Abstract
Context: Tobacco smoking generates a tremendous amount of free radicals that induce oxidative stress (OS) in diabetics (pancreatic islet cells are defective). Salacia oblonga Wall. (Celastraceae) is a proven antioxidant and antidiabetic plant whose mechanism of action is yet to be explored.
Objective: The present study focuses on the protective ability of S. oblonga in tobacco smoke-induced oxidatively stressed pancreatic β-cell line.
Materials and methods: The RINm5f cell line was exposed to tobacco smoke concentrate (TSC) (0.5–10%, 24 h), plant extract (1–75 µg/ml, 3 h), and their combinations. Cell viability was determined through MTT assay. Microscopic analysis was carried out in unstained and nonyl acridine orange-stained cells. The effect of toxic doses of TSC on DNA integrity was analyzed through DNA fragmentation assay. The TSC-induced nitric oxide generation was determined spectrophototmetrically. The expression of anti-apoptotic protein Bcl-X under the above treatment conditions was carried out through RT-PCR.
Results: The LD50 dose for TSC was found to be 1% TSC. Salacia oblonga extracts (10 and 15 µg/ml) were found to be optimum safe doses that significantly increased cell viability and decreased the nitric oxide production in TSC-treated cells. Pre-treatment with plant extract suppressed apoptosis through probable increase in the expression of anti-apoptotic protein Bcl-X in TSC-treated cells. Thus, the overall efficiency of plant extract in recovering cellular damage was proven.
Discussion and conclusion: The results suggest that TSC-induced cellular alterations are related to rise in nitric oxide and Bcl-X mRNA expression and propose that S. oblonga may confer significant cytoprotection against OS-mediated injury in β-cells.
Introduction
The interplay between free radicals and antioxidants in normal and diseased state through generation of oxidative stress (OS) is quite an old area of research. One of the major causes for an increase in OS is tobacco smoke. The latter contains thousands of harmful chemicals including active ingredients like nitrosamines which cause OS in cells due to excess formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). This induces cell death in various cell types including β-cells, which are found to be more vulnerable to OS (Ramkumar et al., Citation2009). β-Cell death has been reported to play an important role in the pathogenesis of diabetes mellitus (Ramkumar et al., Citation2009; Targher et al., Citation1997) and, tobacco smoke may further deteriorate the condition.
In vivo studies have reported that cigarette smoke exposure induces morphological alterations in the pancreas due to various toxic organic and inorganic compounds present in it (Wittel & Hopt, Citation2008). This damage may extent to mitochondria, an organelle that plays a significant role during glucose metabolism and insulin secretion (Rolo & Palmeira, Citation2006). OS might target the mitochondrial genetic makeup and activate other pathways involved in the pathogenesis of diabetic complications. Among various free radicals involved in the generation of OS, nitric oxide (NO) has emerged as a fundamental signaling molecule regulating several essential cellular functions (Jagetia et al., Citation2004). NO is a small hydrophobic molecule that crosses cell membranes without channels or receptors. When both superoxide and NO are synthesized within a few cell diameters of each other, they interact spontaneously to form peroxynitrite. Under pro-inflammatory conditions, simultaneous production of NO and superoxide can increase to 1000-fold, which further increases the peroxynitrite production by 1 000 000-fold and alters DNA integrity (Pacher et al., Citation2007). Moreover, several scientists have reported that the NF-κB-dependent NO production can lead to dysfunction and destruction of β-cells (Song et al., Citation2010). A common pathway involving the mitochondria and outer mitochondrial membrane permeability (OMMP) might be responsible in this peroxynitrite-mediated apoptosis. OMMP might be triggered by pore formation in it by pro-apoptotic proteins like Bax, which is inhibited by anti-apoptotic Bcl-2 (located predominantly in the OMM) and Bcl-X proteins (Pacher et al., Citation2007). Besides the OMMP, the phospholipid Cardiolipin serves as a proton reservoir in the mitochondrial membranes in coordination with oxidative phosphorylation complexes (Mileykovskaya & Dowhan, Citation2009).
Treatment of a diabetic patient with numerous oral diabetes medicines carries a long list of long-term side effects and high expenditure (Anonymous, Citation2009). Whereas several Ayurvedic drugs and formulations composed of anti-diabetic plants or their active constituents have been reported for their protective action on β-cells (Ramkumar et al., Citation2009). Salacia oblonga Wall. (Celastraceae) (SO) is a well-known anti-diabetic herb found mainly in Southern India and Sri Lanka. It has been used as a nutritional adjunct, either as a tea or as a supplement with meals as an antidiabetic for ages (Williams et al., Citation2007). The root bark of this plant is pale yellow and light brown in color and is used as decoction or as powder in the treatment of diabetes and other diseases such as rheumatism, gonorrhea, asthma, and ear diseases (Ismail et al., Citation1997). SO is also reported to possess good antioxidant and anti-inflammatory activities (Ismail et al., Citation1997; Krishnakumar et al., Citation1999). Damage to proteins, nucleic acids, and other biomolecules due to uncontrolled ROS production and lipid peroxidation could be prevented by plant-derived antioxidants such as tannins, flavonoids, and phenolics. They behave as hydrogen donors, reducing agents, and free radical scavengers (Jha et al., Citation2010). The root barks of SO have been demonstrated to have 60–70% hypoglycemic potency of an equal dose of tolbutamide in streptozotocin-induced albino rats (Krishnakumar et al., Citation1999). In the present study, we have investigated the possible cytoprotective effects of S. oblonga extract (SOE) on oxidative stress induced by tobacco smoke concentrate (TSC) in pancreatic β-cells. Also, this study attempts to determine if tobacco smoke can bring about β-cell apoptosis, along with an alteration in Bcl-X gene expression. An approach towards a possible coordinated signaling among RNS, NO, and anti-apoptotic proteins in the above treatment conditions in oxidative-stressed RINm5f cells is initiated.
Materials and methods
Chemicals required
RPMI-1640 medium, fetal bovine serum (FBS), and nonyl acridine orange (NAO) were purchased from Sigma Aldrich, Bengaluru, India. Trypsin-EDTA, 3-4,5-dimethylthiazolyl-2-2,5-diphenyltetrazolium bromide (MTT) were purchased from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Phenol, isoamylalcohol, chloroform, sodium dodecyl sulfate (SDS), sodium nitrite, and Griess reagent were purchased from Central Drug House Pvt. Ltd., New Delhi, India.
Plant material
The S. oblonga extract was purchased from Natural Remedies Private Limited, Bangalore, India, and was verified through purity test analysis by the company, before delivery. It contains a mixture of roots and stems of SO that were extracted with methanol and water (10:1) and obtained as a brown powder.
Cell line and culture conditions
The RINm5f cell line (rat pancreatic β-cell line) was purchased from National Centre for Cell Science, Pune, India. It was cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), streptomycin (100 μg/ml), and penicillin (100 units/ml) at 37 °C in a humidified CO2 incubator. Confluent monolayers of RINm5f cells were trypsinized, centrifuged at 1200 rpm for 5 min, and the pellets were resuspended in the RPMI-1640 medium for use in all the assays.
TSC preparation
The market-purchased bidi rolls were used to prepare TSC by passing the complete vapor phase of bidi smoke obtained from complete combustion of three bidis over 3 ml of incomplete RPMI-1640 medium in a compact set-up. The concentrate (considered as 100% TSC) was made fresh for every experiment.
Effect of TSC on the viability of RINm5f cells
The cell viability was assessed by MTT assay using a microplate reader, as carried out in our earlier studies (Basu et al., Citation2013b). Briefly, 5 × 104 RINm5f cells were seeded per well (Dypbukt et al., Citation1994) in a 96-well cell culture plate and cultured overnight at 37 °C in humidified CO2 incubator, prior to treatment. The cells were exposed to 0.5–10% TSC (24 h) to determine its toxic dose causing 50% cell death. The viability of the cells was determined in terms of absorbance of the test solution on a multi-well scanning spectrophotometer (ELISA Reader, Haryana, India) at 570 nm, in comparison with the control.
Effect of SOE on TSC-induced cytotoxicity in RINm5f cells
To determine the safe dose of SOE, the cells were seeded and cultured as mentioned above. After 24 h of seeding, cells were treated with 1–75 μg/ml SOE (3 h) followed by MTT assay. Further, the cytoprotective effect of SOE in TSC-induced toxicity in RINm5f cells was determined through pre-treatment of cells with related doses followed by TSC exposure, and the experiment was done in triplicates.
DNA fragmentation analysis
Cells (3 × 104) were cultured overnight and then incubated with SOE, TSC, and their combinations as mentioned above, followed by harvesting and centrifugation at 1300 rpm for 5 min. DNA was isolated from these samples as per standard protocol (Sambrook & Russell, Citation2001) and electrophoresed on agarose gel (1%) containing ethidium bromide, for 2 h at 50 V. DNA was visualized by UV illumination and photographed in GelDoc apparatus (BioRad Laboratories, Haryana, India).
Griess nitrite assay
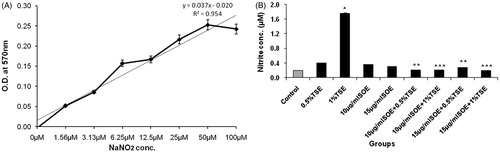
The production of NO in different wells under various treatment conditions was estimated spectrophotometrically as nitrite concentration in cell-free culture supernatants as per standard protocol (Lee et al., Citation2012). Briefly, 10 μl of culture medium was taken from each sample and incubated with 50 μl Griess reagent {1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride} at room temperature for 10 min. The absorbance of the pink-colored chromophore, formed by diazotization of nitrite with sulfanilamide and subsequent coupling with naphthylethylenediamine, was measured at 550 nm. The nitrite content was calculated based on a standard curve of sodium nitrite (NaNO2).
Morphological examination of SOE and TSC-treated RINm5f cells
On exposure of RINm5f cells to 15–20 μg/ml SOE (3 h) and 0.5–1% TSC (24 h), individually and in combination, the cell morphology was examined under an inverted microscope (20× magnification). Microphotographs were taken using a digital camera (Sanyo Electric Co. Ltd., New Delhi, India).
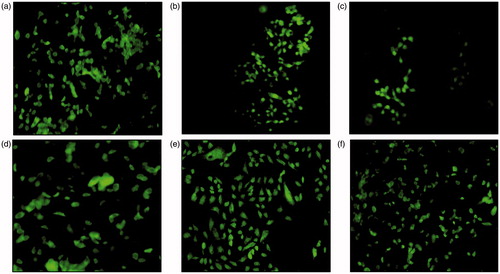
Analysis of mitochondrial membrane integrity through NAO-bound cardiolipin in RINm5f cells
Cardiolipin is localized at contact sites between the mitochondrial inner and outer membrane and the lipophilic cationic probe nonyl-acridine orange (NAO) forms dimers with it. This generates bright green fluorescence that was observed through fluorescence microscope (Olympus, Indore, India) after excitation at 490 nm.
Semi-quantitative reverse-transcribed polymerase chain reaction (PCR) analysis
The expression of the anti-apoptotic gene Bcl-X in various treatment groups was assessed by semi-quantitative polymerase chain reaction (PCR) by the standard method (Sigfrid et al., Citation2003). In brief, the total RNA was isolated from the respective cell pellets by RNA extraction kit of Agilent Technologies, Santa Clara, CA, and stored at −80 °C. RNA was quantified by the 260/280 ratio, and finally run on agarose gel. cDNA was prepared using the high-yield Applied Biosystems kit (Applied Biosystems, Waltham, MA). cDNA was also quantified and further used for PCR analysis. The sequence for forward and backward primers used for Bcl-X was 5′-TTGGACAATGGACTGGTTGA-3′ and 5′-GTAGAGTGGATGGTCAGTG-3′ (Sigma Aldrich, Bengaluru, India).
Statistical analysis
All the data are expressed as mean ± standard deviation (SD) of the number of experiments (n = 3) and tested using one-way ANOVA. The values were considered significant at p values < 0.01.
Results
Effect of SOE and TSC on cell viability of RINm5f cells
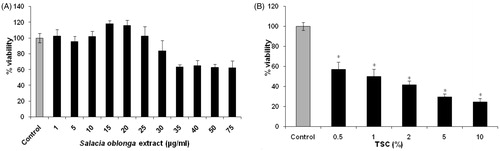
On exposure of RINm5f cells to SOE (1–75 μg/ml, 3 h; ), 10–20 μg/ml SOE increased the cell viability to ≥100%. Further increase in dose decreased the cell viability to almost 60%. Hence, 10 and 15 μg/ml were selected for further studies. However, exposure of cells to 0.5–10% TSC () for 24 h decreased the cell viability to almost 25% in comparison with control. TSC of 0.5% and 1% led to almost 50% cell death and all further studies were performed using these TSC concentrations.
Figure 1. Effect of SOE (A) and TSC (B) on the viability of RINm5f cells. The cells were treated with SOE (1–75 μg/ml, 3 h) and TSC (0.5–10%, 24 h) and the cell viability was assessed through MTT assay. The results were expressed as percentage of the control value from the untreated group. Data were presented as mean ± SD of three experiments (*p < 0.01 versus control).
Effect of pre-treatment with SOE on TSC-induced cytotoxicity in RINm5f cells
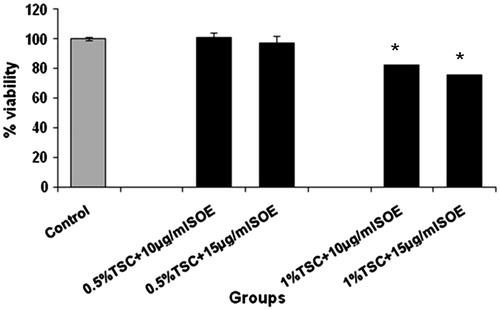
Pre-exposure of cells to 10 and 15 μg/ml SOE () provided significant protection against TSC-induced cytotoxicity, thereby maintaining the cell viability (∼100%).
Figure 2. Effect of SOE on TSC-induced cytotoxicity in RINm5f cells. The cells were pre-treated with SOE (10 and 15 μg/ml) followed by exposure to TSC (0.5% and 1%). The cell viability was determined through MTT assay and the data were presented as mean ± SD of three independent experiments (*p < 0.01 versus 1% TSC).
DNA damage induced after exposure of RINm5f cells to tobacco smoke
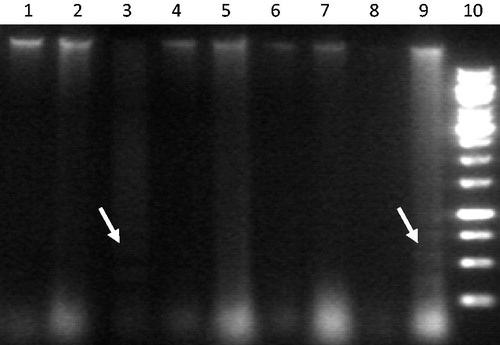
We further investigated whether the TSC-induced decrease in cell viability was due to apoptosis and if SOE (10 and 15 μg/ml) can prevent/overcome the DNA damage. Exposure of cells to 0.5% and 1% TSC caused cleavage of chromosomal DNA into internucleosomal fragments (indicated by laddering), which appeared as a smear on the agarose gel (). Cells exposed to SOE alone showed no DNA fragmentation. SOE pre-treatment effectively prevented as well as repaired the oligonucleosomal DNA fragmentation.
Figure 3. Agarose gel showing DNA fragmentation (white arrows). Lanes show DNA of cells under various treatment regime: in sequence from L to R (1: 10 μg/ml SOE, 2: 15 μg/ml SOE, 3: 0.5% TSE, 4: 10 μg/ml SOE + 0.5% TSE, 5: 15 μg/l SOE + 0.5% TSE, 6: Control, 7: 15 μg/ml SOE + 1% TSE, 8: 10 μg/ml SOE + 1% TSE, 9: 1% TSE, 10: 1 kb DNA ladder).
Morphological changes after exposure to SOE, TSC, and treatment combinations
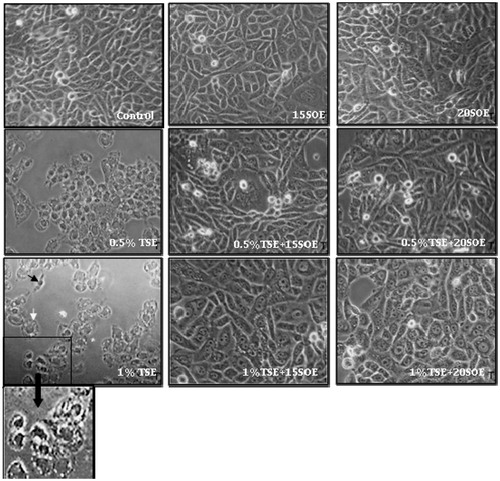
Morphological alterations were observed in 0.5% and 1% TSC-treated cells with almost 50% decrease in cell number (). The cells showed rounding up, cellular elongation and narrowing, condensed nuclei, chromatin margination, and cytoplasmic vacuolization. Cell membrane blebbing, intense vacuolization, loss of plasma membrane integrity, with a typical crescent-like appearance often, were observed on increasing TSC dose to 1%. These observations indicate a combined apoptotic morphology that coincides with our MTT assay and DNA fragmentation analysis data. The cells in the control group appear confluent, closely adhered to the substratum and with an epithelial morphology. Pre-treatment with SOE maintained the cell morphology as well as cell viability almost comparable to control.
Figure 4. Morphological analysis of RINm5f cells treated with TSC, SOE, and its various combinations. Cells were exposed to 0.5% and 1% TSC for 24 h, 15 and 20 μg/ml SOE for 3 h, and their combinations and observed under inverted microscope (20×). Black arrow indicates condensed nuclei and shrunken cytoplasm, and white arrow indicates intense cytoplasmic vacuolization.
Effect of TSC treatment on the NO level in RINm5f cells
To evaluate the molecular basis of TSC-induced oxidative stress and the anti-apoptotic effect of SOE in the cells, we determined the nitrite concentration from NaNO2 standard graph (). Incubation of RINm5f cells with 1% TSC resulted in significant increase in nitrite formation (). SOE pretreatment significantly decreased the TSC-induced nitrite formation and the effect was comparable with control. There were no significant differences in total nitrite levels between the pre-treatment and individual plant extract-treated groups.
Figure 5. Effect of SOE on nitrite generation in TSC-induced oxidatively stressed RINm5f cells. The nitrite generation was estimated spectrophotometrically by the Griess nitrite method. The values are represented as mean ± SD of three independent experiments (*p < 0.01 versus control; **p < 0.01 versus 0.5% TSC; ***p < 0.01 versus 1% TSC).
Effect of TSC-induced oxidative stress at the mitochondrial membrane level in RINm5f cells
In TSC-induced apoptotic condition, the protective effect of SOE on the mitochondrial membrane was assessed through staining of cells with the fluorescent dye NAO. TSC (0.5% and 1%)-treated cells showed highly fluorescent pyknotic nucleus () indicating probable accumulation of mitochondria in and around the condensed nucleus. The TSC-treated group showed smaller cell size and decreased cell number as compared with control. These responses could be effectively abrogated by pre-treatment with 15 μg/ml SOE. A slight difference in fluorescent intensity in indicates that SOE has been able to regain the mitochondrial membrane integrity through enhanced cardiolipin formation, thus overcoming the oxidative stress-induced damage in β-cells.
Role of anti-apoptotic protein in TSC-induced oxidatively stressed RINm5f cells
Treatment of RINm5f cells with 1% TSC revealed increased expression of anti-apoptotic protein Bcl-X on TSC exposure (). SOE exposure of 15 μg/ml increased the expression of protein in comparison with control, thus indicating the possible protective role of the plant extract against oxidative stress. It is surprising to find that there was no expression of Bcl-X mRNA in SOE-pre-treated group.
Discussion
During smoking, burning of tobacco yields a mixture of side- and mainstream smoke that differ in composition and adversely affects the oxidant-antioxidant balance in cells (Wittel & Hopt, Citation2008). It is a fact that β-cells are prone to ROS and RNS attacks due to their low antioxidant profile (Ramkumar et al., Citation2009). OS has been considered as a broad mediator of apoptosis (Ramkumar et al., Citation2009). There are several reports suggesting the beneficial effects of medicinal plants against this OS-induced damage to β-cells (Ramkumar et al., Citation2009). The present study analyzed the toxic effects of TSC-induced oxidative stress in RINm5f cell line.
In our experimental model, while investigating the mechanism of TSC-induced apoptosis in β-cell line and its reversal by the anti-diabetic plant S. oblonga, we analyzed apoptotic parameters like nuclear condensation and DNA fragmentation. Any alteration in cellular stress responses (monitored by mitochondria) play an important role in the initiation of apoptosis wherein multiple pro-apoptotic signals are integrated by this organelle into common apoptotic degradation cascades (Ziegler & Groscurth, Citation2004). The pancreatic β-cell apoptosis is reported to be initiated by two main pathways: the extrinsic or death receptor and the intrinsic or mitochondrial apoptosis pathway (Mehmeti et al., Citation2010). Bcl-X, an important member of the Bcl-2 family, is located in the OMM and regulates cell death. Studies on mice have revealed that the presence of Bcl-XL in β-cells promotes islet cell survival during exposure of cells to various death stimuli (Carrington et al., Citation2009). Hence, we measured cellular changes occurring immediately after various modes of treatment through nitrite level and Bcl-X gene expression. High amount of ROS generated by TSC can provide enough superoxide () and hydrogen peroxide to react with nitrite thus forming nitrate and peroxynitrite. NO exerts inhibitory effects on mitochondrial respiration and brings about several molecular changes with regard to cell survival. Our results showed that TSC-induced NO generation increased to a significant level in β-cell line thereby causing apoptotic changes, as revealed through increased expression of anti-apoptotic protein Bcl-X. Altered cellular morphology coincided with decrease in cell viability. Besides these, SOE-treated cells showed an increased expression of anti-apoptotic protein which can be attributed to the presence of considerable amount of polyphenols in the extract (Basu et al., Citation2013a).
Further in our experimental set up, 0.5% and 1% TSC-treated cells showed a high proportion of mitochondria being concentrated over the nucleus, having lost its distinct cytoplasmic distribution as in control cells. Besides this, in the pre-treatment group, the methanolic-aqueous extract of S. oblonga regained the cell viability by suppressing the Bcl-X expression, indicating that the instinct anti-apoptotic effects of SOE could be achieved through probable regulation of OMM apoptotic factors. Thus, our study shows that the methanolic-aqueous extract of S. oblonga can directly regulate the β-cell biochemical processes in TSC-induced oxidatively stressed condition. This study proves that TSC induces apoptosis in RINm5f cells and the findings of this study may help in further elucidation of the mechanism of TSC-induced β-cell injury at the mitochondrial level.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Anonymous. (2009). Consumer reports health.org, Best buy drugs: Treating type 2 diabetes: The oral diabetes drugs – comparing effectiveness, safety and price. February, 1–27
- Basu S, Pant M, Rachana R. (2013a). Phytochemical evaluation and HPTLC profiling of extracts of Salacia oblonga. Int J Pharm Sci Res 4:1409–18
- Basu S, Pant M, Rachana R. (2013b). Beneficial effects of Salacia oblonga on mitochondrial localization in cells and NADPH oxidase activity in glucose induced cytotoxicity on rat muscle cell line. Int J Biotechnol Bioeng Res 4:321–8
- Carrington EM, McKenzie MD, Jansen E, et al. (2009). Islet β-cells deficient in Bcl-xL develop but are abnormally sensitive to apoptotic stimuli. Diabetes 58:2316–23
- Dypbukt JM, Ankarcrona M, Burkitt M, et al. (1994). Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5f cells, the role of intracellular polyamines. J Biol Chem 269:30553–60
- Ismail TS, Gopalakrishnan S, Begum VH, Elango V. (1997). Anti-inflammatory activity of Salacia oblonga Wall. and Azima tetracantha Lam. J Ethnopharmacol 56:145–52
- Jagetia GC, Rao SK, Baliga MS, Babu KS. (2004). The evaluation of nitric oxide scavenging activity of certain herbal formulations in vitro: A preliminary study. Phytother Res 18:561–5
- Jha MK, Alam MB, Hossain MS, Islam A. (2010). In vitro antioxidant and cytotoxic potential of Costus speciosus (koen.) Smith rhizome. Int J Pharm Sci Res 1:138–44
- Krishnakumar K, Augusti KT, Vijayammal PL. (1999). Hypoglycaemic and anti-oxidant activity of Salacia oblonga Wall. extract in streptozotocin induced diabetic rats. Indian J Physiol Pharmacol 43:510–14
- Lee S, Choi J, Heo S, et al. (2012). Diphlorethohydroxycarmalol isolated from Pae (Ishige okamurae) protects high glucose-induced damage in RINm5F pancreatic β cells via its antioxidant effects. Food Sci Biotechnol 21:239–46
- Mehmeti I, Lenzen S, Lortz S. (2010). Modulation of Bcl-2-related protein expression in pancreatic beta cells by pro-inflammatory cytokines and its dependence on the antioxidative defense status. Mol Cell Endocrinol 332:88–96
- Mileykovskaya E, Dowhan W. (2009). Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta 1788:2084–91
- Pacher P, Beckman JS, Liaudet L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424
- Ramkumar KM, Lee AS, Krishnamurthi K, et al. (2009). Gymnema montanum H. protects against alloxan induced oxidative stress and apoptosis in pancreatic β-cells. Cell Physiol Biochem 24:429–40
- Rolo AP, Palmeira CM. (2006). Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol 212:167–78
- Sambrook J, Russell I. (2001). Molecular Cloning, A Laboratory Manual, 3rd ed., Vol. 1. New York: Cold Spring Harbor Laboratory Press
- Sigfrid LA, Cunningham JM, Beeharry N, et al. (2003). Cytokines and nitric oxide inhibit the enzyme activity of catalase but not its protein or mRNA expression in insulin-producing cells. J Mol Endocrinol 31:509–18
- Song MY, Bae UJ, Lee BH, et al. (2010). Nardostachys jatamansi extract protects against cytokine induced β-cell damage and streptozotocin-induced diabetes. World J Gastroenterol 16:3249–57
- Targher G, Alberiche M, Zenere MB, et al. (1997). Cigarette smoking and insulin resistance in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 82:3619–24
- Williams JA, Choe YS, Noss MJ, et al. (2007). Extract of Salacia oblonga lowers acute glycemia in patients with type 2 diabetes. Am J Clin Nutr 86:124–30
- Wittel UA, Hopt UT. (2008). Cigarette smoke-induced pancreatic damage-experimental data. Langenbecks Arch Surg 393:581–8
- Ziegler U, Groscurth P. (2004). Morphological features of cell death. News Physiol Sci 19:124–8

![Figure 7. TSC-induced mitochondrial dysfunction through mitochondrial anti-apoptotic protein Bcl-X in RINm5f cells [the expression of Bcl-X gene was analyzed through semi-quantitative PCR in following groups: control, 1% TSC treatment (24 h), 15 μg/ml SOE (3 h), and a combination of TSC and SOE].](/cms/asset/1b6ab934-31bd-4b17-8521-798d83c9d397/iphb_a_1046083_f0007_b.jpg)