Abstract
Context: Shorea robusta Gaertn.f. (Dipterocarpaceae) resin is used for treating infected wounds and burns by tribals in India.
Objectives: The objective of this study was to investigate wound-healing activity of S. robusta resin extracts and essential oil in rats.
Materials and methods: Methanol extract (SRME), petroleum ether, benzene insoluble fraction of methanol extract (SRPEBIME), and essential oil (SREO) of S. robusta resin were incorporated in soft yellow paraffin (10% w/w) and applied once daily on incision and excision wounds of Wistar rats. Framycetin ointment (1.0% w/w) was applied to the standard group. Tensile strength (on the 10th day), wound contraction, and scar area (on the 14th day) were recorded. On the 15th day, granulation tissues of excision wounds were analyzed for total protein, hydroxyproline, and hexosamine contents and activities of lipid peroxidation and super oxide dismutase (SOD). Histopathology of the wounds was also studied.
Results and discussion: SRPEBIME and SREO healed incision and excision wounds faster than plain ointment base and framycetin. Tensile strength of SRPEBIME-treated incision wounds was 53% higher than that of control animals. In excision wounds, wound contraction and scar areas were found to be 99% and 7.7 mm2 (SRPEBIME) and 71.7% and 21 mm2 (control). Protein and hydroxyproline contents were higher in SRPEBIME (20.8 and 3.5% w/w) and SREO (17.4 and 2.8% w/w) groups as against 9.95 and 1.48% w/w in control groups. Histopathology revealed complete epithelization and new blood vessel formation in SRPEBIME groups.
Discussion and conclusion: SRPEBIME and SREO have significant wound-healing activities on incision and excision wounds.
Introduction
Shorea robusta Gaertn. f. (Dipterocarpaceae) is a common tree found in Indonesia, Malaysia, the Philippines, and certain parts of Northern and Eastern India. In India, the tree is known as “Sal” (Chitale & Behra, Citation2012). The resin obtained from the stem is aromatic, whitish brown, translucent, and brittle. In India, the leaves of S. robusta are used to expel intestinal worms; the bark powder is applied to treat cuts and wounds by the tribal people of Bihar and Odissa (Ganesan et al., Citation2006). Resin, in combination with honey or sugar, is recommended in dysentery and bleeding piles (Chandel et al., Citation1996). Different monoterpenes and sesquiterpenes were reported from the oil of the resin (Pakniker & Bhattacharya, Citation1961). Various triterpene ursane derivatives were reported from the petroleum ether, benzene insoluble methanol extract (PEBIME) of the resin (Hota & Bapuji, Citation1993, Citation1994). A 70% ethanol extract of the resin was found to have significant anti-inflammatory and antipyretic activities (Wani et al., Citation2012a). Ointments with 10% w/w and 30% w/w of the ethanol extract of the resin were reported to show a dose-dependant improvement of wound contraction, tensile strength of the wound, and hydroxyproline content of wound tissue (Wani et al., Citation2012b). The effect of resin extract in flax seed oil (1:4) on wound healing was studied both alone and in combinations with “amlaki” [Phyllanthus emblica Linn. (Euphorbiaceae)] fruit extract, cow ghee, and “yashada bhasma” (zinc oxide). The combinations were reported to have better healing effect over the resin alone (Datta et al., Citation2011). There was no study on the activity of the essential oil and the triterpene rich non-polar fraction of the methanol extract. The activity of the resin on the lipid peroxidation and superoxide dismutase of the wound tissue has not been reported. Previous studies also did not incorporate activity of the resin on histopathology and hexosamine levels of the wounds. We report here the wound-healing activity of the essential oil, methanol extract, and triterpene-rich petroleum ether, benzene insoluble methanol fraction (SRPEBIME) of S. robusta resin in rats.
Materials and methods
Resin
The resin was collected in June 2007 from S. robusta (“Sal”) trees growing in the forests of Singrauli, Madhya Pradesh, India. The resin was authenticated by Dr. Bhaskar Punjani, Department of Botany, Smt. S.M. Panchal Science College, Talod, Gujarat, India. A specimen of the resin was deposited at the Institute of Pharmacy, Nirma University of Science and Technology, Ahmedabad, India (specimen no. MPH/08/03/045).
Chemicals
Analytical grade chemicals and reagents were used in all the experiments. The sources of the chemicals and reagents are as mentioned in the parenthesis. Folin–Ciocalteu’s phenol reagent and bovine albumin (S.D. Fine Chemicals Limited, Mumbai, India), p-dimethylamino benzaldehyde (Merck Specialties, Mumbai, India), l-hydroxyproline, glucosamine hydrochloride, galactosamine hydrochloride, epinephrine bitartarate, and thio-barbituric acid (Central Drug House, New Delhi, India), framycetin sulfate ointment (Aventis Pharmaceuticals Limited, Mumbai, India), and standard pellet diet (Lipton’s India, New Delhi, India).
Extracts of the resin
Dried resin powder (50 g) of S. robusta was exhaustively extracted with 200 mL of methanol in a Soxhlet apparatus. The extract was completely evaporated to dryness in a rotary evaporator at 40 °C (yield 29.0 g) and stored at 4 °C until further use (SRME). The dried methanol extract (15 g) was successively extracted in cold petroleum ether (boiling range 60–80 °C) followed by benzene and the extracts were collected by decanting. The insoluble residue (10.5 g) was taken for the experiments (SRPEBIME).
Volatile oil of the resin
Dried resin powder (100 g) was subjected to dry distillation without water in a Clevenger glass apparatus and the condensed oil (SREO) was collected (yield 13.0 mL).
Preparation of ointments
Ointments were prepared by incorporating 10% w/w of SRME/10% w/w of SRPEBIME/10% v/w SREO in yellow soft paraffin.
Animals and ethics
The experiments were carried out in the Pharmacology Department of Institute of Pharmacy, Nirma University of Science and Technology, after obtaining necessary clearances from the Institutional Animal Ethics Committee (IAEC No. IPS/PCOG/MPH08/003). Albino rats (Wistar strain) of either sex (180–200 g) were used in the experiments. Animals were procured from the animal breeding house of Zydus Research Centre, Ahmedabad, India. The animals were maintained under 12 h day/night cycle at 25 ± 1 °C and 40–70% RH. Animals were housed individually in polypropylene cages and were kept for acclimatization for a period of 2 weeks before starting the experiments. Animals had free access to standard pellet diet and clean drinking water.
Animal grouping and application of ointments
For the evaluation of the extracts on incision wounds and excision wounds, rats were divided into five groups of six rats each. Animals in groups I, II, III, IV, and V were treated with topical application of simple ointment base (control), ointments with 1% framycetin sulfate (standard), 10% w/w SRME, 10% w/w SRPBIME, and 10% v/w SREO, respectively. Equal quantities (0.5 g) of ointments were applied once daily after cleaning the wounds with a 10% v/v dilute solution of dettol. The wounds were kept open without any dressing during the entire study.
Evaluation of wound healing activity
Wound models reported in the literature for excision (Morton & Malone, Citation1972) and incision (Nayak et al., Citation2006) wound were employed in the present study.
Incision wounds
As reported in the literature, incision wounds were made in ketamine hydrochloride (120 mg/kg, i.p.) anaesthetized animals (Erhlich et al., Citation1969) and the wounds were stitched by bringing the parted skin together (Mukherjee et al., Citation2003). Wounds were treated with respective ointments from day 0 to day 9. Sutures were removed from the wound on the 7th day and the wound-breaking strength was estimated on the 10th day on a digital tensilometer. Briefly, excised piece of skin (1 cm wide) bearing the wound from both incisions was tightened between the two clips of the tensilometer and the force was increased gradually until the wound disrupted (DiPietro & Burns, Citation2003). Skin-breaking strength was expressed as weight (g/cm). The skin pieces with wounds were processed for histological studies on the 10th day.
Excision wound model
Rats were anesthetized with ketamine hydrochloride. A pre-determined area on the dorsal region was shaved with an electric clipper and cleaned with 70% v/v ethanol. A circular piece of full thickness skin was excised to generate a wound of around 325 mm2. Ointments were applied on the entire area of the wound. The area of the wound was traced on a sheet of transparent tracing paper on the day of wound generation (day 0) and on different days until healing was complete. The area of the wound was measured by super-imposing the wound tracing on a graph paper and calculating the squares (Kaushal et al., Citation2007). The absence of residual raw wound and falling of eschar (dead tissue remnants) was considered as the endpoint for complete epithelization (Pandarinathan et al., Citation1998). The percentage of wound contraction on various days was calculated as follows: wound contraction on the Nth day = 100 – (wound area on the Nth day/wound area on day zero) × 100.
Biochemical and anti-oxidant activity studies
On the 5th, 10th, and 15th days after wound formation and subsequent treatment with the ointments, approximately 700 mg of the healing wound tissue (granulated tissue) was harvested from each rat under light ether anesthesia. These tissues were evaluated for total cellular proteins, hydroxy-proline, hexosamine, superoxide dismutase (SOD), and lipid peroxidation.
Estimation of total cellular protein from isolated excision wound tissue
Approximately 100 mg of the wet wound tissue was taken and homogenized. Precipitation, extraction, and estimation of total proteins from the homogenate were done as per reported methods (Lowry et al., Citation1951; Schmidt & Thannhauser, Citation1945).
Estimation of hydroxyproline
The granulated tissue from excision wounds was defatted and hydroxyproline was determined by an established method (Neuman & Logan, Citation1950).
Estimation of hexosamine
Granulated tissue from the excision wounds was defatted and hexosamine content was estimated by the modified Morgan Elson Reaction (Ohkuma et al., Citation1966).
Antioxidant activity
Wet granulated tissue (100 mg) from the excision wounds was homogenized in 10 mL ice cold Tris HCl buffer. Tissue homogenate was centrifuged at 3500 rpm for 15 min and the supernatant was used for the determination of SOD (Misra & Fridovich, Citation1972) and lipid peroxidation (Ohkawa et al., Citation1979) activities.
Histopathological examinations
Rats in the incision model were sacrificed on the 10th day and a portion of the skin containing the incision wound was taken for histopathological studies.
On the 16th day, rats of the excision model were sacrificed and the tissues from the wound site of the individual animals were removed. The samples from incision and excision wounds were separately fixed in 10% formalin for 24 h, dehydrated, cleared through ethanol–xylene series, and embedded in paraffin wax (melting point 56 °C). Serial sections of 10 µm were cut, and stained with hematoxylin and eosin. The sections were studied under light microscope (Prasad & Dorle, Citation2006).
Statistical analysis
Data are presented as mean ± SEM. The data were analyzed using one-way ANOVA followed by “Tukey's Multiple Comparison Test”. A value of p < 0.05 was considered to be significant. All statistical analyses were performed using Graph Pad Prism Version 5 (GraphPad Software Inc., La Jolla, CA).
Results and discussion
Topical application in the form of ointment was preferred to internal medication as this mode of application was reported to be effective in faster wound contraction due to larger availability of the drug at the site of the wound (Sumitra et al., Citation2005). SRME, SRPEBIME, and SREO exhibited significant wound-healing activity in the incision wound model. This is also evident from the high tensile strength of the wounds in the treatment groups on day 10 (). Wounds of SRPEBIME-treated rats had the highest tensile strength followed by those of SREO, framycetin, and SRME. However, the tensile strengths of SRME- and framycetin-treated wounds are significantly higher than those in the control group. Higher tensile strength is indicative of increase in collagen and its maturation leading to the formation of inter and intra-molecular cross-links. The low tensile strength in the control wounds resulted in prolonged wound-healing time.
Table 1. Effect of framycetin, SRME, SRPEBIME, and SREO on the tensile strength of rat incision wounds.
In the excision wound model, treatment with different extracts of S. robusta resulted in faster rate of epithelization and faster wound contraction (). SRPEBIME showed the highest effect followed by SREO, SRME, framycetin, and control in that order. There was almost complete healing on day 14 in the SRPEBIME group while complete healing was observed on days 17, 18, 18, and 23 in SREO, SRME, framycetin, and control groups, respectively. The scar areas were 7.67, 11.00, 10.00, 10.00, and 21.3 mm2 in SRPEBIME, SREO, SRME, framycetin, and control groups, respectively ().
Figure 1. Effect of various extracts of S. robusta resin on the scar area of excision wounds in rats.
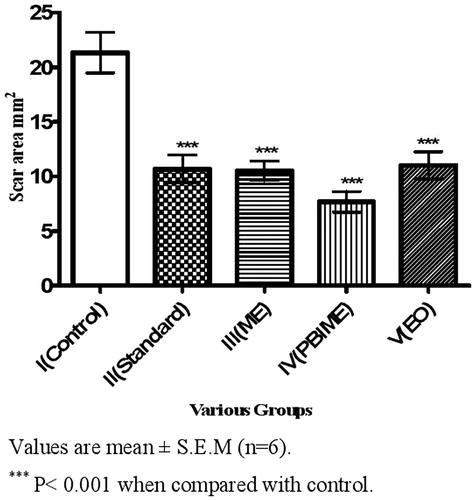
Table 2. Effect of framycetin, SRME, SRPEBIME, and SREO on wound area and wound contraction in rat excision wounds.
The results of the biochemical studies on the wounds are presented in . There was an increase in the protein content and hydroxyproline of all the animals in the excision wound tissue between the 5th and 15th days. However, the increase was much faster between the 5th and 10th days when there was rapid wound healing and wound contraction. SRPEBIME-treated groups had the highest values followed by SREO, framycetin, SRME, and control groups. Hexosamine levels were found to increase in the initial phases of wound healing from days 5 to 10 and decreased significantly at the later stages, i.e., on day 15. These changes are much faster and clearer in the SRPEBIME group.
Table 3. Effect of framycetin, SRME, SRPEBIME, and SREO on various biochemical parameters in the granulation tissue of rat excision wound model.
The healing process of wounds is achieved by the synthesis of connective tissue matrix. Collagen contributes to wound strength and constitutes a major protein of the tissue matrix. Hydroxyproline is liberated due to breakdown of collagen and hence is a good indicator of collagen turnover. Higher collagen and hydroxyproline contents in the tissues point to a faster healing of the wounds resulting in greater tensile strength. It has been suggested that the common factor in wound contraction is the activity of fibroblasts (myofibroblasts) that are found in the granulation tissue of healing wounds (Gabbiani et al., Citation1971). An increase in the levels of hexosamine during the early stages of wound healing followed by restoration to normal levels has been reported (Dunphy & Udupa, Citation1956). Our findings on hexosamine in the excision wounds are similar to those reported by these authors. The results are more striking in SRPEBIME- and SREO-treated rats in which the healing process was comparatively faster.
Excision wounds are akin to open wounds and require considerable fibroplasias to fill the defect by formation of more collagen deposits. Inflammation is an integral part of wound-healing process. In the initial stages, wound contraction is independent of myofibroblast involvement. Later, growth factors facilitate differentiation of fibroblasts into myofibroblasts which are required for wound contraction. Fibroblasts lay down collagen to reinforce the wound as myofibroblasts contract.
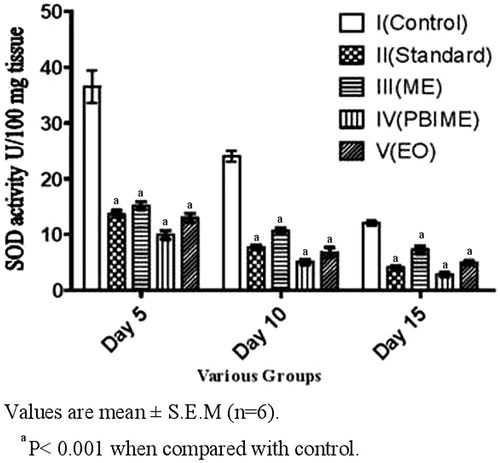
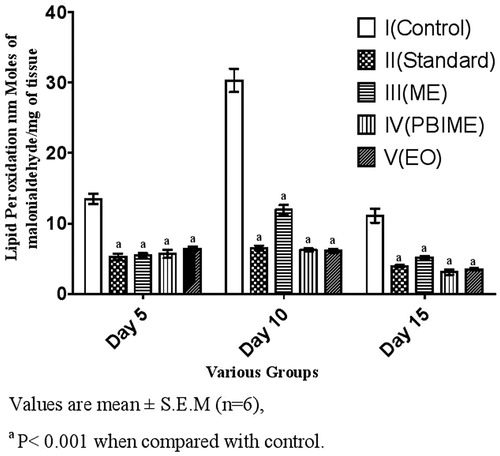
Data of SOD and lipid peroxidation activities of the wound tissues are depicted in and . There was much lower activity of SOD and lipid peroxidation in the granulation tissues of all treated groups; the activity in the SRPEBIME group being the lowest correlating with the wound healing and wound contraction.
Figure 3. Effect of various extracts of S. robusta resin on lipid peroxidation of excision wounds in rats.
Injuries generate superoxides and lipid peroxidation through the activation of neutrophils (Wooliscroft et al., Citation1990). This oxidative stress may cause damage to the growing tissue (collagen and epithelium) at the repair site. SOD activity was found to be less throughout the study in treated wounds indicating lesser inflammation response as compared with high degree of SOD activity in untreated control animals indicating high inflammation.
Lipid peroxidation products were elevated in all the groups from day 5 to 10 but it was nearly twice in control animals as compared with the treated ones. On day 15, lipid peroxidation returned to normal in treated animals, whereas control animals showed higher level of TBARS. Lower level of lipid peroxidation in the treated group indicates antioxidant activity (Sumitra et al., Citation2005), which would help to prevent oxidative damage and promote the healing process.
Histological studies show complete epithelization in SRPEBIME- and SREO-treated groups whereas there was only a partial epithelization in wounds of control animals. In the incision model, cellular architecture in histopathological studies revealed faster healing in SRPEBIME-, SREO-, and SRME-treated animals (). Histology of excised skin revealed greater tissue regeneration in framycetin- and drug–treated groups (II–V) than in the control group. Skin adnexal structures such as the pilosebaceous glands and sweat glands were better preserved in the treated groups (II–V). In these animals, connective tissue is fully formed; collagen fibers are compact and well-aligned, indicating complete healing of the wound ().
Figure 4. Histological changes in the incision wounds on the 10th day after treatment with different extracts of S. robusta. There is incomplete epithelization in the control (A) and framycetin (standard)-treated animals while the incision wounds in SRME (methanol extract), SRPEBIME (PEBIME), and SREO (essential oil)-treated groups showed complete epithelization.
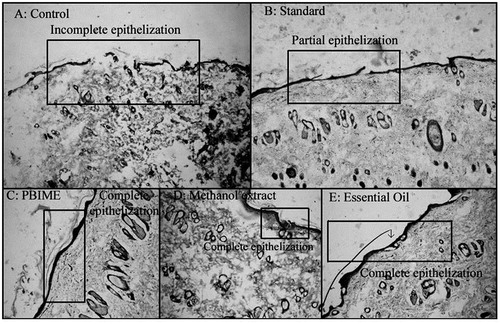
Figure 5. Histological changes in excision wounds of rats on the 16th day after treatment with various extracts of S. robusta resin. Congested blood vessels with no re-epithelization are seen in the control (A); partial epithelization is observed in framycetin (B), and methanol extract (C and D)-treated groups; complete epithelization with the formation of blood vessels (E and F) is seen in animals treated with PEBIME.
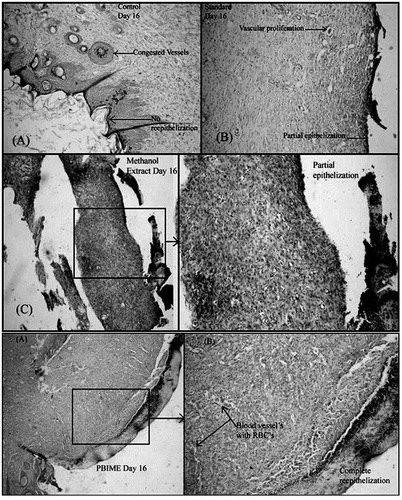
The higher level of hydroxyproline, hexosamine, high tensile strength together with the desired wound contraction and epithelization indicate interplay of different mechanisms leading to faster wound healing in the treated animals. Histopathological studies further support the biochemical and biophysical data of the study.
Wound-healing activity of 70% alcholol extract of S. robusta (Wani et al., Citation2012b) and that of S. robusta in flax seed oil (Datta et al., Citation2011) were reported. While the former studies reported wound healing activity similar to our results, the latter group did not find any significant activity. They found good wound healing activity only when S. robusta was mixed with zinc oxide or Phyllanthus emblica Linn. (Euphorbiaceae) fruit extract. From these studies, it is difficult to attribute the activity to any particular compound/s. SREO contains a number of mono- and sesquiterpenes (Pakniker & Bhattacharya, Citation1961) and SRPEBIME contains different ursane types of triterpenes (Hota & Bapuji, Citation1993, Citation1994). Ursane derivatives like asiaticosides from Centella asiatica Linn. (Apiaceae) are known to have anti-ulcer and wound healing activities. Since high wound healing activity in two different models with improvement in biochemical and anti-oxidant indicators were observed in SRPEBIME and SREO groups in our study, we suggest that the activity might be due to the triterpenes of S. robusta resin.
Conclusion
Our results indicate that the triterpene-rich fraction and essential oil of S. robusta have the highest wound healing activities and confirm the traditional claims on this plant for healing of wounds.
Acknowledgements
The authors are thankful to Dr. Avani F Amin, Director, Institute of Pharmacy, Nirma University for providing the facilities, Dr. Bhaskar Punjani for sample authentication, Dr. Shital S. Shah and Dr. Ashesh Shah for their continuous support during histopathological studies.
Declaration of interest
The authors report no conflicts of interest. The authors are solely responsible for the content and writing of this article.
References
- Chandel KPS, Shukla G, Sharma N. (1996). Biodiversity in Medicinal and Aromatic Plants in India: Conservation and Utilization. New Delhi, India: National Bureau of Plant Genetic Resources
- Chitale VS, Behra MD. (2012). Can the distribution of Sal (Shorea robusta Gaertn.f.) shift in the northeastern direction in India due to changing climate? Curr Sci India 102:1126–35.
- Datta HS, Mitra SK, Patwardhan B. (2011). Wound healing activity of topical application forms based on Ayurveda. eCAM. [Epub ahead of print]. doi:10.1093/ecam/nep015
- DiPietro LA, Burns AL. (2003). Wound Healing Methods and Protocols. New Jersey: Humana Press
- Dunphy JE, Udupa KN. (1956). Wound healing: A new perspective with particular reference to ascorbic acid deficiency. Ann Surg 144:304–16
- Erhlich HP, Hunt TK. (1969). Effect of cortisone and anabolic steroids on tensile strength of healing wound. Ann Surg 170:203–6
- Gabbiani G, Ryan GB, Majno G. (1971). Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27:549–50
- Ganesan S, Venkateshan G, Bhanumathy N. (2006). Medicinal plants used by ethnic group Thottianiackans of Semmalai hills, Tiruchirrapalli district, Tamil Nadu. Indian J Tradit Know 5:245–52
- Hota RK, Bapuji M. (1993). Triterpenoids from the resin of Shorea robusta. Phytochemistry 32:466–8
- Hota RK, Bapuji M. (1994). Triterpenoids from the resin of Shorea robusta. Phytochemistry 35:1073–4
- Kaushal M, Gopalan NK, Rao CM. (2007). Wound healing activity of NOE-aspirin: A pre-clinical study. Nitric Oxide 16:150–6
- Lowry OH, Rosebrough NJ, Farr AL, et al. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75
- Misra HP, Fridovich I. (1972). The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–5
- Morton JJP, Melone MH. (1972). Evaluation of vulnerary activity by an open wound procedure in rats. Arch Int Pharmacod T 176:117–226
- Mukherjee PK, Mukherjee K, Rajesh Kumar M, et al. (2003). Evaluation of wound healing activity of some herbal formulations. Phytother Res 17:265–8
- Nayak S, Nalabothu P, Sandiford S, et al. (2006). Evaluation of wound healing activity of Allamanda cathartica. L. and Laurus nobilis. L. extracts on rats. BMC Compliment Alternat Med 6:1–6
- Neuman RE, Logan MA. (1950). The determination of hydroxyproline. J Biol Chem 184:299–306
- Ohkawa H, Ohisi N, Yagi K. (1979). Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann Biochem 95:351–8
- Ohkuma S, Shinohara T, Furahata T. (1966). Determination of hexasomines by modified Morgan and Elson reaction. Proc Jpn Acad 42:970–4
- Pakniker SK, Bhattacharya SC. (1961). Investigation on resin oil from S. robusta. Perfum Essen Oil Records 52:233–6
- Pandarinathan C, Sajithlal GB, Chandrkasan G. (1998). Influence of Aloe vera on collagen turnover in healing of dermal wounds in rats. Indian J Exp Biol 36:896–901
- Prasad V, Dorle AK. (2006). Evaluation of ghee based formulation for wound healing activity. J Ethnopharmacol 107:38–47
- Schmidt G, Thannbauser S. (1945). A method for the determination of deoxyribonucleic acid, ribonucleic acid, and phosphoproteins in animal tissues. J Biol Chem 161:83–9
- Sumitra M, Manikandan P, Suguna L. (2005). Efficacy of Butea monosperma on dermal wound healing in rats. J Biochem Cell Biol 37:566–73
- Wani TA, Chandrasekhara HH, Kumar D, et al. (2012a). Wound healing activity of ethanolic extract of Shorea robusta Gaertn.f. resin. Ind J Exp Biol 50:277–81
- Wani TA, Chandrasekhara HH, Kumar D, et al. (2012b). Anti-inflammatory and antipyretic activities of the ethanolic extract of Shorea robusta Gaertn.f. resin. Ind J Biochem Biophys 49:963–7
- Wooliscroft JO, Prasad JK, Thomson P, et al. (1990). Metabolic alterations in burn patients: Detection of adenosine triphosphate degradation products and lipid peroxides. Burns 16:92–6