Abstract
Context: 1β-Hydroxyalantolactone (IJ-5) is a sesquiterpene lactone compound isolated from Inula japonica Thunb (Asteraceae). Sesquiterpene lactones have been shown to modulate many processes that influence inflammatory reactions.
Objective: The present study examines the protective effect of IJ-5 on atopic dermatitis (AD) in a mouse model induced by 2,4-dinitrochlorobenzene (DNCB).
Materials and methods: AD-like skin lesions were induced in Balb/c mice by sensitizing once with painting 100 μL of 5% DNCB on their shaved back skin and then challenging with 20 μL of 0.2% DNCB five times on their right ears at 3 d interval starting on day 5 post-sensitization. IJ-5 was administrated intraperitoneally at 10 mg/kg 1 h before each DNCB challenge.
Results: IJ-5 treatment attenuated DNCB-induced dermatitis severity and right ear swelling. The serum levels of IgE, IL-4, and IL-6 in IJ-5-treated mice were reduced by 54.7, 56.5, and 53.0%, respectively, while the mRNA levels of TNFα, IL-1, IL-4, and IL-6 in back skin lesions of IJ-5-treated mice were reduced by 47.7, 61.5, 57.5, and 58.5%, respectively, compared with untreated controls. Histopathological examination showed that IJ-5 treatment decreased DNCB-induced hypertrophy, hyperkeratosis, and infiltration of inflammatory cells in both ear and back skins. Moreover, IJ-5 suppressed the expression of TNFα, IL-1, and IL-6 with IC50 values of 6.58, 9.48, and 7.01 μM, respectively, and inhibited the activation of NF-κB pathway in TNFα-stimulated HaCat cells.
Discussion and conclusion: The present study demonstrates the protective effects of IJ-5 against AD-like skin inflammation and highlights IJ-5 as a potential therapeutic agent for AD.
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease that affects 10–20% of children and is characterized by eczematous lesions (Leung & Berber, Citation2003; Novak, Citation2009). The acute AD lesions present as intensely pruritic erythematous papules associated with excoriation and serous exudate. Repeated inflammation leads to chronic AD lesions characterized by thickened plaques with lichenification and dry, fibrotic papules. Although the pathophysiology of AD still remains incompletely understood, various factors, including genetic predisposition, cutaneous hyperreactivity to environmental triggers, and immunological abnormalities, have been found to contribute to the development and severity of AD. Current treatment of AD generally includes topical corticosteroids and immunosuppressive agents. However, various side effects have prevented long-term use of these therapies (Gottlieb, Citation2005; Rosso & Friedlander, Citation2005). Therefore, there is a need to develop new and effective treatment alternatives for AD.
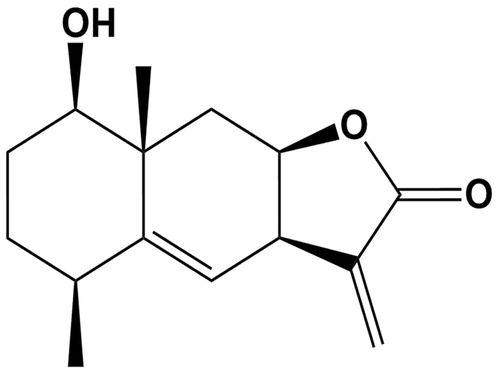
Medicinal plants and the compounds derived from them have been a good source of new anti-inflammatory agents. Inula japonica Thunb. (Asteraceae) is known as Xuanfuhua in Chinese. Its dried aerial part, known as Jinfeicao (its Chinese medicinal name), has long been used in traditional Chinese medicine for the treatment of inflammatory diseases such as tracheitis, bronchitis, and hepatitis (Choi et al., Citation2010; Park et al., Citation2011). Xuanfuhua and other plants of the Asteraceae family are rich in sesquiterpene lactones (SLs); these also constitute the active compounds of many medicinal plants (Merfort, Citation2011). Various SLs have been shown to modulate many processes that influence inflammatory reactions, for example, oxidative phosphorylation in neutrophils, chemotaxis, and migration of lymphocyte, as well as the secretion of histamine, serotonin, and proinflammatory cytokines (Hall et al., Citation1980; Koch et al., Citation2001). Treatment with SLs has been reported to provide therapeutic efficacy in animal models of paw and ear edema, chronic arthritis, gastritis, and colitis (Giordano et al., Citation1992; Hall et al., Citation1980; Schinella et al., Citation1998; Wendel et al., Citation1999). 1β-Hydroxyalantolactone is an SL isolated from the aerial part of Inula japonica in our phytochemical analysis of this medicinal plant (Qin et al., Citation2010). Its chemical structure is shown in . Preliminary pharmacological study suggested that this compound possesses anti-inflammatory property, as it showed a potent inhibitory activity against lipopolysaccharide-induced nitric oxide (NO) production in RAW264.7 macrophages (Qin et al., Citation2010). This study was performed to examine whether IJ-5 suppresses atopic dermatitis (AD)-like skin lesions in mice. In addition, IJ-5 was also tested in vitro to confirm whether it can inhibit the inflammatory response in keratinocytes.
Materials and methods
Reagents
IJ-5 (purity >99% as determined by HPLC and mass spectrometry) was isolated from the aerial part of Inula japonica in our laboratory using methods as previously described (Qin et al., Citation2010). Inula japonica was collected in Anhui province, China, in November 2013, and was authenticated by Professor Baokang Huang, Department of Pharmacognosy, School of Pharmacy, Second Military Medical University. A voucher specimen (No. 2013XFH09) was deposited at School of Pharmacy, Second Military Medical University. Recombinant human TNFα was purchased from Peprotech (Rocky Hill, NJ). 2,4-Dinitrochlorobenzene (DNCB) was purchased from Sigma-Aldrich (St. Louis, MO).
Mice
BALB/c mice (female, 6–8 week old) were purchased from Shanghai SLAC Laboratory Animal Co., LTD (Shanghai, China). All animal experiments were performed in accordance with Institutional Animal Research Committee guidelines (SMMU, License No. 2013061).
Induction of AD-like skin lesions in mice by DNCB and treatment with IJ-5
AD-like skin lesions in mice were induced by DNCB as previously described with small modification (Li & Li, Citation2009). Briefly, BALB/c mice were sensitized once on day 1 by painting 100 μL of 5% DNCB solution (dissolved in a 3:1 mixture of acetone and olive oil) on their shaved dorsal skin. From day 5, the mice were challenged five times every 3 d by painting the inner and outer surfaces of the right ears with 20 μL of 0.2% DNCB. The normal control animals were painted with solvent alone. In the IJ-5 treatment group, 10 mg/kg IJ-5 was administrated through i.p. injection 1 h before every DNCB challenge. Mice were sacrificed on day 22 of the experiment. The right ears and dorsal skin were removed and subjected to histological examination, and blood was collected from the vena cava.
Evaluation of skin dermatitis severity
The severity of dermatitis in the dorsal skin was evaluated 48 h after each challenge according to the criteria described previously (Leung et al., Citation1990). A total clinical severity score for AD-like lesions was defined as the sum of the individual scores graded as 0 (none), 1 (mild), 2 (moderate), and 3 (severe) for each of four signs and symptoms (erythema/hemorrhage, edema, excoriation/erosion, and scaling/dryness).
Evaluation of ear swelling
Ear swelling was evaluated by measuring the weight difference between the right and left ears based on measuring a small round piece cut-out by using a sharp clamp.
Histological examination
The ear and dorsal skin specimens were fixed in 10% buffered-neutral formalin, embedded in paraffin, and cut in 6 mm thick sections. For demonstrating morphologic changes and inflammatory cell infiltration, sections were stained with hematoxylin and eosin.
RNA isolation and quantitative real-time RT-PCR (qRT-PCR) analysis
RNA from skin specimens and cultured cells was extracted with the RNA isoreagent (Takara, Dalian, China) according to the instructions of the manufacturer. For each sample, 500 ng of total RNA was reverse transcribed using PrimeScript RT reagent Kit (Takara, Dalian, China). PCR amplification was performed on an StepOne Plus™ real time PCR system (Applied Biosystems, Waltham, MA) using the SYBR Premix ExTaq™ PCR Kit (Takara, Dalian, China). The sequences of the primers are indicated in . The relative levels of assayed mRNAs were calculated with the comparative Ct method using GAPDH expressions as endogenous control, and were normalized to non-treated control.
Table 1. Primer sequences used in this study.
Cell culture
The human keratinocyte line HaCat cells were obtained from ATCC (Manassas, VA) and cultured in DMEM medium (Sigma, St. Louis, MO) containing 10% heat-inactivated fetal bovine serum (FBS) (GIBCO, Grand Island, NY, BRL) and 1% penicillin and streptomycin at 37°C in 5% CO2. Cells were plated in 6-well plates at 1 × 106 per well and cultured overnight. The cells were treated with IJ-5 (2.5–10 μM) for 1 h, and then stimulated with 20 ng/ml TNFα for 10 min (for Western blotting assay) or 6 h (for qRT-PCR).
Western blotting
After treatment, cells were washed with ice-cold PBS and lysed with lysis buffer (50 mM Tris-HCl pH 8, 150 mM NaCl, 1 mM EDTA, 0.5% NP40, and 0.1% SDS) containing protein inhibitor cocktail (Roche, Basel, Switzerland). Cell lysates (15–30 mg) were separated by SDS-PAGE, electro blotted onto nitrocellulose membrane, and probed with primary antibodies against inhibitor of kappa (IκBα), phosphorylated IκBα (p-IκBα), p38, c-Jun N-terminal kinase (JNK), extra-cellular signal-regulated kinase (ERK), phosphorylated p38 (p-p38), phosphorylated JNK (p-JNK), phosphorylated ERK (p-ERK), and β-actin (Cell Signaling Technologies, Beverly, MA). Blots were visualized using IRDye 800CW Goat Anti-Mouse Secondary Antibody (LI-COR Biotechnology, Lincoln, NE). Detection was performed with an Odyssey infrared imaging system (LI-COR Biotechnology, Lincoln, NE).
Statistical analysis
All quantitative values are given as means ± SD. An unpaired Student’s t-test was used to evaluate the difference between two different treatments, and one-way ANOVA followed by Dunnett's Multiple Comparison Test was used to evaluate the difference between multiple treatments. p < 0.05 was considered to be statistically significant. Statistical analyses were performed using PRISM software (GraphPad Software Inc., La Jolla, CA).
Results
IJ-5 alleviates DNCB-induced inflammatory reactions in dorsal skin and ear
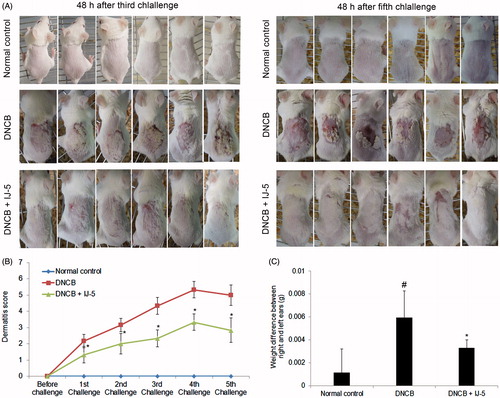
Sensitizing mice once by topically application of 100 μL of 5% DNCB on shaved back skin followed by five consecutive challenges with 20 μL of 0.2% DNCB on the right ear resulted in the development of AD-like clinical symptoms. As shown in , repeated DNCB challenges led to aggravating inflammatory reactions in the painted dorsal skin as evidenced by erythema, hemorrhage, excoriation, erosion, and scaling (). Accordingly, repeated DNCB challenges significantly increased dermatitis scores () and right ear swelling (). Administration of IJ-5 (10 mg/kg, i.p.) 1 h before every DNCB challenge markedly decreased severity of DNCB-induced dermatitis, as assessed by clinical score () and ear swelling ().
Figure 2. Inhibitory effects of IJ-5 on DNCB-induced AD-like symptoms in mice. AD-like skin lesions were induced by sensitizing mice once with topical application of DNCB on their shaved back skin and then challenging mice with DNCB five times on their right ears at 3 d interval starting on day 5 post-sensitization. IJ-5 was administrated intraperitoneally 1 h before each DNCB challenges. (A) The severity of back skin inflammation after DNCB challenge. The photos were taken at 48 h after third and fifth DNCB challenges. (B) The dermatitis score evaluated 48 h after each challenge based on the various AD symptoms. (C) Ear swelling evaluated at 48 h after fifth DNCB challenge by measuring the weight difference between the right and left ears. Data are presented as means ± SD (n = 6). #p < 0.05 versus the normal control group; *p < 0.05 versus the DNCB group.
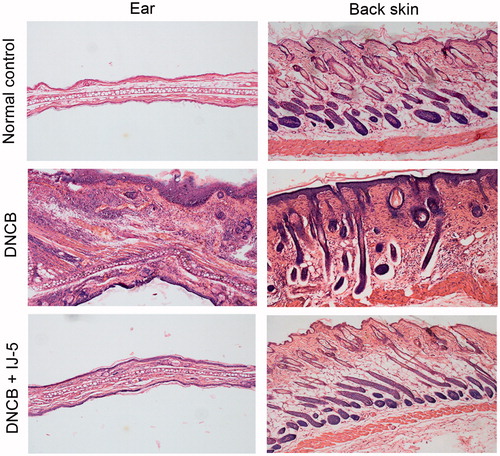
Histopathological analysis showed that DNCB-induced pathological changes of both ear and back skins were characterized by epidermis and dermis thickening, excessive inflammatory infiltration, and moderate edema, which were all markedly alleviated by IJ-5 treatment ().
IJ-5 reduces serum levels of IgE, IL-4, and IL-6 in DNCB-challenged mice
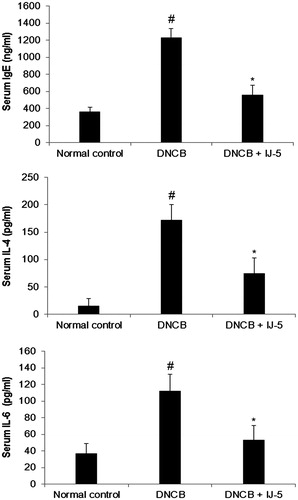
DNCB-challenged mice exhibited significant increase in serum levels of IgE, IL-4, and IL-6 compared with the normal group. In IJ-5-treated mice, the serum levels of IgE, IL-4, and IL-6 were reduced by 54.7%, 56.5%, and 53.0%, respectively, compared with untreated control ().
Figure 4. The effect of IJ-5 on serum levels of IgE, IL-4, and IL-6 in DNCB-challenged mice. The blood samples were collected at 48 h after fifth DNCB challenge and serum levels of IgE, IL-4, and IL-6 were assayed by ELISA. Data are presented as means ± SD (n = 6). #p < 0.05 versus the normal control group; *p < 0.05 versus the DNCB group.
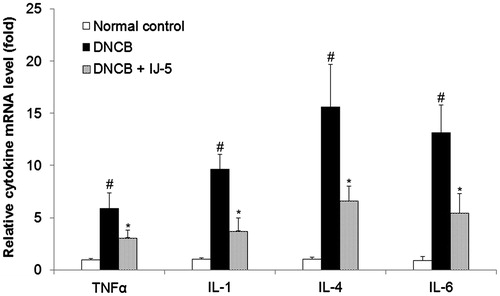
IJ-5 suppresses DNCB-induced expression of cytokines in back skins
To gain a better understanding of the mechanisms underlying the anti-inflammatory effects of IJ-5, we assessed the mRNA level of inflammatory cytokines in skin lesions by qRT-PCR. As shown in , repeated topical application of DNCB significantly increased expression of TNFα, IL-1, IL-4, and IL-6 in back skin lesions. In IJ-5-treated mice, the mRNA levels of TNFα, IL-1, IL-4, and IL-6 were reduced by 47.7, 61.5, 57.5, and 58.5%, respectively, compared with untreated control. These results demonstrate that IJ-5 suppressed DNCB-induced inflammatory cytokine expression, thereby leading to inhibition of the skin inflammation.
Figure 5. The effect of IJ-5 on DNCB-induced expression of cytokines in back skins. The skin specimens were harvested at 48 h after fifth DNCB challenge and the mRNA levels of indicated cytokines were assessed by qRT-PCR. Data are presented as means ± SD (n = 4). #p < 0.05 versus the normal control group; *p < 0.05 versus the DNCB group.
IJ-5 suppresses the expression of inflammatory cytokines in inflammatory HaCat cells by inhibiting NF-κB pathway activation
To further confirm the inhibitory effect of IJ-5 on inflammatory cytokine expression, we tested its activity on in vitro cultured HaCat cells. HaCat cells were stimulated with TNFα to induce the expression of various inflammatory cytokines, including TNFα, IL-1, and IL-6. Treatment with IJ-5 (2.5–10 μM) dose-dependently inhibited TNFα-induced expression of TNFα, IL-1, and IL-6 with IC50 values of 6.58, 9.48, and 7.01 μM ().
Figure 6. The effect of IJ-5 on TNFα-induced expression of inflammatory cytokines and TNFα-triggered signaling cascades in HaCat cells. HaCat cells were treated with 20 ng/ml in the presence of indicated concentrations of IJ-5. (A) After 6 h treatment, mRNA levels of TNFα, IL-1, and IL-6 were detected by qRT-PCR. Data are presented as means ± SD obtained from three independent experiments. #p < 0.05 versus non-stimulated control, *p < 0.05 versus TNFα only. (B) After 10 min treatment, the cells were collected and proteins were extracted, the levels of total IkBα, p38, JNK, and ERK and their phosphorylated levels were detected by Western blotting. This result was a representative of three experiments.
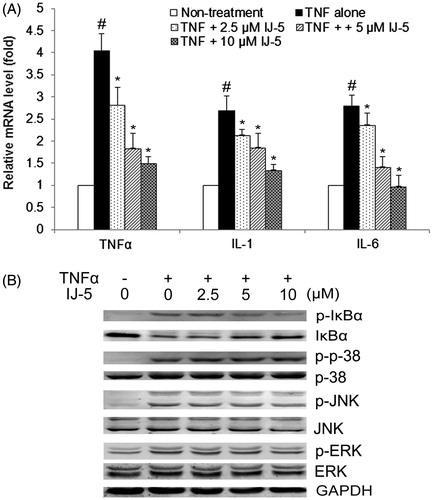
It is well established that TNFα induces inflammatory cytokine expression by triggering several signaling cascades, including NF-κB and MAP kinase (p38, JNK, and ERK) signaling pathways. To probe into the intracellular mechanisms related to the effects of IJ-5, we further tested its effects on TNFα-triggered signaling pathways. The results showed that IJ-5 (2.5–10 μM) dose-dependently inhibited TNFα-induced IκB phosphorylation and degradation in HaCat cells (), indicating IJ-5 can effectively inhibit NF-κB pathway activation. However, determination of MAP kinase activation by immunoblotting with phospho-specific Abs revealed no effect of IJ-5 on TNFα-induced phosphorylation of p38, JNK, and ERK, indicating IJ-5 does not interfere with MAP kinase pathway. Collectively, above data suggest that IJ-5 suppresses inflammatory cytokine expression through specifically interfering with the NF-κB-activating pathway.
Discussion
Topical application of haptens such as DNCB, trinitrochlorobenzene (TNCB), and oxazolone (Ox) in mice is commonly used to induce contact hypersensitivity (CHS) of the skin and these haptens are widely used as animal models for allergic contact dermatitis (Jin et al., Citation2009). Typically, the mice are first sensitized once by painting the hapten on their shaved back or abdominal skin. Five days later, they are challenged on one of the ears to induce skin inflammation. The severity of inflammation can be easily evaluated by ear thickness measurements. The hapten-induced CHS has been thought to evoke primarily a Th1-dominated response (Kehren et al., Citation1999; Larsen et al., Citation2007; Wang et al., Citation2000). However, it has been recently reported that repeated hapten challenges over an extended period causes the skin inflammation to shift from a typical Th1 dominated delayed-type hypersensitivity response to a chronic Th2 dominated inflammatory response that is similar to human AD (Man et al., Citation2008; Matsumoto et al., Citation2004). In the present study, we established a chronic DNCB-induced CHS models in Balb/c mice by sensitizing mice once with topical application of DNCB on their shaved dorsal skin and challenging mice with DNCB five times on their right ears at 3 d interval after 5 d of initial DNCB sensitization. This chronic skin inflammation mouse model displays many of clinical and immunologic features of human AD such as increased systemic IgE levels, altered skin morphology, strong cutaneous immune cell infiltration, a mixed cutaneous cytokine profile with enhanced IL-4 and IL-6 expression as well as strongly increased expression of the proinflammatory cytokines, TNFα, and IL-1β. Therefore, this simple and reproducible model can be used to assess potentially therapeutic agents for the treatment of AD.
To evaluate the protective effect of IJ-5 on the development of chronic AD-like lesions, IJ-5 was intraperitoneally administrated before DNCB challenge. IJ-5 treatment led to a significant inhibition of ear swelling induced by DNCB challenge, reflecting a profound inhibition of the edema and the cell infiltration in ears. The DNCB-induced AD-like histological changes in the back skins, including severe edema, hemorrhage, excoriation, erosion, and scaling, were also prevented by IJ-5 treatment. Accordingly, the total symptom severity scores were reduced by IJ-5 treatment. Histologically, IJ-5 treatment decreased hypertrophy, hyperkeratosis, and infiltration of inflammatory cells in both ear and back skins. These results indicate that IJ-5 treatment produced a resolution of AD-like skin lesions.
IgE hypersynthesis is an immunological hallmark of AD (Leung, Citation1993; Yamanaka & Mizutani, Citation2011). IgE-mediated mast cell activation leads to release of various kinds of inflammatory mediators, which results in infiltration of inflammatory cells into the skin lesion. IgE production is upregulated by Th2 cytokines, such as IL-4 and IL-6. These cytokines enhance B cell proliferation and induce immunoglobulin class switching for IgE production. Besides IgE and Th2 cytokines, the pro-inflammatory cytokines such as TNFα and IL-1β are also involved in the initiation and maintenance of inflammatory processes in the skin. Many cutaneous cells, including keratinocytes, mast cells, and Langerhans cells, can produce a wide array of cytokines, including TNFα, IL-1β, and IL-6 in responses to various stimuli (Pastore et al., Citation2006; Wood et al., Citation1992). These primary pro-inflammatory cytokines in turn induce the production of chemoattractants and adhesion molecules, supporting the recruitment, proliferation, and survival of leukocytes at cutaneous sites (Homey et al., Citation2006). In the present study, we found that the serum IgE level, as well as the serum IL-4 and IL-6 levels, were elevated in DNCB-challenged mice, and IJ-5 treatment significantly reduced the serum levels of IgE, IL-4, and IL-6. Furthermore, mRNA expression of TNFα, IL-1, IL-4, and IL-6 in the back skin lesions were elevated in the DNCB-challenged mice, and the expression of these cytokines were significantly suppressed by IJ-5 treatment. Consistent with the in vivo results, our in vitro study demonstrated that IJ-5 effectively suppressed the expression of TNFα, IL-1, and IL-6 in TNFα-stimulated HaCat cells. Collectively, these results suggest that the protective effects of IJ-5 in DNCB-induced mouse models are associated with downregulations of both Th2 and proinflammatory cytokines.
Since many sesquiterpene lactones are known to be cytotoxic to tumor cells, to determine whether the inhibitory effect of IJ-5 on the expression of cytokines was due to non-specific cytotoxicity, we had studied the effect of IJ-5 on the viability of HaCat cells using the MTT assay. The result showed that treatment of HaCat cells with IJ-5 (2.5–10 μM) for 24 h had no impact on cell viability (data not shown). This result indicates that IJ-5 is not cytotoxic to HaCat cells within the same dose range as it inhibited the expression of cytokines, thus excluding the possibility that the observed inhibition on cytokine expressions by IJ-5 arises from cytotoxicity. Furthermore, in our in vivo study, we observed no mice death, alteration in body weights, and any other obvious adverse effects in IJ-5-treated mice, indicating IJ-5 is safe in mice while exerting an effective anti-inflammatory effect in vivo. Of course, the efficiency and safety of IJ-5 for AD is still waiting for further clinical trials to determine.
The present new finding emphasizes the potential of IJ-5 as an interesting lead compound or the development of new agents for the treatment of AD. IJ-5 is a natural SL compound derived from Inula japonica, a plant with prominent anti-inflammatory activity. Extracts from plants rich in SLs have gained considerable interest for treating human diseases including inflammation of skin and other organs (Heinrich et al., Citation1998). Previous studies on the molecular mechanism of the anti-inflammatory activity of SLs have focused on NF-κB, a key transcriptional factor involved in regulating expression of inflammatory mediators. Several SL compounds have been found to inhibit NF-κB activity by either directly modification of the NF-κB p65 subunit (Garcia-Pineres et al., Citation2001) or to suppress the activity of the upstream IκB kinase complex leading to the stabilization of the NF-κB inhibitors IκB (Kwok et al., Citation2001). In present study, we found that IJ-5 inhibited TNFα-induced IκB phosphorylation and degradation in HaCat cells, indicating that IJ-5 can effectively inhibit NF-κB activation. Interestingly, TNFα-induced phosphorylation of p38, JNK, and ERK was not significantly suppressed by IJ-5, suggesting that this compound does not interfere with MAP kinase signaling cascade. Therefore, it seems that IJ-5 suppresses inflammatory cytokine expression through specifically interfering with the NF-κB-activating pathway. Further studies are required to determine the exact molecular mechanism and to evaluate the therapeutic potential of this natural SL in the prevention and treatment of human AD.
Conclusion
The present study revealed for the first time that IJ-5, a SL compound derived from an anti-inflammatory herb, alleviates AD-like inflammatory symptoms in mice by suppressing the production of Th2 and pro-inflammatory cytokines. Furthermore, its anti-inflammatory activity is associated its capability to suppress NF-κB pathway activation.
Declaration of interest
The authors report that they have no conflicts of interest. The work was supported by the National Natural Science Foundation of China (81072653 and 81373425).
References
- Choi JH, Park YN, Li Y, et al. (2010). Flowers of Inula japonica attenuate inflammatory responses. Immune Netw 10:145–52
- Garcia-Pineres AJ, Castro V, Mora G, et al. (2001). Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem 276:39713–20
- Giordano OS, Pestchanker MJ, Guerreiro E, et al. (1992). Structure–activity relationship in the gastric cytoprotective effect of several sesquiterpene lactones. J Med Chem 35:2452–8
- Gottlieb AB. (2005). Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol 53:S3–16
- Hall IH, Starnes CO, Lee KH, et al. (1980). Mode of action of sesquiterpene lactones as anti-inflammatory agents. J Pharm Sci 69:537–43
- Heinrich M, Robles M, West JE, et al. (1998). Ethnopharmacology of Mexican Asteraceae (Compositae). Annu Rev Pharmacol Toxicol 38:539–65
- Homey B, Steinhoff M, Ruzicka T, et al. (2006). Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol 118:178–89
- Jin H, He R, Oyoshi M, et al. (2009). Animal models of atopic dermatitis. J Invest Dermatol 129:31–40
- Kehren J, Desvignes C, Krasteva M, et al. (1999). Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J Exp Med 189:779–86
- Koch E, Klaas CA, Rungeler P, et al. (2001). Inhibition of inflammatory cytokine production and lymphocyte proliferation by structurally different sesquiterpene lactones correlates with their effect on activation of NF-kappaB. Biochem Pharmacol 62:795–801
- Kwok BH, Koh B, Ndubuisi MI, et al. (2001). The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol 8:759–66
- Larsen JM, Geisler C, Nielsen MW, et al. (2007). Cellular dynamics in the draining lymph nodes during sensitization and elicitation phases of contact hypersensitivity. Contact Dermatitis 57:300–8
- Leung D. (1993). Role of IgE in atopic dermatitis. Curr Opin Immunol 5:956–62
- Leung DY, Bieber T. (2003). Atopic dermatitis. Lancet 361:151–60
- Leung DY, Hirsch RL, Schneider L, et al. (1990). Thymopentin therapy reduces the clinical severity of atopic dermatitis. J Allergy Clin Immunol 85:927–33
- Li Y-M, Li X-R. (2009). Effects of Qingshi Cream on chronic dermatitis-eczema in mice. J Chin Integr Med 7:1164–6
- Man MQ, Hatano Y, Lee SH, et al. (2008). Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: Structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol 128:79–86
- Matsumoto K, Mizukoshi K, Oyobikawa M, et al. (2004). Establishment of an atopic dermatitis-like skin model in a hairless mouse by repeated elicitation of contact hypersensitivity that enables to conduct functional analyses of the stratum corneum with various non-invasive biophysical instruments. Skin Res Technol 10:122–9
- Merfort I. (2011). Perspectives on sesquiterpene lactones in inflammation and cancer. Curr Drug Targets 12:1560–73
- Novak N. (2009). New insights into the mechanism and management of allergic diseases: Atopic dermatitis. Allergy 64:265–75
- Park YN, Lee YJ, Choi JH, et al. (2011). Alleviation of OVA-induced airway inflammation by flowers of Inula japonica in a murine model of asthma. Biosci Biotechnol Biochem 75:871–6
- Pastore S, Mascia F, Girolomoni G. (2006). The contribution of keratinocytes to the pathogenesis of atopic dermatitis. Eur J Dermatol 16:125–31
- Qin J-J, Jin H-Z, Zhu J-X, et al. (2010). New sesquiterpenes from Inula japonica Thunb. with their inhibitory activities against LPS-induced NO production in RAW264.7 macrophages. Tetrahedron 66:9379–88
- Rosso JD, Friedlander SF. (2005). Corticosteroids: Options in the era of steroid-sparing therapy. J Am Acad Dermatol 53:S50–8
- Schinella GR, Giner RM, Recio MC, et al. (1998). Anti-inflammatory effects of South American Tanacetum vulgare. J Pharm Pharmacol 50:1069–74
- Wang B, Fujisawa H, Zhuang L, et al. (2000). CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol 165:6783–90
- Wendel GH, Maria AO, Mohamed F, et al. (1999). Effect of dehydroleucodine in experimental colitis in rats and mice. Pharmacol Res 40:339–44
- Wood LC, Jackson SM, Elias PM, et al. (1992). Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest 90:482–7
- Yamanaka K, Mizutani H. (2011). The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr Probl Dermatol 41:80–92