenan exposure, maximum at 1 h, and then back to normal condition at 3 h. Oral administration of JEE significantly reduced paw edema in a dose- and a time-dependent manner compared with the control group. Indomethacin (10 mg/kg) also inhibited the paw edema.
Figure 6. Effect of JEE on carrageenan-induced paw edema of mice. JEE and indomethacin were administered orally 30 min before carrageenan injection into the left hind paw, and paw volume measured at 3 min, 0.5, 1, 1.5, 2, 2.5, and 3 h intervals after carrageenan injection. Each value represents as means ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 were used to indicate significance compared with control.
Discussion
The increased interest in herbal medicine for the treatment of various inflammatory disorders has drawn attention to their potential safety and efficacy. In the search of natural plants for anti-inflammatory activities, we found that JEE was very effective in suppressing LPS-induced production of NO/iNOS and PGE2/COX-2 by inhibiting activation of the NF-κB and MAP kinase signaling pathways. In the animal model of acute inflammation, JEE was also effective in reducing ear and paw edema of mice.
Excessive amounts of NO and PGE2 have been implicated in the pathology of a variety of inflammatory disorders. Since modulation of NO and PGE2 releases is the major factor during the inflammatory process, we first examined the effects of JEE on the production of NO and PGE2. LPS stimulation resulted in a marked induction of NO and PGE2 production when compared that in the untreated cells. However, pre-treatment with JEE resulted in markedly decreased the production of NO and PGE2 in LPS-stimulated RAW 264.7 cells (). To search for the underlying mechanism of these inhibitory effects of JEE on NO and PGE2 production, we examined its effect on protein expression levels of iNOS and COX-2 and found that there was reduced protein expression, thereby suggesting that the reduced production of NO and PGE2 might be attributable to inhibition of iNOS and COX-2 expression.
Pro-inflammatory cytokines play a critical role in the inflammatory response to pathologic stimuli. LPS or pro-inflammatory cytokines caused a significant increase in NO and PGE2 production. Therefore, reduced iNOS synthesis can lead to decreased LPS-stimulated pro-inflammatory cytokine production in macrophages and the expression of pro-inflammatory cytokines is dependent on COX-2 as well as iNOS in inflammation (Wu et al., Citation2003; Yang et al., Citation2012). In this study, pre-treatment with JEE resulted in a decrease in cytokine production and mRNA levels, suggesting that JEE caused a decrease in the release of these cytokines by suppressing their mRNA expression ().
NF-κB is an important transcription factor regulating the expression of inflammatory mediators, including iNOS, COX-2, IL-1β, and IL-6 (Hambleton et al., Citation1996). In resting cells, NF-κB exists in an inactive form associated with IκB. Activation by stimuli including LPS induces a cascade of events leading to phosphorylation of IκB and its degradation; NF-κB translocates into the nucleus and binds DNA-binding sites in regulatory regions of target genes (Lappas et al., Citation2002; Surh et al., Citation2001). Therefore, NF-κB-targeted therapeutics might be effective in treating inflammatory diseases since a variety of pharmacologic agents have been reported to inhibit one or more activation steps in the signaling pathway (Zingarelli et al., Citation2003). In the current study, we demonstrated that JEE suppresses the phosphorylation and the degradation of IκBα and translocation of NF-κB (). Taken together, the reduced production of inflammatory mediators by JFF might act through its interference with the NF-κB signaling pathway.
The expression of inflammatory mediators is also controlled by the intracellular signaling pathways, MAP kinases (Uto et al., Citation2010). MAP kinases not only play an important role in iNOS and COX-2 expression but are also involved in regulation of pro-inflammatory cytokine production (Johnson & Lapadat, Citation2002; Medzhitov & Horng, Citation2009). In addition, several studies have shown that MAPKs play a critical role in the activation of NF-κB (Chan & Riches, Citation2001). Since JEE suppressed NF-κB activation, we investigated the question of whether the MAP kinase pathway was involved in attenuation of inflammatory mediators. Our study showed that JEE was able to attenuate LPS-stimulated activation of MAP kinases in RAW 246.7 cells (). Based on our finding, we speculate that the anti-inflammatory effect of JEE is due at least in part to attenuation of NF-κB and MAP kinase activation.
Animal experiments confirmed the anti-inflammatory effect of JEE in in vivo. Since TPA-induced ear edema and carrageenan-induced paw edema are well-established models (Yonathan et al., Citation2006) for screening the anti-inflammatory drugs in vivo, we used these models in this study. As shown in and , topical application and oral administration of JEE effectively attenuated acute inflammation on ear and paw edema of mice.
Phytosterols including β-sistosterol, campersterol, stigmasterol, and fucosterol found in seeds, roots, and branches of various plants have been reported to exert anti-inflammatory activity through the inhibition of inflammatory mediators by NF-κB and MAP kinases (Bak et al., Citation2012; Chao et al., Citation2010; Othman & Moghadasian, Citation2011; Yoo et al., Citation2012). In our study, only one compound, stigmasterol, has been identified from JEE so far (data not shown). Although stigmasterol might be an active component, other compounds from JEE may have anti-inflammatory activities. Therefore, we are currently isolating other compounds from JEE to identify active compounds.
Conclusion
The results of our present study provide the first evidence that JEE inhibited production of inflammatory mediators including NO, PGE2, and pro-inflammatory cytokines in LPS-stimulated RAW 264.7 cells. These inhibitory effects are attributable to the prevention of phosphorylation and the degradation of IκBα, thereby suppressing NF-κB activation and the MAP kinase signaling pathways. In acute inflammation of mouse edema models, JEE reduced the development of TPA-induced ear edema and carrageenan-induced paw edema. Therefore, these findings suggest that JEE might be a potential anti-inflammatory agent.
Declaration of interest
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ009067), Rural Development Administration, Republic of Korea.
References
- Bak MJ, Hong SG, Lee JW, Jeong WS. (2012). Red ginseng marc oil inhibits iNOS and COX-2 via NFkappaB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules 17:13769–86
- Behery FA, Naeem ZE, Maatooq GT, et al. (2007). Phenanthrenoids from Juncus acutus L., new natural lipopolysaccharide-inducible nitric oxide synthase inhibitors. Chem Pharm Bull (Tokyo) 55:1264–6
- Carlson RP, O'Neill-Davis L, Chang J, Lewis AJ. (1985). Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions 17:197–204
- Chan ED, Riches DW. (2001). IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am J Physiol Cell Physiol 280:C441–50
- Chao WW, Kuo YH, Lin BF. (2010). Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-kappaB transactivation inhibition. J Agric Food Chem 58:2505–12
- De Young LM, Kheifets JB, Ballaron SJ, Young JM. (1989). Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26:335–41
- Ghosh S, Hayden MS. (2008). New regulators of NF-kappaB in inflammation. Nat Rev Immunol 8:837–48
- Guha M, Mackman N. (2001). LPS induction of gene expression in human monocytes. Cell Signal 13:85–94
- Hambleton J, Weinstein SL, Lem L, DeFranco AL. (1996). Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA 93:2774–8
- Hanawa F, Okamoto M, Towers GH. (2002). Antimicrobial DNA-binding photosensitizers from the common rush, Juncus effusus. Photochem Photobiol 76:51–6
- Handy RL, Moore PK. (1998). A comparison of the effects of L-NAME, 7-NI and L-NIL on carrageenan-induced hindpaw oedema and NOS activity. Br J Pharmacol 123:1119–26
- Johnson GL, Lapadat R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–12
- Lappas M, Permezel M, Georgiou HM, Rice GE. (2002). Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod 67:668–73
- Liao YJ, Zhai HF, Zhang B, et al. (2011). Anxiolytic and sedative effects of dehydroeffusol from Juncus effusus in mice. Planta Med 77:416–20
- Lu YC, Yeh WC, Ohashi PS. (2008). LPS/TLR4 signal transduction pathway. Cytokine 42:145–51
- Medzhitov R, Horng T. (2009). Transcriptional control of the inflammatory response. Nat Rev Immunol 9:692–703
- Othman RA, Moghadasian MH. (2011). Beyond cholesterol-lowering effects of plant sterols: Clinical and experimental evidence of anti-inflammatory properties. Nutr Rev 69:371–82
- Park HH, Kim MJ, Li Y, et al. (2013). Britanin suppresses LPS-induced nitric oxide, PGE2 and cytokine production via NF-kappaB and MAPK inactivation in RAW 264.7 cells. Int Immunopharmacol 15:296–302
- Ritchlin CT, Haas-Smith SA, Li P, et al. (2003). Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest 111:821–31
- Singhuber J, Baburin I, Khom S, et al. (2012). GABA(A) receptor modulators from the Chinese herbal drug Junci Medulla – The pith of Juncus effusus. Planta Med 78:455–8
- Surh YJ, Chun KS, Cha HH, et al. (2001). Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res 480–481:243–68
- Uto T, Suangkaew N, Morinaga O, et al. (2010). Eriobotryae folium extract suppresses LPS-induced iNOS and COX-2 expression by inhibition of NF-kappaB and MAPK activation in murine macrophages. Am J Chin Med 38:985–94
- Wu CH, Chen TL, Chen TG, et al. (2003). Nitric oxide modulates pro- and anti-inflammatory cytokines in lipopolysaccharide-activated macrophages. J Trauma 55:540–5
- Yang YI, Shin HC, Kim SH, et al. (2012). 6,6′-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE production and inflammatory cytokine expression in macrophages: The inhibition of NFkappaB. Int Immunopharmacol 12:510–17
- Yonathan M, Asres K, Assefa A, Bucar F. (2006). In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J Ethnopharmacol 108:462–70
- Yoo MS, Shin JS, Choi HE, et al. (2012). Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-kappaB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem 135:967–75
- Zhou HY, Shin EM, Guo LY, et al. (2008). Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK inactivation. Eur J Pharmacol 586:340–9
- Zingarelli B, Sheehan M, Wong HR. (2003). Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med 31:S105–11
