Abstract
Context: Some Launaea species (Asteraceae) are used traditionally to treat liver oxidative stress.
Objective: The present study investigates the protective effects of isolated compounds from Launaea spinosa Sch. Bip. (Asteraceae) against oxidative stress on t-BHP-induced HepG2 cells.
Materials and methods: Major phenolic content from flowering aerial parts of L. spinosa was isolated and identified. The protective effects of isolated compounds (10 and 20 μM) against oxidative stress induced by tert-butyl hydroperoxide (t-BHP) in HepG2 cells were investigated through the measurement of aspartate aminotransferase (AST), alanine transaminase (ALT), and superoxide dismutase (SOD) levels.
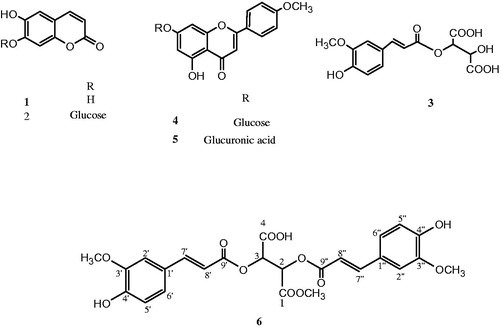
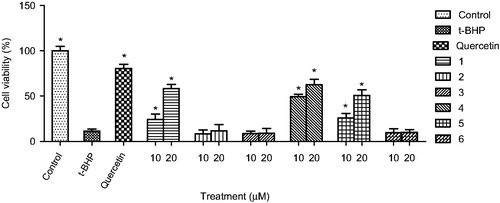
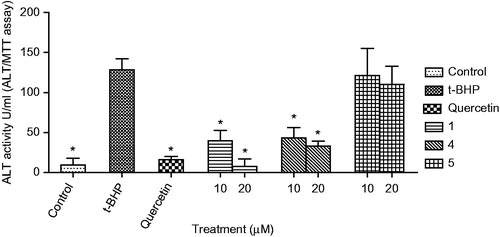
Results: A new phenolic compound identified as 2,3-diferulyl R,R-(+) methyl tartrate (6), in addition to five known metabolites, esculetin (1), esculetin-7-O-d-glucoside (cichoriin) (2), fertaric acid (3), acacetin-7-O-d-glucoside (4), and acacetin-7-O-d-glucuronic acid (5), were isolated. Oxidant-induced damage by 200 μM t-BHP in HepG2 cells was inhibited by compounds 1, 4, and 5 (10 and 20 μM), or quercetin (10 μM; positive control). The protective effects of compounds 1, 4, and 5 were associated with decreasing in AST, ALT, and SOD levels. Compound 4 (20 μM) decreased the AST level from 128.5 ± 13.9 to 7.9 ±1.8 U/mL. Meanwhile, compound 1 (20 μM) decreased ALT activity from 20.3 ± 7.0 to 7.6 ± 2.4 U/mL, while compound 5 decreased SOD levels from 41.6 ± 9.0 to 28.3 ± 3.4 mU/mg.
Conclusion: The major phenolic compounds isolated from L. spinosa displayed a significant cytoprotective effect against oxidative stress, leading to maintenance of the normal redox status of the cell.
Introduction
Oxidative stress in a cell is a state characterized by excess production of reactive oxygen species (ROS) and/or a reduction in antioxidant defenses responsible for metabolism; it can ultimately cause cell death. ROS are formed as a natural by-product of the normal metabolism and under normal circumstances; the cell is able to maintain equilibrium between formation and removal of ROS through enzymatic pathways or via antioxidants (Barzilai et al., Citation2002). The liver is one of the most important organs in the body, playing a pivotal role in regulating various physiological processes. It is involved in several vital functions, such as metabolism, secretion, and storage in addition to its vast capacity to detoxify noxious substances (Abdallah et al., Citation2013). In particular, ROS play a critical role in disease induction and progression of liver diseases such as hepatocellular carcinoma, viral, and alcoholic hepatitis (Vitaglione et al., Citation2005). Because oxidative stress plays a central role in liver diseases, antioxidants have been proposed as therapeutic agents to counteract liver damage (Vitaglione et al., Citation2005).
Many medicinal plants contain large amounts of antioxidants such as polyphenols, which can play an important role in adsorbing and neutralizing free radicals (Abdallah et al., Citation2013; Anderson et al., Citation2001; Ezzat et al., Citation2012). The genus Launaea (tribe Lactucaea, family Asteracea) comprises about 40 species. Many of its plants are used in folk medicine as hepatoprotective, bitter stomachic, for skin diseases, as antitumor and as insecticides (Khan et al., Citation2012). The genus Launaea is rich in different secondary metabolites, such as terpenoids (Abdel Fattah et al., Citation1990; Abdel Salam et al., Citation1986; Hook et al., Citation1984; Sarg et al., Citation1982; Sokkar et al., Citation1993), phenolics (El-Bassuony & Abdel-Hamid, Citation2006; Giner et al., Citation1992; Sarg et al., Citation1986), flavones (Gupta & Ahmed, Citation1985; Mansour et al., Citation1983), and coumarins (Saleh et al., Citation1988; Sarg et al., Citation1987).
In the present study, the potential cytoprotective activity of phenolic compounds isolated from Launaea spinosa Sch. Bip. (Asteraceae) was assessed against oxidative stress induced by tert-butyl hydroperoxide (t-BHP) in HepG2 cells in order to relate in vitro antioxidant activity with cytoprotective effects. The cytoprotective activity of the most effective compounds on HepG2 was further investigated through the determination of aspartate aminotransferase (AST), alanine transaminase (ALT), and superoxide dismutase (SOD) activities.
Material and methods
General
Optical rotation was measured on a Jasco P-2000 Polarimeter (JASCO, Easton, MD) (solvent MeOH). UV spectra were recorded in MeOH solutions (Mabry et al., Citation1970) on a UV IKON 940 spectrophotometer (IKON Inc., Malvern, PA). NMR experiments were performed on a Bruker DRX-600 spectrometer (Bruker Inc., Billerica, MA) relative to TMS in DMSO. Mass spectra were measured on Acquity UPLC system (Waters, Milford, MA) using a MicrOTOF-Q hybrid quadrupole time-of-flight mass spectrometer (Bruker Daltonics, Billerica, MA). Column chromatographic separation (CC) was performed on Sephadex LH-20 (Pharmacia Fine Chemicals Inc., Uppsala, Sweden), silica gel 60 for column Chromatography (70–230 mesh, Fluka, Steinheim, Germany), Silica gel 100 C18 – reversed phase (70–230 mesh, Fluka, Steinheim, Germany). TLC was performed on pre-coated TLC plates with silica gel 60 F254 (Merck, Darmstadt, Germany).
Plant material
Aerial parts of L. spinosa were collected from Wadi Hagol, Suiz governorate, Egypt, in 2012. The plant was kindly identified by Dr. M. Gibali, Senior Botanist, Faculty of Sciences, Cairo University, Cairo, Egypt. A herbarium specimen (2012-0411) was prepared and kept at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Cairo, Egypt.
Extraction, isolation, and identification
The air-dried powdered flowering aerial parts of L. spinosa (2 kg) were extracted with methanol (4 × 5 L) at room temperature, until exhaustion. The combined extracts were filtered and concentrated to give a brown residue (150 g). The residue was suspended in distilled water (500 mL) and partitioned successively with chloroform (4 × 500 mL) and n-butanol (4 × 500 mL) to yield 90 and 40 g, respectively. The n-butanol (flavonoid rich) fraction was suspended in water, precipitated with excess absolute EtOH, filtered, and dried (10 g). The residue was chromatographed over silica gel 60 H for vacuum liquid chromatography VLC (5 × 7.5 cm, 50 g) with chloroform, chloroform–MeOH mixtures, with a gradual increase in polarity up to 100% MeOH. Fractions 200 mL each were collected and monitored by TLC. The spots were visualized under UV and after spraying with p-anisaldehyde followed by heating. Similar fractions were pooled to give four main fractions (A–D). Fraction A (20% MeOH, 1.5 g) was rechromatographed over Sephadex LH-20 column (Sigma-Aldrich, St. Louis, MO) (25 × 2 cm, 50 g), using MeOH as an eluent to afford compound 1 (30 mg). Fraction B (40% MeOH, 2 g) was chromatographed over Sephadex LH-20 column (Sigma-Aldrich, St. Louis, MO) (25 × 2 cm, 50 g), using MeOH–water (1:1) as an eluent to afford compound 2 (50 mg). Fraction C (60% MeOH, 2 g) was chromatographed over silica gel 100 C18 – reversed phase using MeOH–water, 1:9 v/v as an eluent to afford compounds 3 (17 mg) and 4 (22 mg). Fraction D (80% MeOH, 1.5 g) was chromatographed over silica gel 60 column (25 × 2 cm, 50 g), using CHCl3:MeOH (9:1 v/v) as an eluent to give two subfractions: I and II. Subfraction I was purified over sephadex LH-20 column using CHCl3–MeOH (1:1) as an eluent to yield compound 5 (30 mg). Subfraction II was purified over silica gel 100 C18 – reversed phase using MeOH–water, 2:8 v/v as an eluent to afford compound 6 (17 mg).
2,3-Diferulyl R,R-(+) methyl tartrate (6) (): yellow amorphous powder; +33 (c. 0.1, MeOH); UV (MeOH) λmax (log ε) 320(2.74), 275(2.98), 261(2.88), nm. For 1H (600 MHz, DMSO-d6), 13C NMR (150 MHz, DMSO-d6) and HMBC (DMSO-d6): see (). ESI-MS (negative): m/z 515.1189 [M-H]− (calcd. 515.1210).
Table 1. 1H and 13C-NMR and HMBC spectral data of compound (6) (DMSO).
Cell culture
HepG2 cells (human hepatocellular carcinoma cell line) were obtained from the American Type Culture Collection (Manassas, VA). HepG2 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin. The cells were cultured in a humidified atmosphere maintained with 5% CO2 at 37°C. For the cellular treatment, HepG2 cells were cultured in DMEM excluding FBS to reduce direct interaction between the tested chemicals and FBS.
Measurement of HepG2 cell viability
To evaluate the hepatoprotective activity against oxidative stress, the viability of HepG2 cells injured with tert-butyl hydroperoxide (t-BHP) was determined. HepG2 cells (1 × 104 cells per well) were plated onto 96 well plates, incubated in DMEM supplemented with FBS for 24 h. Then, the cells were pre-incubated with compounds (1–6) (10 or 20 μM) and quercetin (10 μM) in serum-free DMEM for 24 h. Cells were washed with Dulbecco's phosphate buffered saline (DPBS), and treated with t-BHP (200 μM) in serum-free DMEM for 3 h. Cell viability () was determined by the measurement of mitochondrial dehydrogenase activity using the EZ-Cytox cell viability assay kit (Daeil Lab Service, Seoul, Korea) as previously described (Kang et al., Citation2011; Kim et al., Citation2011).
Measurement of ALT and AST activities
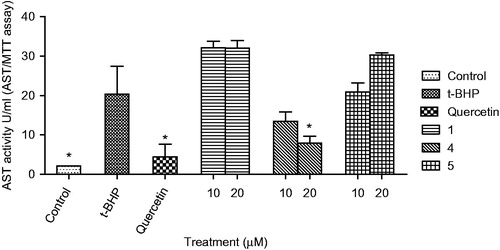
HepG2 cells were cultured in DMEM containing 10% FBS under 5% CO2 at 37°C. The protective effect of compounds on HepG2 cells injured by t-BHP was measured using aspartate aminotransferase kit (BioVision, San Francisco, CA) and alanine aminotransferase kit (BioVision, San Francisco, CA). Briefly, HepG2 cells were dispensed into 96-well plates at the concentration of 1 × 104 cells per well. The test compounds (10 or 20 μM) and quercetin (10 μM) were added into HepG2 cells, and pre-incubated for the next treatment for 24 h. Then the cultured media were replaced to the media containing t-BHP (200 μM), incubated for 3 h, and then AST and ALT were measured in culture broth ( and ).
Measurement of SOD
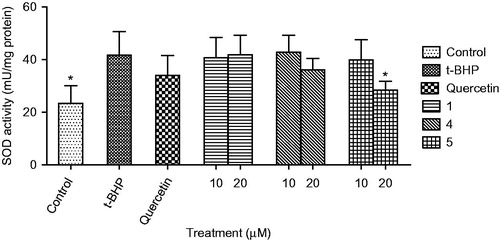
HepG2 cells were cultured in DMEM containing 10% FBS and 1% ampicillin–streptomycin solution under 5% CO2 at 37°C. The protective effect of compounds on HepG2 cells injured by t-BHP was measured using superoxide dismutase enzyme activity kit (Cell Biolabs, San Diego, CA). Briefly, HepG2 cells were dispensed into 100 mm dish at the concentration of 2 × 106 cells per well. The test compounds (10 or 20 μM) and quercetin (10 μM) were added into HepG2 cells and pre-incubated for 24 h. Then the cultured media were replaced to the media containing t-BHP (200 μM), incubated for 3 h and then SOD activity was measured in cell lysates ().
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test using the GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA). p Values < 0.05 were considered statistically significant.
Results
Phytochemical investigation of the flavonoid-rich fraction from flowering aerial parts of L. spinosa led to the isolation of a new diferulyl methyl tartrate ester (6) in addition to five known metabolites (1–5) (). The structures of the known compounds were identified based on the comparison of their spectral data with those previously published and confirmed through co-chromatography with authentic samples. They were identified as esculetin (1) (El-Bassuony & Abdel-Hamid, Citation2006), esculetin-7-O-d-glucoside (cichoriin) (2) (El-Bassuony & Abdel-Hamid, Citation2006), fertaric acid (3) (Qian et al., Citation1993), acacetin-7-O-d-glucoside (4) (Mabry et al., Citation1970), and acacetin-7-O-d-glucuronic acid (5) (Mabry et al., Citation1970).
In this study, the protective effects of the bioactive compounds isolated from L. spinosa on oxidative stress were investigated. Treatment of HepG2 cells with t-BHP (200 μM) significantly decreased cell viability to 11.5% of the control cells. Pre-treatment with only compounds 1, 4, and 5 (10 and 20 μM), or quercetin (10 μM) significantly protected HepG2 cell death induced by t-BHP in a dose-dependent manner (). Compound 4 showed the strongest protective activity among the compounds from L. spinosa. The cytoprotective effect of compounds 1, 4, and 5 on oxidative stress was further confirmed through measurement of the changes in aspartate aminotransferase (AST) (), alanine transaminase (ALT) (), and superoxide dismutase (SOD) () activities before and after exposure of the cells to t-BHP. Exposure of HepG2 cells to t-BHP significantly (p < 0.05) increased AST, ALT, and SOD levels to 20.3 ± 7.0 U/mL, 128.5 ± 13.9 U/mL, and 41.6 ± 9.0 mU/mg, respectively, in comparison with control untreated cells. Pretreatment with 10 μM of quercetin significantly ameliorated t-BHP-induced damage through decreasing AST, ALT, and SOD activities to 4.4 ± 3.1 U/mL, 15.8 ± 3.9 U/mL, and 33.9 ± 7.6 mU/mg, respectively. Compound 4 at dose 20 μM significantly normalized elevated AST activity, whereas its level decreased to 7.9 ± 1.8 U/mL. Meanwhile, compounds 1 and 4 at the two dose levels significantly decreased ALT activity, whereas compound 1 at 20 μM showed the highest protection to HepG2 cells. It decreased ALT activity to 7.6 ± 2.4 U/mL, a value which is more potent than quercetin. Moreover, compound 5 showed more activity on SOD levels than quercetin, it significantly decreased its level to 28.3 ± 3.4 mU/mg.
Discussion
Compound 6 was obtained as a yellowish amorphous powder. It was suggested to be ferulyl methyl tartrate derivative on the basis of UV spectral data and mass analysis. Its UV spectrum (in methanol) exhibited a characteristic band at ≈ 320 nm, along with an extra strong maximum at 261, 275 nm, indicative of a cinnamoyl derivatives moiety (Abdallah et al., Citation2013). Its negative HRESI-MS showed a molecular ion peak at m/z 515.1189 [M-H]− (calcd. 515.1210), corresponding to a molecular formula of Fragmentation of the major ion peak showed three major diagnostic fragments at m/z 193.0517 [ferulic acid] − corresponding to a molecular formula of
, 339.0734 [feruolyl methyl tartrate] − corresponding to a molecular formula of
, and 321.0734 [feruolyl methyl tartrate–H2O] − corresponding to a molecular formula of
.
1H NMR spectrum of 6 () indicated the presence of two ferulyl moieties with trans configuration (E). This was evident from the two overlapping ABX system in the aromatic region for 1,3,4-trisubstituted phenyl at δH 7.33 (d, J = 8.4 Hz) and δH 7.32(d, J = 8.4 Hz) assignable to H-2′ and 2″, respectively, δH 6.65 (m) and δH 6.78(m) assignable to H-5′ and 5″, respectively, and δH 7.08 (m) and δH 7.11(m) assignable to H-6′ and 6″, respectively. In addition, two AX-spin-coupled systems of two trans (E)-olefinic double bonds (d, J = 15.6 Hz) were observed at δH 7.49, 7.55, 6.48, and 6.44 were assigned for of H-7′, H-7″, H-8′, and H-8″, respectively. Three oxygenated methyl singlets at δH 3.74, δH 3.80, and 3.63 correspond to δC 55.5, 55.6, and 52.0, respectively. A long-range correlation in HMBC confirmed the connection of two methoxyl groups to the aromatic ring, which indicated the presence of two ferulic acid moieties. Their positions were confirmed to be at C-3′ and 3″, respectively, through their long-range correlation in HMBC with carbons at δC 146.8 and 147.9. The position of the third oxygenated methyl was attached to carbon of the carboxylic group (C-1) at δC 166.7 through long-range correlation in HMBC.
1H NMR displayed also two broad singlets δH at 5.37 and 5.76 corresponding to H-2 and H-3 of tartaric acid. Their downfield shifts from their typical value were due to bonding with the carboxylic group of ferulic acid (Ali et al., Citation2012). This evidence was further confirmed from key long-range three bond HMBC correlations. The HMBC exhibited correlations between H-2 of tartaric acid at δH 5.37 and C-9′ (δC 166.0) and H-3 at δH 5.76 and C-9″ (δC 165.9) (Ali et al., Citation2012). The optical activity (dextro) and chemical shift of the two protons at the asymmetric carbons of tartaric acid confirmed its stereochemistry to be R,R-(+) (Qian et al., Citation1993). Based on the above spectral data, compound 6 was identified as 2,3-diferulyl R,R-(+) methyl tartrate.
In this study, the protective effects of the bioactive compounds isolated from L. spinosa on oxidative stress were investigated. Oxidative stress can be defined as an imbalance between the oxidant and the antioxidant system. Under normal circumstances, the levels of ROS are low enough to be removed by the natural defense systems of the cell. However, when ROS is induced by oxidants to such an extent that cellular defenses are overwhelmed, the cells are exposed to oxidative stress, consequently leading to cell injury (Alia et al., Citation2005). Numerous studies noted that t-BHP induces an array of cellular dysfunctions, including generation of peroxyl radicals, peroxidation of membrane lipids, glutathione and protein thiol deletion, and DNA damage, and eventually leading to cell death (García-Alonso et al., Citation2006). To evaluate the cytoprotective activity against oxidative stress, the cell viability in HepG2 cells damaged with t-BHP was measured. Quercetin, a natural antioxidant, was used as a positive control (Alia et al., Citation2006; Kang et al., Citation2011).
In this work, the cytoprotective effects of the phenolic compounds isolated from L. spinosa were examined on oxidative stress. Many studies have suggested that cytoprotective activity of flavonoids (4 and 5) devoted to their antioxidant activity, which is due to their ability to reduce free radical formation and also to scavenge free radicals. Esculetin (1) efficiently attenuated the oxidative stress induced cell damage via its anti-oxidant properties (Zhang, Citation2008). Moreover, it significantly reduced CCl4-induced hepatic apoptosis in rats, probably by exerting a protective effect against hepatocellular apoptosis with its free-radical scavenging ability and inhibiting the mitochondrial-dependent apoptotic pathway in CCl4-induced liver apoptosis in rats (Tien et al., Citation2011).
Conclusion
The cytoprotective effect of L. spinosa against oxidative stress induced by t-BHP on HepG2 cells is at least partly due to its constituents of phenolic compounds which displayed cytoprotection as evidenced by the reduction in AST, ALT, and SOD levels.
Declaration of interest
The authors report no conflicts of interest. This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under Grant no. 207/166/1433. The authors thank DSR for technical and financial support.
References
- Abdallah HM, Mohamed MA, Abdou AM, et al. (2013). Protective effect of Centaurea pallescens Del. against CCl4-induced injury on a human hepatoma cell line (Huh7). Med Chem Res 22:5700–6
- Abdel Fattah H, Zaghloul A, Halim A, Waight E. (1990). Steroidal and triterpenoid constituents of Launaea resedifolia L. Egypt J Pharm Sci 31:81–91
- Abdel Salam N, Mahmoud Z, Kassem F. (1986). Sesquiterpene lactones, coumarins and flavonoids of Launaea tenuiloba Boiss grown in Egypt. J Pharm Sci 27:275–82
- Ali K, Iqbal M, Korthout HA, et al. (2012). NMR spectroscopy and chemometrics as a tool for anti-TNFα activity screening in crude extracts of grapes and other berries. Metabolomics 8:1148–61
- Alia M, Mateos R, Ramos S, et al. (2006). Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur J Nutr 45:19–28
- Alia M, Ramos S, Mateos R, et al. (2005). Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). J Biochem Mol Toxicol 19:119–28
- Anderson KJ, Teuber SS, Gobeille A, et al. (2001). Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J Nutr 131:2837–42
- Barzilai A, Rotman G, Shiloh Y. (2002). ATM deficiency and oxidative stress: A new dimension of defective response to DNA damage. DNA Repair 1:3–25
- El-Bassuony AA, Abdel-Hamid N. (2006). Antibacterial coumarins isolated from Launaea resedifolia. Chem Plant Raw Mater 1:65–8
- Ezzat SM, Abdallah HM, Fawzy GA, El-Maraghy SA. (2012). Hepatoprotective constituents of Torilis radiata Moench (Apiaceae). Nat Prod Res 26:282–5
- García-Alonso J, Ros G, Jesús Periago M. (2006). Antiproliferative and cytoprotective activities of a phenolic-rich juice in HepG2 cells. Food Res Int 39:982–91
- Giner RM, Díaz J, Máñez S, et al. (1992). Phenolics of Spanish Launaea species. Biochem Syst Ecol 20:187–8
- Gupta D, Ahmed B. (1985). Asplenetin, a flavone and its glycoside from Launaea asplenifolia. Phytochemistry 24:873–5
- Hook F, Behari M, Gupta R, Matsumoto T. (1984). Chemical constituents of Launaea nudicaulis. Indian Drugs 21:366–8
- Kang K, Jho EH, Lee HJ, et al. (2011). Youngia denticulata protects against oxidative damage induced by tert-butylhydroperoxide in HepG2 cells. J Med Food 14:1198–207
- Khan RA, Khan MR, Ahmed M, et al. (2012). Hepatoprotection with a chloroform extract of Launaea procumbens against CCl4-induced injuries in rats. BMC Complement Alternat Med 12:114
- Kim SM, Kang K, Jho EH, et al. (2011). Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother Res 25:1011–17
- Mabry TJ, Markham KR, Thomas MB. (1970). The Systematic Identification of Flavonoids. Berlin: Spriger
- Mansour R, Ahmed AA, Saleh NA. (1983). Flavone glycosides of some Launaea species. Phytochemistry 22:2630–1
- Qian T-X, Yan Z-H, Li L-N. (1993). Mono-feruloyl-R,R-(+)-tartaric acid form Salvia. J Chin Pharm Sci 2:148–50
- Saleh M, Habib A, El-Ghazooly M, et al. (1988). Chemical constituents of Launaea nudicaulis (L.), Egypt. J Pharm Sci 29:507–13
- Sarg T, Ateya A, Dora G. (1987). Constituents of Egyptian medicinal plants. Fitoterapia 58:133–4
- Sarg T, Omar A, Ateya A, Hafiz S. (1986). Phenolic constituents of Launaea nudicaulis (L.). Egypt J Pharm Sci 25:35–40
- Sarg T, Omar A, Khafagy S, et al. (1982). 11β,13-dihydrolactucin, a sesquiterpene lactone from Launaea mucronata. Phytochemistry 21:1163
- Sokkar N, Aboutable E, Kubelka W, et al. (1993). Terpenoids from Launaea spinosa (Forssk). Plant Med Phytother 26:368–74
- Tien Y-C, Liao J-C, Chiu C-S, et al. (2011). Esculetin ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. Int J Mol Sci 12:4053–67
- Vitaglione P, Morisco F, Caporaso N, Fogliano V. (2005). Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr 44:575–86
- Zhang R. (2008). Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharmacol Sin 29:1319–26