Abstract
Context: Carum copticum seeds have been prescribed in the traditional system of medicine for the treatment of immune disorders, such as asthma and rheumatism.
Objective: The objective of this study was to determine immunomodulatory effects of the alcoholic extract and isolated compounds in Swiss albino mice.
Materials and methods: Seeds of C. copticum were extracted with 95% v/v alcohol. The immunomodulatory activity of the crude extract was evaluated at the doses of 100, 300, and 500 mg/kg body weight of mice, administered in mice once daily (orally) for 25 days. Volatile oil of C. copticum was isolated by steam distillation and was characterized by GLC and HPLC. Bio-assay-guided fractionation and isolation were carried out and the isolated compounds were characterized and subjected to immunomodulatory activity studies.
Results: The n-hexane fraction yielded p-cymene, carvacrol, and α-pinene. The LD50 value of the crude extract was found to be 4500 mg/kg and the values reported for p-cymene, carvacrol, and α-pinene in the literature were 4750, 810, and 3700 mg/kg, respectively. The oral administration of crude extract, n-hexane fraction (HEF), and isolated oils at the dose of 500, 150, and 50 mg/kg body weight, respectively, showed a significant increase in the HA titers, DTH-response, and phagocytosis. The stimulatory effect observed, on humoral and cellular immunity, was compared with the standard (levamisole treated) and control groups.
Discussion and conclusion: The results obtained in the study endorse the traditional use of the seeds of C. copticum and the isolated constituents act as immunostimulants.
Introduction
Carum copticum L. (Apiaceae), commonly called Bishop's weed, is widely cultivated for its uses as a spice and in medicine. The plant is indigenous to India, Iran, and Egypt. The fruits are harvested from February to March and are separated when dried. The seeds are grayish-brown in color (Boskabady & Shaikhi, Citation2000; Chopra, Citation1982; Devasankaraiah et al., Citation1974). Its essential oil was in great demand in the past due to its antiseptic properties. The seeds of C. copticum consist of proteins, fat, minerals, fibers, and carbohydrates. Calcium, phosphorus, iron, carotene, thiamine, riboflavin, and niacin are among the vitamins and minerals present in the seeds. The volatile oil is rich in cymene, terpene, thymine, and steroptin. The oil is almost colorless to brownish liquid, with a characteristic odor and a sharp hot taste. If the liquid is allowed to remain undisturbed, a part of thymol may separate in the form of crystals. Thymol is used as a condiment and the oil is used as an antiseptic. Carum copticum seeds increase virtility and cure premature ejaculation (Dubey, Citation1980; Khare, Citation2001; Peter, Citation2004). Carum copticum seeds have high medicinal value and possess various pharmacological activities such as antiasthamatic or bronchodilatory (Boskabady et al., Citation2003, Citation2007), antihypertensive, antispasmodic, hepatoprotective (Gilani et al., Citation2005), antitussive (Boskabady et al., Citation2005), antibacterial (Kaur & Arora, Citation2009; Singh et al., Citation2002; Rani & Khullar, Citation2004), anticataract (Biswas et al., Citation2001), antihistaminic (Boskabady & Shaikhi, Citation2000), analgesic (Dashti-Rahmatabdi et al., Citation2007), cholinomimetic (Devasankaraiah et al., Citation1974), anticancer (Yin et al., Citation2012), antiepileptic (Rajput et al., Citation2013; Rezvani et al., Citation2011), antioxidant (Deb et al., Citation2012; Zahin et al., Citation2010), antifungal (Alizadeh et al., Citation2010), antihyperlipidaemic (Javed et al., Citation2006), and antifilarial (Mathew et al., Citation2008). Seeds of C. copticum have been prescribed for the treatment of asthma and rheumatism in the traditional system of medicine (Nadkarni, Citation2002; Norman, Citation1990). Rheumatoid arthritis is a chronic, systemic autoimmune disorder that causes the immune system to attack the joints resulting in inflammation (arthritis) and destruction. It is usually described as Type-III hypersensitivity. Asthma is an immune disorder resulting from an immune response to inhaled allergens (Thomas et al., Citation2007). In this study, the immunomodulatory activity of C. copticum seeds was evaluated, and the responsible constituents were isolated and characterized, to verify its traditional use.
Materials and methods
The authentic marker of thymol was procured from S.D. Fine Chemicals, India. All the solvents used were of analytical and HPLC grade. Melting ranges were determined by the open capillary method, using the Edutek melting point apparatus, and are uncorrected. IR spectra were recorded on Shimadzu FTIR-8400S (Shimadzu Corporation, Kyoto, Japan), in the range of 4000–400 cm−1, using KBr pellet technique. NMR spectra were obtained on a Bruker ADVANCE DRX 300 MHz spectrometer at room temperature (Bruker Corporation, Billerica, MA) (δ in ppm, J in Hz), with TMS as the internal standard, ESIMS and DART-MS were carried out on a JEOL SX 102/DA-600 mass spectrometer (JOEL Inc., Tokyo, Japan). Optical and specific rotations were determined on Rudolph Research Analytical Autopol-III automatic polarimeter (Rudolph Research Analytical, Hackettstown, NJ). Gas chromatograms were recorded on Perkin Elmer Autosystem-XL chromatograph (PerkinElmer, Inc., Waltham, MA) with a flame ionization detector (FID). HPLC chromatograms were recorded on the D-7000 HPLC system with a PDA detector at SAIF, CDRI, Lucknow.
Plant material
Seeds of C. copticum were procured on 27 March 2009 from the local market of Lucknow, India. The seeds were identified and authenticated by Dr. A. K. S. Rawat, Scientist and Head, Pharmacognosy and Ethnopharmacology Division, National Botanical Research Institute, Lucknow, India. The plant herbarium was submitted to NBRI (Ref. no. NBRI/CIF/88/2009).
Preparation of the alcoholic extract
Powdered C. copticum seeds (400 g) were packed in a Soxhlet apparatus and subjected to hot percolation for about 4 days, using 800 ml of ethanol (95% v/v) as the solvent. The solvent was evaporated under vacuum, using IKA-RV 10 basic rotary evaporator, and the extract was further dried in a desiccator under reduced pressure for complete drying (Sonar et al., Citation2012a,Citationb).
Fractionation and isolation of the alcoholic extract
The ethanolic extract (100 g) was suspended in distilled water (200 ml) and then extracted, in a separatory funnel, with n-hexane, chloroform, and n-butanol. All the fractions were concentrated under vacuum, using rotary evaporator, and then placed in a desiccator at reduced pressure for complete drying. The n-hexane fraction (20 g) was subjected to column chromatography over silica gel (60–120 mesh), using n-hexane with ethyl acetate as eluent with increasing polarity. Fractions of 10 ml each were collected and similar fractions were pooled after monitoring their TLC. A mixture of compounds was obtained from the eluent of n-hexane:ethyl acetate (85:15) as a yellow-colored oily liquid. This mixture was further subjected to chromatography over silica gel (60–120 mesh) column, using chloroform and methanol as eluents with increasing polarity, to obtain three oily liquids. This procedure was repeated to obtain sufficient quantity of isolated oils for the pharmacological activity studies.
Isolation and analysis of volatile oil
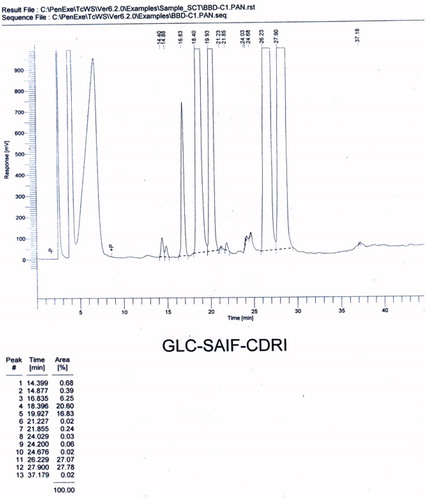
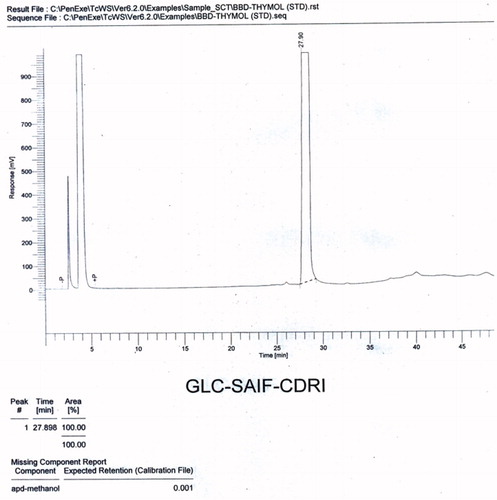
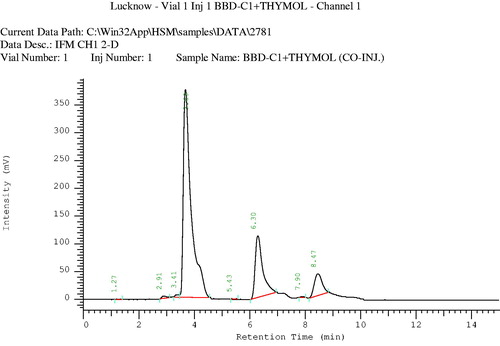
Volatile oil was extracted from the powdered (50 g) seeds of C. copticum by steam distillation, using Clevenger apparatus (PerkinElmer, Inc., Waltham, MA). The volatile oil obtained was a transparent liquid with a pungent smell. Samples containing 20 µg/ml concentration of volatile oil and the standard were prepared in chloroform for chromatographic studies. The GLC analysis of the volatile oil was performed on a Perkin Elmer autosystem-XL chromatograph (PerkinElmer, Inc., Waltham, MA), with a flame ionization detector (FID) at a temperature of 250 °C, equipped with a 10 ft long OV-1-packed column. The oven temperature program was set at 80 °C for 2 min and then was raised at 4 °C per min to 120 °C for 3 min, at 6 °C per min to 180 °C for 5 min, and then 8 °C per min to 220 °C and held for 30 min. Nitrogen was used as the carrier gas, with a flow rate of 20 ml/min. The injector mode was split injection and the volume of the sample used was 2 µl. Major components of the volatile oil were identified and confirmed by comparing their retention indices with those of the authentic compounds ( and ).
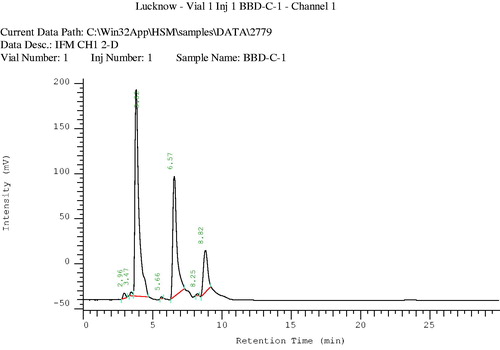
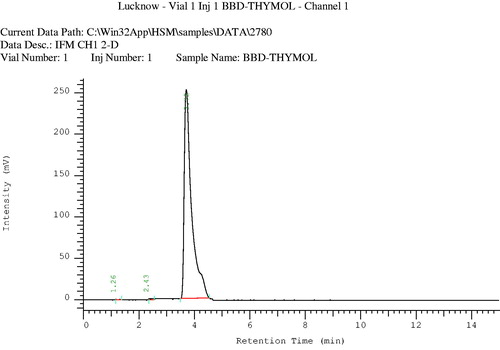
HPLC analysis of the volatile oil was performed to identify its major components. Chromatographic conditions were C-18 column, L-7100 type pump, pressure 0–420 kgf/cm2, mobile phase acetonitrile:water (70:30), injection volume: 4 µl, flow rate: 0.8 ml/min, run time 30 min, calculation method: area %, and peak identification window: % time ().
Animals
Male Swiss albino mice, weighing 25–30 g, were used for the assessment of immunomodulatory activity. These animals were obtained from the Laboratory Animal Service Division of Central Drug Research Institute, Lucknow. The animals were housed in polypropylene cages with steel nets in a temperature-controlled room, under standard living conditions of 25 ± 2 °C and 12 h light/dark cycles, and fed with a standard pellet diet and water ad libitum. The experimental protocol was duly approved by the Institutional Animal Ethics Committee (BBDNITM/IAEC/05/2010) and the experiments were performed as per the guidelines.
Acute toxicity studies
The mice were fasted overnight and then treated orally with the crude alcoholic extract of the seeds of C. copticum. The dose was gradually increased to establish the dose required to observe lethality in 50% of the animals (LD50). The animals were observed for 7 days following the treatment. The number of the death of mice was noted for each group. The LD50 value was calculated according to the reported protocol (Lorke, Citation1983). The control group received only vehicle (3% gum acacia suspension).
Immunomodulatory activity
Immunomodulatory activity of the crude extract, fractions, and isolated oils of the seeds of C. copticum was evaluated by the sheep RBC antigenic challenge model in mice. The parameters of the activity were primary and secondary humoral immune responses, delayed-type hypersensitivity (DTH) reaction, and phagocytosis.
The animals were divided into five groups, consisting of six animals in each group (n = 6):
Group 1 = Control, received only vehicle (3% gum acacia suspension).
Group 2 = Positive control, received levamisole (2.5 mg/kg body weight of mice).
Group 3 = Extract-treated group, received 100 mg/kg body weight of mice.
Group 4 = Extract-treated group, received 300 mg/kg body weight of mice.
Group 5 = Extract-treated group, received 500 mg/kg body weight of mice.
A total of five groups of animals were used to evaluate the activity of different fractions (150 mg/kg body weight of mice) and isolated compounds (50 mg/kg body weight of mice), in the same manner as the above studies.
The above groups were administered the respective material once daily (orally), from day 1 to day 25. Mice from all the groups received an antigenic challenge, with intraperitonial (i.p.) injection of sheep RBC (SRBC) in phosphate buffer saline (10% approx., 0.1 ml), on day 7 and day 14 of the treatment. SRBCs were obtained by centrifugation of the blood of sheep at 4000 rpm, using BL-120-Refrigerated centrifuge (Biolab, Lawrenceville, GA). The RBCs were preserved in Alsever’s solution. They were then suspended in phosphate-buffered saline for use.
Humoral immune response
The blood was withdrawn by puncturing the retro-orbital plexus of all antigenically challenged mice on day 14 and day 21of the treatment. The serum was separated from the collected blood to determine the primary and secondary antibody levels in it. For this, 20 µl of serum was mixed with 180 µl of phosphate-buffered saline, in 96-well microtitre plates, to get 10-fold dilution of the antibodies present in the serum. Further, 2-fold dilutions of this diluted serum were similarly carried out so that the antibody concentration of any of the dilutions was half of the previous dilution. SRBC (1%, 100 µl) were added to each of these dilutions. After mixing, the plates were incubated at room temperature for 4h and examined for hemagglutination. The reciprocal of the highest dilution of the test serum giving agglutination was taken as the antibody titer or HA titer, which is defined as the “reciprocal” of the last dilution with positive reaction. The level of the antibody titer on day 14 of the experiment was considered as the primary humoral immune response, and the one on day 21 of the experiment was considered as the secondary humoral immune response (Fakeye et al., Citation2008).
Cell-mediated immune response
DTH reaction
This was assessed by the foot pad reaction method in mice. The increase in paw thickness, induced by an injection of SRBC (20 μl, 1% v/v) in the sub-plantar region of right hind paw of each mouse’ on day 22, was assessed after 48 h, i.e., on day 24. The increase in paw thickness was considered as the DTH reaction and as an index of cell-mediated immunity. The thickness of left hind paw, injected similarly with phosphate buffer saline, served as the control (Bafna & Mishra, Citation2006).
Phagocytosis
On day 25 of the experiment, cervical dislocation euthanasia was done on mildly anaesthetized mice. The peritoneal macrophages of treated mice were harvested by flushing the peritoneal cavity with 3.0 ml of Hank balanced salt solution (HBSS), containing 1% bovine serum albumin. The macrophages present in the aliquots were incubated on glass slides, at 37 °C for 30 min, in a humidified chamber. The glass slides were washed thoroughly to remove non-adherent cells. The adhered cells were incubated with 50 µl of heat killed (boiled for 30 min in normal saline) Candida albicans cells, at 37 °C for 30 min. Finally, the cells on the slides were stained with Wright’s dye, after thorough washing with HBSS, to microscopically determine the adherent cells containing yeast cells. Three hundred cells were counted and were expressed as percent phagocytosis (Joharapurkar et al., Citation2004; Sonar et al., Citation2013).
Immunomodulatory activity studies for the fractions and the isolated compounds were performed in the same manner as above, at the dose of 150 mg/kg body weight of mice and 30 mg/kg body weight of mice, respectively.
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM). All the treated-groups were compared with control and standard (levamisole treated) by one way-analysis of variance (ANOVA) with Tukey’s multiple comparison tests. The significance level was set at p < 0.05 with the help of GraphPad Instat® software (GraphPad Inc., La Jolla, CA).
Results and discussion
The alcoholic extract of the seeds of C. copticum was subjected to preliminary phytochemical tests to identify the various phytoconstituents like alkaloids, phytosteroids, carbohydrates, and phenolic compounds (Evans, Citation2009; Vogel, Citation2000).
A mixture of compounds was isolated from the n-hexane fraction of the extract, on elution with n-hexane:ethyl acetate (85:15), as a yellow-colored oily liquid. This mixture was further subjected to chromatography over a silica gel (60–120 mesh) column, using chloroform and methanol as eluents with increasing polarity, to afford three compounds (). Compound 1, a light-yellow oily liquid obtained from the eluent of chloroform:methanol (99:01), was identified as p-cymene. Its IR spectrum showed the peaks of aromatic ring (1641 and 1541 cm−1). The PMR spectrum showed a singlet sharp peak at 7.26 ppm indicating the methyl group at para position. The M+1 and M+2 peaks were observed in the mass spectrum. The CMR spectrum depicted the carbons of aromatic rings and aliphatic substituent. Compound 2, a light brown oily liquid obtained from the eluent of chloroform:methanol (98:02), was identified as carvacrol. Its IR spectrum showed the peaks of hydroxyl group (3433 cm−1) and aromatic ring (1620 cm−1). The PMR spectrum displayed the signals of aromatic protons (6.627–7.278 ppm) and a singlet for hydroxyl proton (5.080 ppm). The M+1 peak was obtained in the mass spectrum. Compound 3, a brown-colored oily liquid was obtained from the eluent of chloroform:methanol (98:02), was identified as α-pinene. The absorption peak in IR spectrum at 2923 cm−1 represented the presence of vinyl group and that at 2854 cm−1 represented the alkyl group. The absorption signals in PMR spectrum at 5.27–5.39 ppm represent the protons of alkene. Signals of methyl and methylene were obtained at 1.22–1.68 ppm. The M+1 peak was obtained in the mass spectrum. CMR spectrum gave the signals of the carbon of cycloalkene. The volatile oil of C. copticum was isolated and its major constituent was determined by gas chromatography and HPLC. Chromatographic analysis represented that thymol was the major component of the volatile oil (27.78%). Its peak in the gas chromatogram was obtained at RT (retention time) 27.90 min (). The RT of thymol in the volatile oil was compared with that in the chromatogram obtained for the standard thymol (). As per the HPLC chromatogram, there were three major components in the volatile oil (), with thymol being the chief constituent. RT of thymol of the volatile oil was found to be identical to that of authentic thymol (). Thymol was further confirmed by co-chromatography (). The RT of the peak of thymol was obtained at 3.72 min. The values of refractive index and optical rotation of volatile oil were also found to be similar to the reported values (Anonymous, Citation1996).
Table 1. Spectral data of isolated compounds from n-hexane fraction of Carum copticum.
In the acute toxicity studies, the crude extract was tested orally in mice at doses of 1500, 3000, and 4500 mg/kg. The LD50 value of the extract was found to be 4500 mg/kg. The LD50 values of p-cymene, carvacrol, and α-pinene have been reported in the literature as 4750, 810, and 3700 mg/kg, respectively (Azizi et al., Citation2012; O’Neil, Citation2006). All the doses used in the immunomodulatory activity studies were lower than the observed LD50 values. As per the observations, no noticeable behavioral side effects were observed in the animals during the studies. The high LD50 value also warrants that the extract and the isolated compounds are safe and non-toxic.
In the immunomodulatory activity studies, animals treated with different doses of the crude extract (300 and 500 mg/kg body weight), n-hexane fraction (150 mg/kg body weight), and isolated compounds 2 and 3 (30 mg/kg body weight) showed a significant increase in the primary and secondary antibody syntheses, DTH-response, and phagocytosis (). The agglutination of the humoral responses, as evidenced by an enhancement of antibody responsiveness to SRBC, indicated enhanced responsiveness of macrophages and B-lymphocytes that are closely associated with antibody production. DTH responses were significant at the doses of 300 and 500 mg/kg body weight of crude extract, HEF and compounds 2 and 3. This indicated the overall stimulatory effect of the extract, HEF, and compounds 2 and 3 on cellular immunity when compared with the levamisole-treated group. The results of the in vitro polymorphonuclear (PMN) function test (phagocytosis) showed a significant increase in the per cent phagocytosis. The extract, HEF, and isolated compounds 2 and 3 exhibited significant phagocytosis at different doses. This may be due to the extract, HEF, and isolated compounds 2 and 3 enhancing phagocytic efficacies of the macrophages, causing greater engulfment of the added Candida cells in comparison with the control, thereby stimulating a non-specific immune response.
Table 2. Immunomodulatory effects of seeds of Carum copticum.
Conclusion
On the basis of the results obtained in this study, it can be concluded that the alcoholic extract of C. copticum seeds, HEF, α-pinene, and carvacrol act as an immunostimulants. These agents may hold the potential of being developed into drug entities of natural origin, which may be used to stimulate immunity, as an accessory to the therapy in immune disorders.
References
- Alizadeh A, Zamani E, Sharaifi R, et al. (2010). Antifungal activity of some essential oils against toxigenic Aspergillus species. Commun Agric Appl Biol Sci 75:761–7
- Anonymous. (1996). Indian Pharmacopoeia. New Delhi, India: The Government of India, Ministry of Health & Family Welfare
- Azizi Z1, Ebrahimi S, Saadatfar E, et al. (2012). Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav Pharmacol 23:241–9
- Bafna AR, Mishra SH. (2006). Protective effect of bioactive fraction of Sphaeranthus indicus Linn. against cyclophosphamide induced suppression of humoral immunity in mice. J Ethnopharmacol 104:426–9
- Biswas NR, Gupta SK, Das GK, et al. (2001). Evaluation of ophthacare eye drops – A herbal formulation in the management of various ophthalmic disorders. Phytother Res 15:618–20
- Boskabady MH, Alizadeh M, Jahanbin B. (2007). Bronchodilatory effect of Carum copticum in airways of asthmatic patients. Therapie 62:23–9
- Boskabady MH, Jandaghi P, Kiani S, Hasanzadeh L. (2005). Antitussive effect of Carum opticum in guinea pigs. J Ethnopharmacol 97:79–82
- Boskabady MH, Ramazani M, Tabei T. (2003). Relaxant effects of different fractions of essential oil from Carum copticum on guinea pig tracheal chains. Phytother Res 17:1145–9
- Boskabady MH, Shaikhi J. (2000). Inhibitory effect of Carum copticum on histamine (H1) receptors of isolated guinea-pig tracheal chains. J Ethnopharmacol 69:217–27
- Chopra RN. (1982). Chopra's Indigenous Drug of India. Calcutta, India: Academic Publishers
- Dashti-Rahmatabdi MH, Hejazian SH, Morshedi A, Rafati A. (2007). The analgesic effect of Carum copticum extract and morphine on phasic pain in mice. J Ethnopharmacol 109:226–8
- Deb DD, Parimala G, Devi SS, Chakrabarti T. (2012). Role of Carum copticum seeds in modulating chromium-induced toxicity on human bronchial epithelial cells and human peripheral blood lymphocytes. Exp Toxicol Pathol 64:889–97
- Devasankaraiah G, Hanin I, Haranath PS, Ramanamurthy PS. (1974). Cholinomimetic effects of aqueous extracts from Carum copticum seeds. Br J Pharm 52:613–14
- Dubey SK. (1980). Text Book of Materia Medica. Calcutta, India: Sree Bhartee Press
- Evans WC. (2009). Trease and Evans Pharmacognosy. New Delhi, India: Saunders Elsevier Limited
- Fakeye OT, Pal A, Bawankule DU, Khanuja SPS. (2008). Immunomodulatory effect of extracts of Hibiscus sabdariffa L. (Family Malvaceae) in a mouse model. Phytother Res 22:664–8
- Gilani AH, Jabeen Q, Ghayur MN, et al. (2005). Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum compticum seed extract. J Ethnopharmacol 98:127–35
- Javed I, Iqbal Z, Rahman ZU, et al. (2006). Comparative antihyperlipidaemic efficacy of Trachyspermum ammi extracts in albino rabbits. Pak Vet J 26:23–9
- Joharapurkar AA, Wanjari MM, Dixit PV, et al. (2004). Pyrogallol: A novel tool for screening immunomodulators. Indian J Pharmacol 36:355–9
- Kaur GJ, Arora DS. (2009). Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement Altern Med 9:1–10
- Khare CP. (2001). Encyclopedia of Indian Medicinal Plants. Berlin, Heidelberg, New York: Springer-Verlag
- Lorke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol 54:275–87
- Mathew N, Misra-Bhattacharya S, Perumal V, Muthuswamy K. (2008). Antifilarial lead molecules isolated from Trachyspermum ammi. Molecules 13:2156–68
- Nadkarni KM. (2002). Indian Materia Medica. Mumbai, India: Popular Prakashan
- Norman J. (1990). The Complete Book of Spices: A Practical Guide to Spices and Aromatic Seeds. London: Dorling Kindersley Limited
- O’Neil MJ. (2006). The Merck Index an Encyclopedia of Chemicals, Drugs & Biological. New Jersey, USA: Merck Research Laboratories
- Peter KV. (2004). Handbook of Herbs and Spices. Cambridge, England: Woodhead Publishing Limited
- Rajput MA, Khan RA, Feroz Z. (2013). Evaluation of antiepileptic activity of the methanol extract of Trachyspermum ammi (L.). Arch Biol Sci 65:815–19
- Rani P, Khullar N. (2004). Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phytother Res 18:670–3
- Rezvani ME, Roohbakhsh A, Mosaddegh MH, et al. (2011). Anticonvulsant and depressant effects of aqueous extracts of Carum copticum seeds in male rats. Epilepsy Behav 22:220–5
- Singh G, Kapoor IP, Pandey SK, et al. (2002). Studies on essential oils: Part 10; antibacterial activity of volatile oils of some spices. Phytother Res 16:680–2
- Sonar PK, Singh R, Khan S, Saraf SK. (2012a). Isolation, characterization and activity of the flowers of Rhododendron arboreum (Ericaceae). E J Chem 9:631–6
- Sonar PK, Singh R, Bansal P, et al. (2012b). R. arboreum flower and leaf extracts: RP-HPTLC screening, isolation, characterization and biological activity. Rasayan J Chem 5:165–72
- Sonar PK, Singh R, Verma A, Saraf SK. (2013). Rhododendron arboreum (Ericaceae): Immunomodulatory and related toxicity studies. Orient Pharm Exp Med 13:127–31
- Thomas JK, Richard AG, Barbara AO. (2007). Kuby Immunology. Berlin, Heidelberg, New York: W.H. Freeman and Company
- Vogel AI. (2000). Elementary Practical Organic Chemistry: Qualitative Organic Analysis. New Delhi, India: CBS Publishers and Distributors
- Yin QH, Yan FX, Zu XY, et al. (2012). Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology 64:43–51
- Zahin M, Ahmad I, Aqil F. (2010). Antioxidant and antimutagenic activity of Carum copticum fruit extracts. Toxicol In Vitro 24:1243–9