Abstract
Context: Plants of the genus Cistanche Hoffmg. et Link (Orobanchaceae) are usually used as ethno-medicine in Eastern Asia. Pharmacology studies have shown that Cistanche possesses an androgen-like effect; however, the exact mechanism is unclear.
Objective: The present study determines the effect of ethanol extract of Cistanche tubulosa (Schenk) R. Wight stem (CTE) on hormone levels and testicular steroidogenic enzymes in rats.
Materials and methods: Phenylethanoid glycoside content of CTE was detected by UV spectrophotometry. Rats were fed with different doses of CTE (0.2, 0.4, and 0.8 g/kg) by intragastric administration for 20 d. Sperm parameters were measured by staining and counting method. The level of progesterone and testosterone in serum was quantified by radioimmunoassay. The expression levels of cholesterol side-chain cleavage enzyme (CYP11A1), 17α-hydroxylase/17, 20-lyase (CYP17A1), and a liver metabolic enzyme (CYP3A4) in the microsome were assessed by immunohistochemical staining or/and western blot analysis.
Results: The study illustrates that the administration of CTE (0.4 and 0.8 g/kg) increased sperm count (2.3- and 2.7-folds) and sperm motility (1.3- and 1.4-folds) and decreased the abnormal sperm (0.76- and 0.6-folds). The serum level of progesterone and testosterone in rats was also increased by CTE administration (p < 0.05). Results of immunohistochemistry and western blot analysis confirmed that the expression of CYP11A1, CYP17A1, and CYP3A4 was enhanced by CTE (p < 0.05). It was also found that high-dose of CTE can cause mild hepatic edema.
Conclusion: Our results suggest that the increase in sex hormone levels could be mediated by the induction of testicular steroidogenic enzymes.
Introduction
Cistanche Hoffmg. et Link (Orobanchaceae) is a genus of medicinal plant that has been used in China and other eastern Asian countries since the fifteenth century for the treatment of disease conditions (in traditional Chinese medicine theory) such as impotence, seminal emission, infertility, general weakness with lassitude of the loins and knees, and chronic constipation (Wang et al., Citation2012). C. deserticola Y. C. Ma and Cistanche tubulosa (Schenk) R. Wight have similar chemical constituents and pharmacological activities, and they have been documented in the Chinese Pharmacopoeia (Editorial Committee of Chinese Pharmacopoeia, Citation2010). The effect of C. deserticola in terms of increasing organ indices of the male reproductive system was evaluated in healthy rats (He et al., Citation1996a,Citationb). Testicle is the main tissue responsible for androgen secretion; the reproductive system of the castrated rats was improved well by C. deserticola administration (He et al., Citation1996a,Citationb). The above findings indicate that C. deserticola may contribute for the development of male reproductive system through an androgen-like or sex hormone regulation effect (He et al., Citation1996a,Citationb). Previous studies have also indicated that C. deserticola can contribute for bone formation in osteoblasts (Li et al., Citation2012). Androgen is well known for its beneficial influence on skeletal development and maintenance in both women and men (Hofbauer & Khosla, Citation1999). Cistanche tubulosa is being considered as an alternative to C. deserticola, since it is on the verge of extinction due to over-exploitation for decades. Therefore, the effect of C. tubulosa on sex hormone levels and its safety evaluation are indispensable.
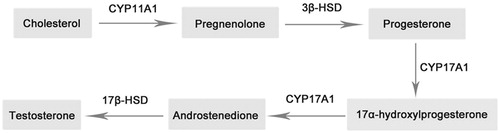
The biosynthesis of testosterone begins with cholesterol in the mitochondrial inner membrane, where steroidogenic enzyme cytochrome P450 cholesterol side chain cleavage enzyme (CYP11A1) converts cholesterol to pregnenolone. After a series of reactions, CYP17A1 catalyzes the final conversion of androstenedione to testosterone () (Bosmann et al., Citation1996; Ge & Hardy, Citation2007; Payne & O’Shaughnessy, Citation1996). CYP3A4 is the main liver metabolic enzyme for progesterone and especially testosterone metabolism (Yamazaki & Shimada, Citation1997). In order to determine the mechanism of sex hormone synthesis, the effect of C. tubulosa on the two key steroidogenic enzymes (CYP11A1 and CYP17A1) and the metabolic enzyme (CYP3A4) was evaluated (Hanukoglu, Citation1992).
The present study investigates the direct effects of C. tubulosa extract (CTE) on progesterone and testosterone levels as well as on the expression of two steroidogenic enzymes (CYP11A1 and CYP17A1) and metabolic enzyme (CYP3A4) in male rats.
Materials and methods
Animals
A total of 60 male Sprague–Dawley rats weighing 100–120 g were used in the study. The animals were kept at a constant temperature (23 ± 1 °C) with a 12 h light-dark cycling in polypropylene cages and allowed free access to water and food. All animal procedures were performed in accordance with the university guidelines for care and use of laboratory animals.
Preparation of C. tubulosa extract
The stems of C. tubulosa were kindly provided and confirmed by Associate Prof. Jian-Jun Hu (Tarim University, Xinjiang, China). The C. tubulosa was collected during florescence (in May) from the desert area in South of Xinjiang Autonomous Region. The dried stems were crushed into powder and sieved through a 200-mesh sieve. Cistanche tubulosa powder (50 g) was extracted twice by 250 mL 70% ethanol and concentrated, then frozen at −80 °C and lyophilized to dryness. The powder was stored at 4 °C until further use.
Analysis of the chemical components of C. tubulosa extract
Phenylethanoid glycosides (PhGs) are the major active compounds of C. tubulosa and the content was detected by ultraviolet spectrophotometry (Wang et al., Citation2008). Echinacoside (111670-200503, National Institutes for Food and Drug Control, China) was used as a standard compound of PhGs in the detection (Editorial Committee of Chinese Pharmacopoeia, Citation2010). The extract of C. tubulosa (2 g) was dissolved in 100 mL of distilled water and subjected to D101 macroporous resin to yield distilled water (200 mL), 20% ethanol (200 mL), and 70% ethanol (200 mL). The 70% ethanol part was then concentrated to 50 mL, and was frozen at −80 °C and dried to powder at −60 °C. The powder was dissolved in 50 mL methanol until further use (mother solution). The solution in 40-fold dilution was measured at 330 nm with a spectrophotometer. Standard solutions were prepared by adding known volumes of echinacoside (0.04, 0.08, 0.16, 0.32, and 0.64 mg) to 5 mL of methanol.
Treatment of animals
The rats were randomly divided into four groups: (1) control group (treated with normal saline), (2) CTE low-, (3) middle-, and (4) high-dose groups (treated with 0.2, 0.4, and 0.8 g/kg of CTE, respectively). Materials were fed to rats by intragastric administration for 20 d. On the day after the last administration, the rats were anesthetized with pentobarbital and blood was collected by cardiac puncture. Organs, tissues, and glands, including liver, testicles, epididymis, seminal vesicles, prostate gland, and preputial gland, were removed and weighed. The organ indices were calculated using the following equation:
Organ index (%) = 100 × (organ weight/body weight)
Unilateral testicles were fixed in Bouin’s fixative at room temperature for H&E and immunohistochemical staining, and other testicles were stored at −80 °C for microsome preparation. Livers were fixed in formalin for H&E staining.
Blood samples were collected and incubated in an upright position at room temperature for 30 min to allow for clotting and then centrifuged for 15 min at 2000 rpm. The serum was stored at −20 °C until further use.
Detection of semen parameters
To obtain sperm suspension, the epididymis of rats was collected and minced in normal saline, and then cultured for 15 min at 37 °C. The sperm suspension was collected for the detection of semen parameters.
For the assay of the sperm count, a total of 450 µl 10% formalin fixative were added to 50 µl of sperm suspension. Then 10 µl of the solution was added onto a blood counting chamber, the sperm heads were counted and expressed as million/ml of suspension.
Sperm viability was detected by the staining method, 10 µl of sperm suspension were added onto a glass slide. Then, 10 µl of the staining solution (eosin Y and nigrosin) was added and incubated for 2 min. The slides were observed by optical microscope (400 × magnification). For each sample, a total of 200 sperm were evaluated and the viability percentages were calculated.
A total of 10 µl sperm suspension was added onto glass slide, the sperm motility was observed by optical microscope (400 × magnification). Ten visual fields were selected from each sample and 200 sperm were evaluated for motility. Standard of classification was as follows: (1) progressive motility (PR), good athletic ability, straight line or curve movement, the speed can be ignored; (2) non-progressive motility (NP), bad athletic ability, spin around or swing; (3) immobility (IM), no movement.
A total of 50 µl sperm suspension was fixed onto a glass slide and stained by 1% eosin solution for 10 min. Sperm morphology was observed by optical microscope (400 × magnification). A total of 200 sperm were evaluated and the rate of sperm deformation was calculated.
Radioimmunoassay
Progesterone and testosterone levels were quantified directly from prepared serum using radioimmunoassay (RIA) kits (Beijing Sino-UK Institute of Biological Technology, Beijing, China) according to protocol of the manufacturer.
Immunohistochemical staining
Fixed tissues were cut into paraffin sections (5 µm) and the immunohistochemistry was performed to evaluate the expression of CYP11A1 and CYP17A1 using a commercial kit (Fuzhou Maixin Bio-technology Co., Ltd., Fuzhou, China) according to manufacturer’s instructions. Antibodies against CYP11A1 and CYP17A1 were diluted in PBS (1:200) and the second antibody was available in the kit. Four sections were selected from each group, and 10 visual fields were selected from each section and positive cells were counted qualitatively by a pathologist.
Preparation of testicular and liver microsomal protein
Microsomal preparation was carried out essentially as described by Jiang et al. (Citation2012). The testicles or liver samples were minced and homogenized in four volumes of ice-cold TMS buffer (20 mM Tris–HCl, 5 mM MgCl2, and 0.25 M sucrose, pH 7.5). The homogenates were centrifuged at 9000g for 20 min at 4 °C. Supernatants were removed and mixed with 88 mM CaCl2 solution (10:1, v/v), centrifuged at 27 000 g for 20 min at 4 °C. Pellets containing crude membrane fractions were suspended in 0.1 M Tris buffer with 20% glycerol. Microsomes were stored at −80 °C until further use. Protein concentrations were determined by Coomassie brilliant blue staining using bovine serum albumin as a standard.
Western blot analysis
CYP11A1, CYP17A1, and CYP3A4 expressions were assessed by western blot analysis. Testicular or liver microsomal proteins were separated by SDS-PAGE on a 12% gel and transferred to polyvinylidene fluoride (PVDF) membrane. The membranes were blocked in 5% (w/v) powdered low fat skim milk in Tris-buffered saline with 0.05% Tween-20 (TBST) for 2 h, and further incubated with rabbit anti-rat CYP11A1 and CYP17A1 (Boster Biological Technology, Wuhan, China) and CYP3A4 (Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) polyclonal anti-bodies (1:200) in TBST at 4 °C for 8 h, followed by goat anti-rabbit (1:3000) peroxidase secondary antibody (Tianjin Sungene Biotech Co., Ltd., Tianjin, China) diluted in TBST at 37 °C for 1 h. Chemiluminescent detection was performed using Bio-Rad ChemiDocTM MP Imaging System (Bio-Rad Laboratories, Hercules, CA). Gray value of the bands was analyzed by Image J2× software (National Institutes of Health, Bethesda, MA).
Hepotoxicity assays
To detect the hepatotoxicity of CTE on rat, liver index, hepatic histopathology, alanine aminotransferase (ALT) level were assessed. Paraffin sections were prepared under a standard procedure and histological features were observed by light microscope after H&E staining. Sections were examined by a pathologist who was blinded to identification of samples. The serum ALT levels were measured using an ALT/GPT kit (Biosino Bio-technology and Science Inc. Beijing, China) according to the manufacturer’s protocol.
Statistical analysis
All data were expressed as means ± SD, and were analyzed with one-way repeated measures analysis of variance (ANOVA). All statistical parameters were calculated using SPSS version 19.0 software (SPSS Inc., Chicago, IL). Differences with a p value less than 0.05 were considered statistically significant.
Results
Content of phenylethanoid glycosides in C. tubulosa extract
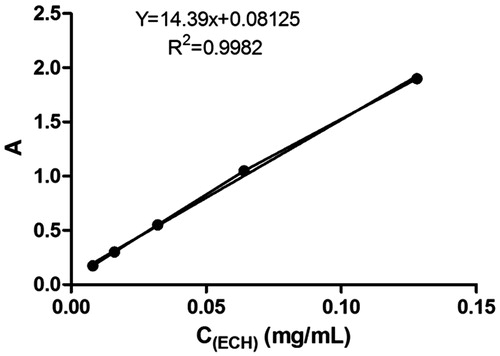
The standard curve exhibited a good linear relationship between absorbance and echinacoside concentration from 0.008 to 0.128 mg/mL (Y = 14.39 x + 0.08125, R2 = 0.9982) (). The content of PhGs in CTE was 41.52% by calculation.
Effect of C. tubulosa extract on organ indices
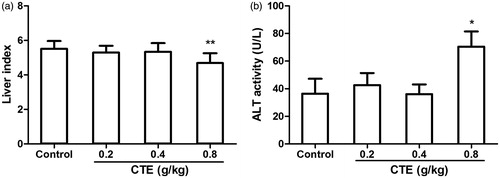
Variances of male reproductive organ indices between control group and all treatment groups were not significantly different (). A significant difference (p < 0.05) of liver index was observed between control group and high-dose (0.8 g/kg) CTE treatment groups.
Table 1. Effect of Cistanche tubulosa extract on organ indices.
Effect of C. tubulosa extract on semen parameters
As shown in , sperm count of rats was increased by treatment of 0.4 and 0.8 g/kg CTE (p < 0.05), while the sperm viability showed no significant difference between each group. Administration with CTE also showed significant induction of sperm motility and inhibition of abnormal sperm (p < 0.05).
Table 2. Effect of Cistanche tubulosa extract on semen parameters.
Effect of C. tubulosa extract on progesterone and testosterone levels
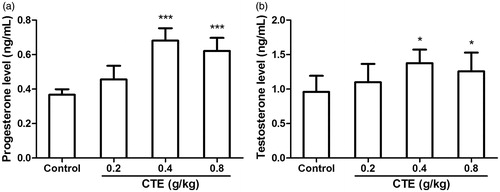
The effect of CTE on serum progesterone and testosterone levels is shown in . Compared with the control group, middle- and high-dose of CTE caused increase (p < 0.001) of progesterone level (1.9- and 1.7-fold). Testosterone levels of middle- and high-dose groups exhibited increase of 1.5- and 1.3-fold, respectively (p < 0.05).
Figure 3. Effect of CTE on progesterone (a) and testosterone (b) levels. Values are means ± SD. *p < 0.05, ***p < 0.001 versus control.
Effect of C. tubulosa extract on the expression of CYP11A1 and CYP17A1
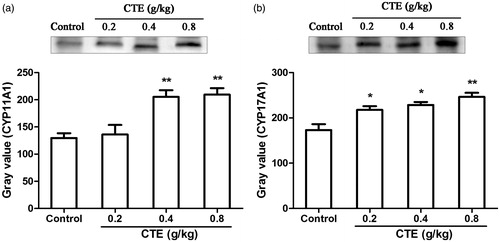
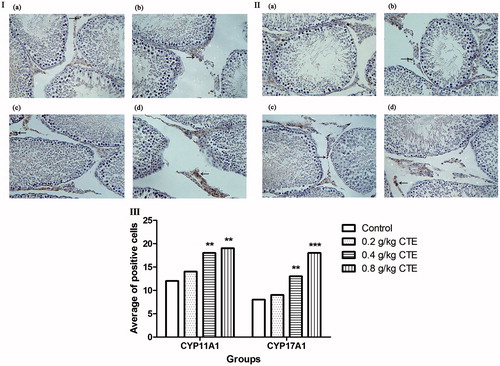
CYP11A1 and CYP17A1 were expressed in Leydig cells; immunohistochemical staining was used to detect the level of the proteins; and staining of positive cells in middle- and high CTE-dose groups were stronger than the control group (). After middle- and high-dose CTE administration, CYP11A1 expression levels were increased by 1.5- and 1.6-fold, and CYP17A1 levels were increased by 1.7- and 2.3-fold, respectively.
Figure 4. CYP11A1 and CYP17A1 expressions detected by immunohistochemical staining. (a) Control group; (b) low-dose CTE group; (c) middle-dose CTE group; (d) high-dose CTE group. (I) CYP11A1 expression; (II) CYP17A1 expression; (III) Average number of positive cells. Arrows indicate positive cells (IHC, ×400). Values are means ± SD. **p < 0.01, ***p < 0.001 versus control.
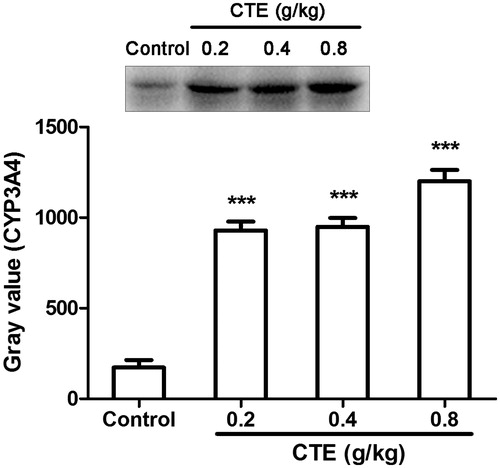
In western blot assays, CYP11A1 levels of low-, middle-, and high-dose of CTE were increased by 1.1-, 1.6-, and 1.6-fold, and expression of CYP17A1 were increased by 1.3-, 1.3-, and 1.4-fold (). The expression of CYP3A4 was significantly increased by 5.3-, 5.5-, and 7.0-fold ().
Hepatotoxicity of C. tubulosa extract
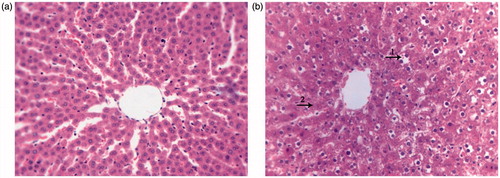
The liver index of high-dose CTE treatment group was decreased and serum ALT was increased when compared with the control group (). High-dose CTE caused mild hepatic edema (). The liver structure of the control group was normal; hepatocytes maintained cellular morphology and were arranged in visible hepatic cords (). Conversely, hepatocytes in high-dose CTE group () were scattered and more than 50% of hepatocytes showed various degrees of edema. A few hepatocytes exhibited fatty degeneration. Some hepatocytes displayed cell death with pyknotic nuclei and other evidence of cell necrosis including karyolysis and diffuse cellular debris.
Discussion
Effects of CTE on sex hormone levels, testicular steroidogenic enzymes, and the expression of hormone metabolic enzyme in liver in male rats were investigated in this study. Our results show that the sex hormone levels were enhanced by inducing CYP11A1 and CYP17A1 expressions. The metabolism of sex hormones was also influenced by inducing CYP3A4 expression. Slight liver injury was also observed while administering high dose of CTE (0.8 g/kg).
Cistanche tubulosa was proved to possess a hypocholesterolemic effect (Shimoda et al., Citation2009; Xiong et al., Citation2013). The mRNA level of CYP11A1 was up-regulated by CTE (400 mg/kg) (Shimoda et al., Citation2009), consistent with previous reports; however, the mechanism of transcriptional regulation was unclear. As with the other steroidogenic P450s, the regulation of CYP11A1 and CYP17A1 is relatively complex, and their induction has been known to be caused through cAMP signaling pathway (Guo et al., Citation2003; Košir et al., Citation2012). The promoters of CYP11A1 and CYP17A1 contain sequences that bind to the steroidogenic factor (SF-1) that in turn plays an important role in the tissue specific and hormonally regulated expression of steroidogenic genes (Ortiz de Montellano, Citation2005). Other factors, including cAMP response element binding (CREB), activator protein 1 (AP-1), specificity protein (SP-1, SP-3), are also involving in activation of CYP11A1 and CYP17A1 transcriptions. The assembly of various transcription factors forming protein-DNA complexes appears to be a key step in CYP11A1 and CYP17A1 transcription (Ortiz de Montellano, Citation2005). The target protein of CTE to affect the CYP11A1 and CYP17A1 expression needs to be further investigated.
As shown in , CTE increases the expression of CYP11A1 and CYP17A1 in dose-dependent manner, while the testosterone level of the high-dose group was lower than the middle-dose group. That might be due to the facts that metabolisms of progesterone and testosterone were also influenced. Liver cytochrome P450 is a superfamily of enzymes that catalyze the oxidation of organic substances, such as drugs and steroidal hormones (Ortiz de Montellano, Citation2005). The high enzyme levels in liver and other tissues can be induced by various compounds including drugs, pesticides, and carcinogens. The results showed that the main hormone metabolic enzyme (CYP3A4) was also induced by CTE. In healthy rats, there is a feedback regulation of hormones. The authors hypothesized that CYP3A4 expression was increased in this study to metabolize excessive hormones. That might be the reason that the increase of progesterone and testosterone levels was not significant. If CTE was administrated to rats with low sex hormones levels, the effect might be more obvious.
Systemic toxicity and safety evaluations of CTE have not been fully investigated, and our present study showed that high-dose CTE caused liver edema. Kim et al. (Citation2012) found that Cistanche herba induced testicular damage including decreasing the number of sperm and testosterone level, damaging the histologic structure of testicle; however, in our work, the testicular structure was normal in histological examination (data not shown). Although the doses used in this study were similar to the report by Kim et al. (Citation2012), the species of herbal plant was different. The growth conditions, harvest season, and processing method were unclear that may lead to different quality or clinical pharmacological effects (Wang et al., Citation2012).
Conclusion
CTE treatment showed a significant induction in the level of sex hormones in male rats, which was probably contributed by triggering effect the CYP11A1 and CYP17A1 expressions. As a traditional Chinese medicine and food stimulant, C. tubulosa could be further developed into new drugs, functional foods or dietary supplements in the future, however, systematic investigation of C. tubulosa safety should be assessed and the possible safety risk should be taken into consideration.
Acknowledgements
The authors thank Prof. Jian-Jun Hu for his help in collecting and confirming the stem of Cistanche tubulosa.
Declaration of interest
The work was supported by the Open Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. SKLNMKF201221), Ministry of Education and State Administration of Foreign Experts Affairs “Overseas Teacher” project (No. MS2011XBNL057), China, the High-end Foreign Experts Recruitment Program (GDW20146100228), China and Supported by the Key Construction Program of International Cooperation Base in S&T, Shaanxi Province (2015SD0018), China.
References
- Bosmann HB, Hales KH, Li X, et al. (1996). Acute in vivo inhibition of testosterone by endotoxin parallels loss of steroidogenic acute regulatory (StAR) protein in Leydig cells. Endocrinology 137:4522–5
- Editorial Committee of Chinese Pharmacopoeia. (2010). Pharmacopoeia of the People’s Republic of China. Beijing, China:China Medical Science and Technology Press
- Ge RS, Hardy MP. (2007). Regulation of Leydig cells during pubertal development. In: Payne AH, Hardy MP, eds. The Leydig Cell in Health and Disease. Totowa: Humana Press, 55–70
- Guo IC, Hu MC, Chung BC. (2003). Transcriptional regulation of CYP11A1. J Biomed Sci 10:593–8
- Hanukoglu L. (1992). Steroidogenic enzymes: Structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol 43:779–804
- He W, Shu XF, Zong GZ, et al. (1996a). Studies on kidney reinforcing and yang supporting action of Cistanche deserticola Y. C. Ma before and after preparation. Chin J Chin Mater Med 21:534–7
- He W, Zong GZ, Wu GL, Chen MH. (1996b). Preliminary study on the male hormone-like function of Cistanche deserticola. Chin J Chin Mater Med 21:5
- Hofbauer LC, Khosla S. (1999). Androgen effects on bone metabolism: Recent progress and controversies. Eur J Endocrinol 140:271–86
- Jiang B, Cai F, Gao SH, et al. (2012). Induction of cytochrome P450 3A by Shexiang Baoxin Pill and its main components. Chem Biol Interact 195:105–13
- Kim SW, Yoo SH, Lee HJ, et al. (2012). Cistanches herba induces testis cytotoxicity in male mice. Bull Environ Contam Toxicol 88:112–17
- Košir R, Zmrzljak UP, Bele T, et al. (2012). Circadian expression of steroidogenic cytochromes P450 in the mouse adrenal gland involvement of cAMP-responsive element modulator in epigenetic regulation of Cyp17a1. FEBS J 279:1584–93
- Li TM, Huang HC, Su CM, et al. (2012). Cistanche deserticola extract increases bone formation in osteoblasts. J Pharm Pharmacol 64:897–907
- Ortiz de Montellano PRO. (2005). Cytochrome P450 Structure, Mechanism, and Biochemistry. New York:Kluwer Academic/Plenum Publishers
- Payne AH, O’Shaughnessy PJ. (1996). Structure, function and regulation of steroidogenic enzymes in the Leydig cell. In: Payne AH, Hardy MP, Russell LD, eds. The Leydig Cell. Vienna: Cache River Press, 259–86
- Shimoda H, Tanaka J, Takahara Y, et al. (2009). The hypocholesterolemic effects of Cistanche tubulosa extract, a Chinese traditional crude medicine, in mice. Am J Chin Med 37:1125–38
- Wang T, Zhang XY, Xie WY. (2012). Cistanche deserticola Y. C. Ma, “Desert Ginseng”: A review. Am J Chin Med 40:1123–41
- Wang LN, Chen J, Yang MH, et al. (2008). Quantitative determination of total phenylethanoid glycosides in Cistanche desertiola. Northwest Pharm J 23:67–9
- Xiong WT, Gu L, Wang C, et al. (2013). Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J Ethnopharmacol 150:935–45
- Yamazaki H, Shimada T. (1997). Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys 346:161–9