Abstract
Context: Toxicological screening of natural compounds for medicinal purposes.
Objectives: The objective of this study is to evaluate the toxicity of methyl ferulate (MF), methyl p-coumarate (MpC), and pulegone 1,2-epoxide (PE) with in vitro and in vivo assays.
Materials and methods: The in vitro toxicity of MF, MpC, and PE was assessed at a concentration of 10 mg/ml with the Ames assay using two strains of Salmonella typhimurium TA98 and TA100. Human red blood cells (RBC) were used to determine the hemolytic activity of these compounds. The cytotoxicity of above compounds was determined with brine shrimp lethality bioassay (BSLB) at the concentrations of 0.1–20 mg/ml. While dermal and ocular irritation studies were conducted on healthy rabbits (n = 8) for 96 and 12 h post-topical application of test compounds, respectively.
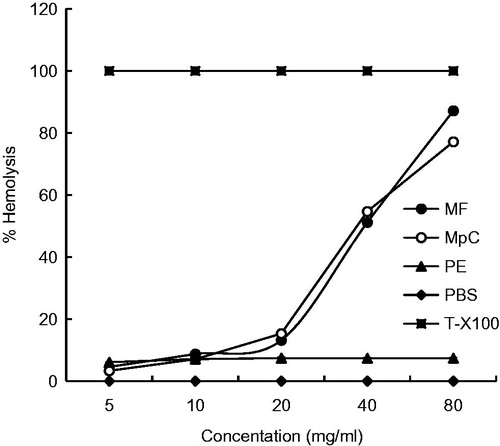
Results: PE produced 6–8% hemolysis of RBCs at all the tested concentrations while MF and MpC produced 10–5% hemolysis up to 20 mg/ml, and 50–85% hemolysis at concentrations of 40 and 80 mg/ml, respectively. The Ames assay indicated that MF, MpC, and PE were non-mutagenic as the test values were not significantly higher as compared with background values of the assay. BSLB suggested the lethal concentration (LC50) values of MF, MpC, and PE as 4.38, 6.74, and 25.91 mg/ml, respectively. In vivo ocular and dermal irritation scores of MF, MpC, and PE were comparable with ethanol (control) in rabbits indicating the non-irritant nature of these natural compounds.
Conclusion: The present studies suggest that these compounds are non-toxic/non-irritant and might be used for medicinal purposes.
Introduction
Toxicological screenings is routinely conducted on the chemicals intended for therapeutic purposes. Natural compounds have potential therapeutic effects such as antimicrobial (Lima et al., 2006; Oliveira et al., Citation2006) antinociceptive, and anti-inflammatory activities (Sousa et al., Citation2004). These effects are probably due to great structural diversity of the essential oils constituents in plants (Calixto, Citation2005).
Methyl ferulate (MF), C11H12O4, is insoluble in water but very soluble in ethanol and diethyl ether. MF is found in Stemona tuberosa Lour (Stemonaceae) and Ferula assa-foetida L. (Apiaceae) (Phuong et al., Citation2014). MF has antitumor activity against liver and breast cancers (Kampa et al., Citation2004; Lee, Citation2005) while it also has antithrombotic, antioxidant, and anti-inflammatory activities (Karamac et al., Citation2005; Phuong et al., Citation2014). Methyl p-coumarate (MpC), C10H10O3, is insoluble in water but very soluble in ethanol and diethyl ether. p-Coumaric acid is found in Gentum cleistostachyum Linn (Gnetaceae) (Yao et al., Citation2005). It has antioxidant properties and is found to reduce stomach cancer (Ferguson et al., Citation2005). Pulegone, C10H16O, is colorless naturally occurring, monoterpene compound extracted from essential oils of many plants such as pennyroyal, Nepeta cataria L. (Lamiaceae) and Mentha piperita L. (Lamiaceae). It is soluble in alcohol and is difficult to dissolve in water at room temperature. Chemically, Schizonepeta tenuifolia Briq. (Lamiaceae) contains several constituents in essential oil, among them pulegone was up to 47.73% and this oil has many pharmacological functions (Chun et al., Citation2010). Pulegone 1,2-epoxide (PE) has vasorelaxant activity (Lima et al., Citation2012).
Some medicinal compounds have limited availability in nature emphasizing the need to synthesize these natural compounds in the laboratories. MF, MpC, and PE are a few synthetically prepared natural compounds with limited published toxicity data. Therefore, this study evaluates these compounds for their toxicity with commonly available tests.
Materials and methods
Chemicals and reagents
All chemicals were of analytical grade. Natural compounds (MF, MpC, and PE) were prepared according to Machado et al. (Citation2015) and Lima et al. (Citation2014) in the laboratory of Prof. Dr. Damião Pergentino de Sousa, University of Paraíba, João Pessoa, PB, Brazil. Ethanol, d-glucose, potassium dichromate, sodium chloride, and potassium dihydrogen phosphate were purchased from Merck, Darmstadt, Germany. Disodium hydrogen phosphate and potassium chloride were procured from Bio Basic Inc, Markham, Canada. Bromocresol purple, d-biotin, l-histidine, and sodium azide were purchased from Scharlau Chemie S.A., Barcelona, Spain. Triton X-100 was procured from IBI Scientific, Peosta, IA. While, d-biotin, magnesium sulfate, trisodium citrate, sea salt, and ammonium sulfate were obtained from Sigma-Aldrich, Hamburg, Germany.
Hemolytic assay
Hemolytic activity of MF, MpC, and PE was studied according to the method used by Shahid et al. (Citation2013) and Zuber et al. (Citation2014). Freshly obtained heparinized human blood (3 ml) was collected from volunteers after their consent and counseling. Blood was centrifuged for 5 min at 1000g, the plasma was discarded, and cells were washed three times with 5 ml of chilled (4 °C) sterile isotonic phosphate buffered saline (PBS) pH 7.4. Red blood cells (RBCs) were maintained at 108 cells/ml for each assay. Each test compound (100 μl) was mixed with washed cells (108 cells/ml) separately. Samples were incubated for 35 min at 37 °C and agitated after 10 min. Immediately after incubation the samples were placed on ice for 5 min then centrifuged for 5 min at 1000g. Supernatant (100 μl) was taken from each tube and diluted 10 time with chilled (4 °C) PBS. Triton X-100 (0.1% v/v) was used as a positive control and PBS was used as a negative control. The absorbance was noted at 576 nm using μQuant (Bioteck, Jamestown, NY). The percent RBCs lysis for each sample was calculated
where Ab is the absorbance of the sample and Ac is the absorbance of the control.
Ames assay
This test kit method was based on the validated Ames bacterial reverse mutation test but was performed entirely in liquid culture by using the fluctuation test (Mohd-Fuat et al., Citation2007). Two mutant strains, Salmonella typhimurium TA98 and TA100, were used. The bacteria were maintained on nutrient agar at 3 ± 1°C. The bacteria were inoculated in nutrient broth and incubated at 37 °C for 18–24 h prior to the test. The following chemicals were used in this test: Davis–Mingioli salt (5.5 times concentrated), d-glucose (40%, w/v), bromocresol purple (2 mg/ml), d-biotin (0.1 mg/ml), and l-histidine (0.1 mg/ml). Sodium azide (NaN3, 0.5 μg/100 μl) for S. typhimurium TA100 and potassium dichromate (K2Cr2O7, 30 μg/100 μl) for S. typhimurium TA98. All chemicals were kept at 3 ± 1 °C until used.
Preparation of reagent mixture
Davis–Mingioli salt (21.62 ml), d-glucose (4.75 ml), bromocresol purple (2.38 ml), d-biotin (1.19 ml), and l-histidine (0.06 ml) were mixed aseptically in a sterile bottle. Reagent mixture, natural compounds (MF, MpC, and PE), sterile distilled water, and standard mutagen were mixed in several bottles in specific amounts as given in and inoculated with an overnight culture broth of S. typhimurium test strains. The content of each bottle was dispensed into each well of a 96-well micro-titration plate and the plates were incubated at 37 °C for 4 d. Observations were made by analyzing the color changes of the dye.
Table 1. Set-up of the Ames assay for MF, MpC, and PE (10 mg/ml) by using S. typhimurium TA98 and TA100.
Brine shrimp lethality bioassay (BSLB)
BSLB was performed according to the procedure given by Ullah et al. (Citation2013). Shrimp eggs were hatched in a plastic jar of about 3 l volume. Egg suspensions (2 mg) were introduced into the plastic jar. Brine solution (3.8% sea salt), oxygen, and light were provided to the environment for hatching of eggs. After a period of 24 h, eggs were hatched to nauplii. Different concentrations of the MF, MpC, and PE were made, i.e., 0.1, 1, 2.5, 5, 10, and 20 mg/ml. Larvae (n = 10) were added into each sterilized test tube along with 4.5 ml of brine solution and then 0.5 ml of each sample was poured into test tubes. These test tubes were incubated for 24 h at room temperature with proper aeration. In this assay, brine solution was taken as negative control. Potassium dichromate (PD) was used as positive control while brine solution (BS) was used as negative control. After 24 h, the nauplii were examined against a lightened background with a magnifying glass and an average number of survived larvae were determined.
Ocular irritation
To determine the degree of ocular irritation, the chemicals were instilled into one eye (0.1 ml of liquid) of each test rabbit (n = 8). The contralateral eye was used as the control (Klaassen, Citation2008). The eyes of the rabbits were examined at 4 and 12 h post-chemical application to evaluate the following irritation scores. 0 = No redness, dilation and secretion; 1 = very slight redness, dilation, and secretion; 2 = mild to moderate redness, dilation, and secretion; 3 = moderate to severe redness, dilation, and secretion.
Dermal irritation
The ability of compounds to irritate the skin after topical exposure was determined in rabbits (n = 8). Hairs were clipped from the back of rabbits 24 h prior to the experiments. Rabbits were divided into two groups (n = 4) for topical application of MF, MpC, and PE (4%) under non-occluded and occluded conditions with repeated daily application for 4 d. The erythema scores were recorded on the 5th day as follows: 0 = no significant change; 1 = very slight erythema (barely perceptible); 2 = slight erythema (pale red in defined area); 3 = moderate to severe erythema (red in well-defined area); and 4 = severe erythema (beet red in defined area) (Klaassen, Citation2008). Skin biopsies were preserved in 10% buffered formalin for microscopic examination. All the experiments were carried out in accordance with the guidelines of the directorate of graduate studies and institutional animal ethical committee.
Microscopic examination of skin
Formalin fixed skin biopsies were processed through graded ethanol and embedded in paraffin blocks. Dermal sections were oriented perpendicular to the plane of section in the block and 6 μm thick transverse sections were cut and mounted on glass slides and then stained with hematoxylin and eosin (H & E). Microscopic examination of skin slides was completed on Olympus PM-10 ADS automatic light microscope (Olympus Optical Co., Tokyo, Japan) with a 40 × objective.
Statistical analysis
Data obtained were subjected to analysis of variance (ANOVA) to determine the significance (p < 0.05) among the parameters (Microsoft Excel 2007). Values are given in means ± SD of each measurement. Probit analysis was applied on BSLB data.
Results
Hemolytic assay
The hemolytic assay was performed by using Triton X-100 as positive control and PBS as negative control. The compound with a hemolysis value of less than 10% was considered non-hemolytic while values more than 25% hemolysis were assumed as toxic. indicates that PE produced 6–8% hemolysis of RBCs at all the tested concentrations while MF and MpC produced 10–15% hemolysis up to 20 mg/ml and 50-85% at concentrations of 40 and 80 mg/ml, respectively.
Ames assay
In the Ames assay, yellow or turbid wells were scored as positive, while purple wells were scored as negative. For the natural compound to be mutagenic, the number of positive wells should be significantly higher than the number of positive wells in the background plate (spontaneous mutations). It was found that there were 20 yellow wells for TA98 and 25 yellow wells for TA 100 in the background plate which indicated spontaneous mutation while these positive wells were 94 and 93, respectively, in the 96 wells standard plate (). In this way, MF was found to be non-mutagenic because there were only 34 yellow wells out of 96 wells plate incubated with TA98 but with TA100 there were only 8 yellow wells. MpC was also found to be non-mutagenic because there were only 28 and 34 yellow wells out of 96 well plates incubated with TA98 and TA100, respectively. Similarly, PE was non-mutagenic because positive wells were not significantly higher than the background plate as shown in .
Table 2. Mutagenic activity of MF, MpC, and PE (10 mg/ml) by using S. typhimurium TA98 and TA100.
BSLB
The natural compounds caused mortality of shrimp larvae after 24 h. The natural compounds classified as toxic if lethal concentration 50% (LC50) value was less than 1 mg/ml and non-toxic for a value more than 1 mg/ml. The log concentration of the chemicals and percent mortalities are presented in . PD showed higher mortality at the lowest concentration and acted as positive control and BS exhibited no mortality thus was used as negative control. The LC50 values for these compounds as calculated with the Probit method were 4.38, 6.74, and 25.91 mg/ml for MF, MpC, and PE, respectively, as shown in .
Figure 2. Plot of log concentration MF, MpC, PE, and PD versus percent shrimp mortality after 24 h of exposure.
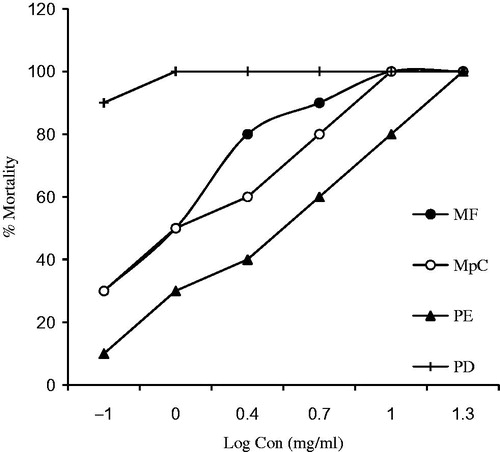
Table 3. LC50 values MF, MpC, and PE using the Probit method.
Ocular irritation
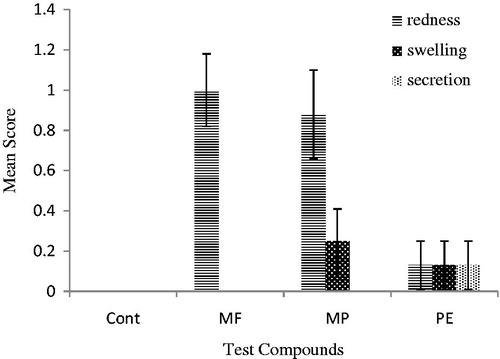
Ocular irritation was tested on rabbits. Natural compounds (4%) showed slight redness, dilation, and secretions after 4 and 12 h of exposure as presented in and , respectively. In the present study, all the studied compounds produced slight signs of redness at 4 and 12 h. Slight dilations and secretions were visible after 4 h that diminished at 12 h of exposure.
Figure 3. Comparison of redness, dilation, and secretion’s mean score ± SE of MF, MpC, PE, and control after 4 h of ocular application in rabbits (n = 8). 0 = No redness, dilation and secretion; 1 = very slight redness, dilation and secretion; 2 = mild to moderate redness, dilation and secretion; 3 = moderate to severe redness, dilation and secretion.
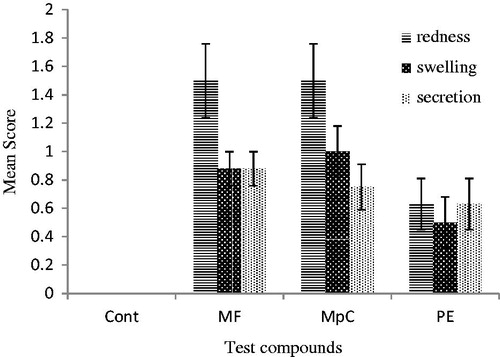
Figure 4. Comparison of redness, dilation, and secretion’s mean score ± SE of MF, MpC, PE, control after 12 h ocular application in rabbits (n = 8). 0 = No redness, dilation and secretion; 1 = very slight redness, dilation and secretion; 2 = mild to moderate redness, dilation and secretion; 3 = moderate to severe redness, dilation and secretion.
Dermal irritation
Erythema scores 96-h post non-occluded and occluded topical application of MF, MpC, and PE are presented in that indicates non-significant differences in dermal irritation.
Table 4. Mean ± SEM erythema scores 96-h post non-occluded versus occluded topical application of MF, MpC, and PE in rabbits (n = 4).
Microscopic examination of skin
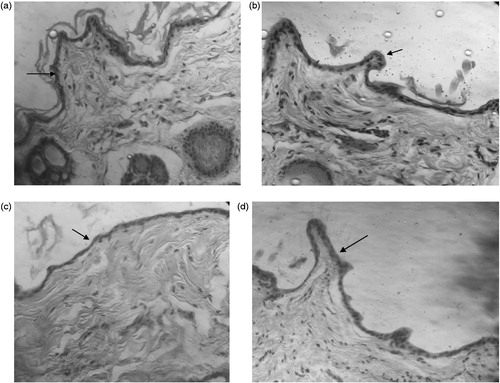
The microscopic examination of rabbit skin after topical application of MF, MpC, and PE exhibited non-significant changes with respect to ethanol control ().
Discussion
Hemolytic assay
The trend of using natural substances for therapeutic purposes is increased in recent years. The biocompatibility of these substances is an important consideration during formulation development. The formulations with a hemolysis value of less than 10% were considered non-hemolytic while values with more than 25% hemolysis were considered as hemolytic (Amin & Dannenfelser, Citation2006). In our study, MF and MpC were found to be hemolytic at higher concentrations and non-hemolytic at lower tested concentrations while PE was non-hemolytic at all the tested concentrations (). This type of hemolytic activity may be due to the lysis of the cell or some disturbances in the membrane integrity of RBCs (Powell et al., Citation2000) and may be due to the reduced xenobiotic compounds, such as phenols, that are capable of promoting hemolysis through oxidation of hemoglobin, forming metahemoglobin (Bukowska & Kowalska, Citation2004). Surfactants cause hemolysis through dissolution of the erythrocyte plasma membrane might be due to increased fragility or osmotic lysis by increased permeability of the plasma membrane (Aparicio et al., Citation2005). The erythrocyte lysis in the rats was also induced by peroxyl radical produced by different sources (Cheung et al., Citation2003).
The hemolysis produced by MF and MpC at higher concentrations in our present study is supported by the findings of Tamir et al. (Citation1984) who reported that high concentrations of citral produced 100% hemolysis as compared with low concentrations. This behavior is considered because of two mechanisms. At high concentrations, a non-specific steroid terpenoid or glutathione depletion occurs while at low concentrations a free radical mechanism might be involved (Tamir et al., Citation1984). All our tested compounds produced less than 10% hemolysis up to 20 mg/ml concentrations indicating that all these compounds are non-hemolytic. Generally, such compounds are used in pharmaceutical/cosmetic preparations at a concentration of about 1% (10 mg/ml) so all these tested compounds might be safe for therapeutic uses.
Ames assay
Since mutagenic compounds can potentially induce cancer, the identification of chemicals or compounds which are capable of inducing mutation is crucial in the safety assessment (Sugimura, Citation2000). The Ames assay is a widely used bacterial assay with high predictive value when comparing with rodent carcinogenicity test and for the identification of chemicals that can produce gene mutations (Leach et al., Citation1993). Salmonella mammalian microsome test (Ames test) was used to test the mutagenic properties of 76 artificial flavoring compounds. In the present study, all the tested compounds were non-mutagenic. Anyhow, there are a few reports in the literature indicating the mutagenic potential of some natural compounds such as alkaloid β-carboline that was reported to be mutagenic and genotoxic as well (Boeira et al., Citation2002). Pulegone was not mutagenic in S. typhimurium strains TA98 and TA100 (Andersen & Jensen, Citation1984); these results coincide with the results of the present study. The Ames test was also performed to evaluate the antigenotoxic and genotoxic properties of essential oils of Origanum onites L. (Lamiaceae). No mutagenic activity was found in screening with Salmonella typhimurium strains TA98 and TA100 (Ipek et al., Citation2005).
BSLB
The importance of medicinal plants and traditional health systems in solving the health care problems is gaining increasing attention. Some of these plants have been subjected to the isolation of the active ingredients (chemical compounds) and their subsequent modification (Krishnaraju et al., Citation2005). BSLB is commonly used to calculate the LC50 value of toxic substances and to evaluate the biologically active natural compounds (Pisutthanan et al., Citation2004). The crude extracts and pure substances were classified as toxic if the LC50 value was less than 1 mg/ml and non-toxic if the LC50 value was more than 1 mg/ml (Meyer et al., Citation1982). The pesticidal compounds exhibit good activity in BSLB (Mazid et al., Citation2008) and due to its low cost and simplicity, it is preferably used to evaluate the cytotoxic potential of natural compounds.
In the present study, six different concentrations of MF, MpC, and PE were used which showed LC50 values as 4.38, 6.74, and 25.91 mg/ml, respectively (). This study is supported by the findings that the presence of C–C double bond was the key factor for larvicidal activity of pulegone against Culex pipiens (Michaelakis et al., Citation2012). In our study, the LC50 values of all the tested compounds were more than 1 mg/ml so these were considered nontoxic.
Ocular irritation
In epidemiological studies, strained, burning, itchy eyes, eye secretions, and lacrimation along with redness are major signs of eye irritation (Brightman & Moss, Citation2000). Some unidentified irritants are present in oxidation products of terpenes (e.g., limonene). In indoor environments, these allergens are mostly responsible for a fraction of the reported eye and airway complaints (Nøjgaard et al., Citation2005).
In our finding, MF and MpC were found slightly irritant to eyes; signs of eye secretions, swelling, and redness were observed similar to the findings of Assad et al. (Citation2002). PE was non-irritant to rabbit eyes ( and ).
Dermal irritation
Humans are exposed to a variety of toxic insults such as pesticides or drugs during their professional life style. Some chemicals are less volatile and, therefore, persist on the exposed skin of human or animals for longer periods of times. The skin is naturally protected to different toxic agents by virtue of stratum corneum (SC), i.e., the superficial layer of epidermis. Because of the lipid nature of SC, most of the hydrophobic chemicals can reside on the SC for variable times and can result in local and systemic toxicity (Brand & Muller, Citation2002; Muhammad et al., Citation2005; Van der & Revier, Citation2005).
It was reported that most skin allergy reactions are due to fragrance ingredients used in daily life (Heisterberg et al., Citation2011). In the present investigation, skin irritation test was performed following the Draize test under occluded and non-occluded conditions. MF was found slightly irritant to skin under occluded and non-occluded conditions. Mean score for erythema after 96 h post occluded and non-occluded topical application was 1.5 and 1.75, respectively (). MpC was found slightly irritant to skin under occluded and non-occluded conditions. Mean score for erythema after 96 h post occluded and non-occluded topical application was 1.75 and 1.5, respectively. Pulegone is mostly found in peppermint oil and used as flavoring and aromatic ingredient in fragrances (Barba et al., Citation2013). In the skin irritation test, pulegone was found non-irritant to skin. However, this has been reported to induce a high grade of edema, redness, and itching on the skin surface when used in the patch test on hairy skin of a rabbit (Assad et al., Citation2002). Microscopic examination of skin biopsies in the present in vivo studies revealed intact/normal stratum corneum and epidermis () suggesting that topical application of various natural compounds on rabbit’s skin were non-irritant after repeated exposure for 4 d under both occluded and non-occluded conditions.
Conclusion
Our studies suggested that MF, MpC, and PE were non-hemolytic as well as non-mutagenic. The LC50 values for these compounds were more than 1 mg/ml indicating that all these compounds were non-toxic to brine shrimp larvae. Dermal and ocular irritation studies also suggested that these compounds were non-irritant. The present investigation suggests that these compounds are non-toxic/non-irritant and might be used for their pharmaceutical benefits.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Amin K, Dannenfelser RM. (2006). In vitro hemolysis: Guidance for the pharmaceutical scientist. J Pharm Sci 95:1173–6
- Andersen PH, Jensen NJ. (1984). Mutagenic investigation of peppermint oil in the Salmonella/mammalian-microsome test. Mutat Res 138:17–20
- Aparicio RM, José García-Celma M, Pilar Vinardell M, Mitjans M. (2005). In vitro studies of the hemolytic activity of microemulsions in human erythrocytes. J Pharm Biomed Anal 39:1063–7
- Assad M, Chernyshov A, Leroux MA, Rivard CH. (2002). A new porous titanium-nickel alloy: Part 2. Sensitization, irritation and acute systemic toxicity evaluation. Biomed Mater Eng 12:339–46
- Barba C, Santa-María G, Herraiz M, Martínez RM. (2013). Direct enantiomeric analysis of Mentha essential oils. Food Chem 141:542–7
- Boeira JM, Viana AF, Picada JN, Henriques JA. (2002). Genotoxic and recombinogenic activities of the two β-carboline alkaloids harman and harmine in Saccharomyces cerevisiae. Mutat Res 500:39–48
- Brand RM, Mueller C. (2002). Transdermal penetration of atrazine, alachlor, and trifluralin: Effect of formulation. Toxicol Sci 68:18–23
- Brightman HS, Moss N. (2000). Sick building syndrome studies and the compilation of normative and comparative values. In: Spengler JD, Samet JM, McCarthy JF, eds. Indoor Air Quality Handbook. New York: McGraw-Hill, 3.1–3.32
- Bukowska B, Kowalska S. (2004). Phenol and catechol induce pre-hemolytic and hemolytic changes in human erythrocytes. Toxicol Lett 152:73–84
- Calixto JB. (2005). Twenty-five years of research on medicinal plants in Latin America: A personal review. J Ethnopharmacol 100:131–4
- Cheung LM, Peter CKC, Vincent ECO. (2003). Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem 81:249–55
- Chun MH, Eun KK, Kang RL, et al. (2010). Quality control of Schizonepeta tenuifolia by solid-phase microextraction gas chromatography/mass spectrometry and principal component analysis. Microchem J 95:25–31
- Ferguson LR, Shuo-tun Z, Harris PJ. (2005). Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29. Mol Nutr Food Res 49:585–693
- Heisterberg MV, Menné T, Andersen KE, et al. (2011). Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis 64:258–64
- Ipek E, Hulya Z, Sezer O, et al. (2005). Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem 93:551–6
- Kampa M, Alexaki VI, Notas G, et al. (2004). Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res 6:63–74
- Karamac M, Bucinski A, Pegg RB, Amarowicz R. (2005). Antioxidant and antiradical activity of ferulates. Czech J Food Sci 23:64–8
- Klaassen CD. (2008). Casarett and Doull’s Toxicology: The Basic Science of Poisons. 7th ed. New York: McGraw-Hill
- Krishnaraju AV, Tayi VNR, Dodda S, et al. (2005). Assessment of bioactivity of Indian medicinal plant using brine shrimp (Artemia salina) lethality assay. Int J Appl Sci Eng 3:125–34
- Leach FS, Nicolaides NC, Papadopoulos N, et al. (1993). Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75:1215–25
- Lee YS. (2005). Role of NADPH oxidase-mediated generation of reactive oxygen species in the mechanism of apoptosis induced by phenolic acids in HepG2 human hepatoma cells. Arch Pharm Res 28:1183–9
- Lima TC, da Silva TK, Silva FL, et al. (2014). Larvicidal activity of Mentha x villosa Hudson essential oil, rotundifolone and derivatives. Chemosphere 104:37–43
- Lima TC, Marcelo MM, José MBF, et al. (2012). Structural relationships and vasorelaxant activity of monoterpenes. DARU J Pharm Sci 20:3
- Lima IO, Oliveira RAG, Lima EO, et al. (2006). Atividade antifúngica de óleos essenciais sobre espécies de Candida. Rev Bras Farmacogn 16:197–201
- Machado KC, Oliveira GL, de Sousa ÉB, et al. (2015). Spectroscopic studies on the in vitro antioxidant capacity of isopentyl ferulate. Chem Biol Interact 225:47–53
- Mazid MA, Datta BK, Nahar L, Sarker SD. (2008). Assessment of antibacterial activity and brine shrimp toxicity of two Polygonum species. Ars Pharm 49:127–34
- Meyer BN, Ferrigni NR, Putnam JE, et al. (1982). Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med 45:31–4
- Michaelakis A, Papachristos D, Kimbaris A, Polissiou M. (2012). Larvicidal evaluation of three Mentha species essential oils and their isolated major components against the West Nile virus mosquito. Hellenic Plant Protect J 4:35–43
- Mohd-Fuat AR, Kofi EA, Allan GG. (2007). Mutagenic and cytotoxic properties of three herbal plants from Southeast Asia. Trop Biomed 24:49–59
- Muhammad F, Monteiro-Riviere NA, Baynes RE, Riviere JE. (2005). In vivo jet fuel exposure on subsequent in vitro dermal absorption individual aromatic and aliphatic hydrocarban fuel constituents. J Toxicol Environ Health A 68:719–97
- Nøjgaard JK, Christensen KB, Wolkoff P. (2005). The effect on human eye blink frequency of exposure to limonene oxidation products and methacrolein. Toxicol Lett 156:241–51
- Oliveira FP, Lima EO, Siqueira Júnior JP, et al. (2006). Effectiveness of Lippia sidoides Cham. (Verbenaceae) essential oil in inhibiting the growth of Staphylococcus aureus strains isolated from clinical material. Rev Bras Farmacogn 16:510–16
- Phuong NTM, Trinh TC, Dang NQ. (2014). Anti-inflammatory activity of methyl ferulate isolated from Stemona tuberosa Lour. Asian Pac J Trop Med 7:S327–31
- Pisutthanan S, Pinyupa P, Nisit P, et al. (2004). Brine shrimp lethality activity of Thai medicinal plants in the family Meliaceae. Naresuan Univ J 12:13–18
- Powell WA, Catranis CM, Maynard CA. (2000). Design of self-processing antimicrobial peptides for plant protection. Lett Appl Microbiol 31:163–8
- Shahid M, Bukhari SA, Gul Y, et al. (2013). Graft polymerization of guar gum with acryl amide irradiated by microwaves for colonic drug delivery. Int J Biol Macromol 62:172–9
- Sousa OV, Soares-Júnior DT, Del-Vechio G, et al. (2004). Atividades antinociceptiva e antiinflamatória do óleo essencial de cascas de Duguetia lanceolata St. Hil., Annonaceae. Rev Bras Farmacogn 14:11–4
- Sugimura T. (2000). Nutrition and dietary carcinogens. Carcinogenesis 21:387–95
- Tamir I, Abramovici A, Milo-Goldzweig I, Segal R. (1984). The hemolytic activity of citral: Evidence for free radical participation. Biochem Pharmacol 33:2945–50
- Ullah MO, Haque M, Urmi KF, et al. (2013). Antibacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac J Trop Biomed 3:1–7
- Van der MD, Riviere JE. (2005). Comparative studies on the effects of water, ethanol and water/ethanol mixtures on chemical partitioning into porcine stratum corneum and silastic membrane. Toxicol In Vitro 19:69–77
- Yao CS, Lin M, Liu X, Wang YH. (2005). Stilbene derivatives from Gnetum cleistostachyum. J Asian Nat Prod Res 7:131–7
- Zuber M, Shazia T, Tahir J, et al. (2014). Biocompatibility and microscopic evaluation of polyurethane–poly(methyl methacrylate)–titnanium dioxide based composites for dental applications. J Appl Polym Sci 131:39806