Abstract
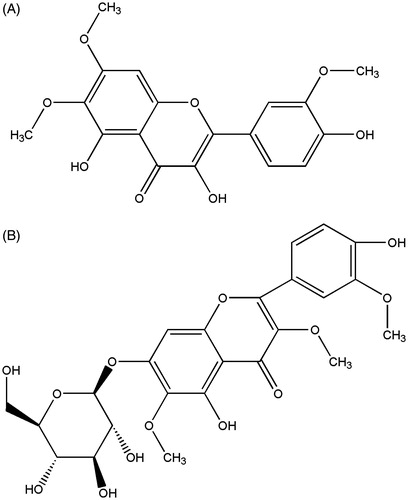
Context: Gastric cancer remains highly prevalent, but treatment options are limited. Natural products have proved to be a rich source of anticancer drugs. Chrysosplenium nudicaule Ledeb. (Saxifragaceae) is a perennial herb that grows in the highlands of China. It has been used as a traditional Chinese medicine to treat digestive diseases for hundreds of years. Recent studies revealed that this herb had anticancer activity, and the flavonoids were speculated to be the effective components. 6,7,3′-Trimethoxy-3,5,4′-trihydroxy flavone (TTF) and 5,4′-dihydroxy-3,6,3′trimethoxy-flavone-7-O-β-d-glucoside (DTFG) are flavonoid compounds isolated from Chrysosplenium nudicaule.
Objective: This study examined the effect of TTF and DTFG on SGC-7901 human stomach cancer cell in vitro to determine the anticancer and induction of apoptosis properties of TTF.
Materials and methods: The proliferation of cells treated with 32, 16, 8, 4, and 2 μg/mL of TTF or DTFG for 24, 48, and 72 h was assessed by the MTT assay. After being treated with TTF, the apoptosis of SGC-7901 cells was assessed by acridine orange staining, ultrastructure, electrophoresis of DNA fragmentation, and flow cytometry.
Results: Results indicated that TTF inhibited the growth of cancer cells with an IC50 value of 8.33 μg/mL after 72 h incubation. However, DTFG showed no inhibitory effect on the growth of the cancer cell. Further studies on TTF also confirmed that it was able to induce apoptosis of SGC-7901 cells at a concentration as low as 4 μg/mL.
Discussion and conclusion: The apoptotic effect of TTF makes it a promising candidate for future chemotherapeutic application in treating stomach cancer.
Introduction
Gastric cancer, the second leading cause of cancer-related deaths worldwide, is a severe threat to human health and is considered one of the major health problems globally. The incidence of this disease is the highest among the malignant tumors worldwide and remains an increasing problem in China. In addition, the low successful rate in early diagnosis has led to a lower chance of cure surgically which left chemotherapy to be the only treatment for those patients. Consequently, the patients suffer the side effects and complications due to the strong toxic effect of standard chemotherapy (Beijers et al., Citation2012). Therefore, alternative anticancer drugs with alleviated toxicity, such as phytochemicals, have attracted much attention recently (Jeong et al., Citation2011). For example, Choedon et al. (Citation2011) have provided evidence to demonstrate Thapring, a Tibetan herbal formulation, is able to kill hepatoma cells by the use of MTT. Thus, the use of natural products in determining effective antitumor agents has been a valuable way to discover new drug candidates (Li et al., Citation2014; Mishra & Tiwari, Citation2011).
Chrysosplenium nudicaule Ledeb.(Saxifragaceae) is widespread in the northwest and southwest mountain regions of China according to the Flora of China (Wu & Pan, Citation1992) and has been used for years in traditional Tibetan medicine to treat digestive system diseases. Anticarcinogenic activity was reported by Zong et al. (Citation2000) through screening 110 Tibetan medicine plants; it was found that C. nudicaule was one of the five plants with antitumor activity (Arisawa et al., Citation1991, Citation1992). Further study established that the flavonoid compound, 6,7,3′-trimethoxy-3,5,4′-trihydroxy flavones isolated from C. nudicaule, was able to induce apoptosis in human leukemia K562 cells (Wang et al., Citation2005). However, C. nudicaule is commonly used to alleviate gastrointestinal symptoms; we conclude that the compounds or others isolated from the plants may show stronger anticancer activities to gastrointestinal tumors than other tumors. We are interested in searching for compounds isolated from C. nudicaule possessing antigastric cancer activity. In this study, we isolated two flavonoid compounds, 6,7,3′-trimethoxy-3,5,4′-trihydroxy flavone (TTF) and 5,4′-dihydroxy-3,6,3′trimethoxy-flavone-7-O-β-d-glucoside (DTFG), from C. nudicaule. Their potential anticancer activity was evaluated by the use of SGC-7901 human stomach cancer cell.
Materials and methods
Chemicals
The two flavonoids including TTF and DTFG () were isolated and identified as described previously (Yang et al., Citation2003). Briefly, Chrysosplenium nudicaule (M2007-1001) were obtained from Qinghai province in China and identified by Rongfu Huang (Institute of Northwest Plateau Biological, Chinese Academy of Sciences, Qinghai, China) in 2007. Air dried herb (1600 g) was thereafter pulverized and soaked for 7 d in 95% ethanol three times successively. Ethanol extract (260 g) was collected and extracted subsequently in the following order, petroleum ether, chloroform, ethyl acetate, n-butanol followed by dissolving in hot water. After elution with chloroform and methanol, 0.102 g of TTF and 0.01 g of DTFG were obtained from 21 g, and 65 g, respectively, of crude extract by silica gel column chromatography. Then two compounds were identified through ultraviolet spectrum (UV), infrared spectroscopy (IR), mass spectrum (MS), and nuclear magnetic resonance (1H-NMR and 13C-NMR). Both the TTF and the DTFG are in the form of yellow powder and shows poor solubility in water. Therefore, they were dissolved in dimethyl sulfoxide (DMSO) with a concentration of 0.1% (v/v). This concentration of DMSO has been confirmed to have no detrimental effect on cell growth (Ma et al., Citation2006).
Cell culture
The SGC-7901 human stomach cancer cell line was obtained from Xiaohui Wen (School of Basic Medical Sciences, Lanzhou University, Lanzhou, China) in 2006. The SGC-7901 cell line was cultured in an RPMI 1640 medium (Sigma, St. Louis, MO) supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% heat inactivated fetal calf serum (Sijiqing, Hangzhou, China). The cells were grown under saturated humidity conditions at 37 °C and 5% CO2 (Lou et al., Citation2012).
MTT assay cell proliferation
The inhibitory effect of the two compounds on human stomach cancer cells was determined via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to measure the cell viability (Lu et al., Citation2014). SGC-7901 cells in the logarithmic growth phase were collected, washed once with phosphate buffer solution (PBS), and resuspended in a fresh culture medium. The cells (1 × 104/well) were then seeded in quadruplicate on a sterile 96-well tissue culture plate. After the cells attached to the culture plate, 10 μL of normal saline (negative control), or one of the compounds with final concentrations of 32, 16, 8, 4, and 2 μg/mL were added. After incubating for 24, 48, and 72 h, the MTT assay was used to determine cell viability. Four hours before completion of the incubation period, 10 μL of MTT (5 mg/mL) (Sigma, St. Louis, MO) was added into each well. The MTT was reduced to formazan, which was then dissolved in DMSO. The absorbance of formazan dye was quantified at 570 nm by using a microplate reader (Sanco, Shanghai, China). The experiment was repeated three times. The results were expressed as the mean percentage of cell growth inhibition rate (%), which was obtained via the following formula: Growth inhibition rate (IR) = [1 − (absorbance TTF treated/Absorbance negative control)] × 100%. The IC50 value was defined as the drug concentration required to inhibit 50% of cell growth based on the 100% viability of untreated cells.
Acridine orange (AO) staining for cell apoptosis morphology
AO staining (Ma et al., Citation2006) of the SGC-7901 cells was performed to confirm the TTF-induced apoptosis pattern. Cells treated with 8 μg/mL of TTF for 48 h were harvested and washed twice with PBS. The cells were then incubated in the dark for 3 min with 0.01% AO solution at room temperature and observed immediately under a fluorescence microscope (Olympus, Tokyo, Japan).
Transmission electron microscopy (TEM) for ultrastructure observation
Apoptosis cell morphology was determined by using TEM (Meng et al., Citation2014). Cells were treated with TTF (8 μg/mL) or without for 48 h at 37 °C and 5% CO2, and then washed twice with PBS. The cells were fixed with 3% glutaraldehyde for 12 h at 4 °C. After two washings to remove the primary fixative, the cells were fixed with 1% osmic acid for 1 h. After the cells were centrifuged, the pellets were washed twice and pre-stained with 2% uranyl acetate for 1 h. The dye was discarded afterward and the cells were washed twice. They were then dehydrated in various concentrations of alcohol (35, 50, 75, and 90%) for 5 min. The cells were then embedded in epoxy resin, and ultra-thin sections were cut. The sections were further double-stained with lead citrate/uranyl acetate and then examined under a JEM-100CX transmission electron microscope (JEOL Ltd., Tokyo, Japan).
Agarose gel electrophoresis of DNA fragmentation
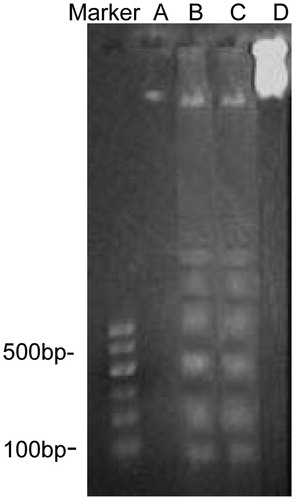
SGC-7901 cells treated with various concentrations of TTF (4, 8, and 16 μg/mL) for 48 h were harvested through trypsinization and were washed twice with PBS. To detect the DNA ladder, DNA fragments were extracted using a commercial Quick Genomic DNA Extraction Kit (Sangon Biotech, Shanghai, China) according to the instruction manual. Each DNA sample (6 μL) was loaded with 1.5% agarose gel containing 0.5 μg/mL of ethidium bromide. Electrophoresis was performed at 80 V for 1 h. DNA stripes were visualized and photographed under an ultraviolet gel documentation system (Shanghai Jiapeng Technological Co. Ltd., Shanghai, China).
Flow cytometry (FCM) analysis of the cell cycle
Cell-cycle phase analysis was performed with propidium iodide staining coupled with FCM (Ma et al., Citation2006). In each analysis, 1 × 106 cells were harvested through trypsinization, treated with TTF (4, 8, and 16 μg/mL) for 48 h, and then washed twice with PBS. After being fixed with 70% ethanol at 4 °C overnight, the cells were washed twice to remove the ethanol. The fixed cells were stained with 50 mg/L of propidium iodide at room temperature in the presence of 50 g/L of RNase A for 30 min. The distribution of the cells in each stage of the cell cycle was then analyzed by using a Coulter Epics XL (Beckman Coulter, Los Angeles, CA).
Statistical analysis
All statistical analysis was conducted with SPSS 10.0 software (SPSS Inc., Chicago, IL). One-way analysis of variance was used for the MTT assay. The data were expressed as mean ± standard deviation of the mean (SD). p Values < 0.05 were considered statistically significant.
Results
Effects of TTF and DTFG on cell proliferation
TTF inhibited growth of SGC-7901 human stomach cancer cell
The flavonoid compound is one of the most abundant compounds of C. nudicaule (Wangchuk et al., Citation2011). To determine whether TTF and DTFG can inhibit the growth of the SGC-7901 human stomach cancer cell, a MTT assay was employed to detect cell viability in the presence of TTF or DTFG. Cell proliferation was significantly inhibited after being incubated with TTF at various concentrations for 48 h (p < 0.05) and 72 h (p < 0.05) as shown in . Longer incubation time (e.g., 72 h) showed higher growth inhibition rate (IR). After treated with TTF for 24, 48, and 72 h, the corresponding IC50 values were at 142.17, 29.23, and 8.33 μg/mL, respectively.
Table 1. The inhibitory effect of TTF from C. nudicaule on SGC-7901 cell line in vitro.
DTFG did not show any inhibition on growth of SGC-7901 cell
Similarly, the effect of DTFG on the growth of SGC-7901 human stomach cancer cell was detected by MTT assay. However, no growth inhibition was observed with incubation of cells and DTFG at different concentrations (data not shown).
Effect of SGC-7901 human stomach cancer cell apoptosis induced by TTF
The observation of apoptosis morphology
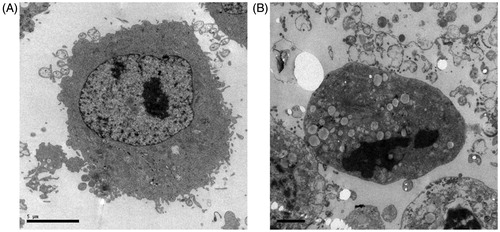
To further investigate the mechanism behind the “killing” effect of TTF on cancer cell, apoptosis was monitored following the TTF treatment of SGC-7901 cell. To determine apoptosis morphology, cells were stained with AO and observed under a fluorescence microscope following a 48 h treatment with 8 μg/mL of TTF. The non-TTF-treated cells were regularly shaped, had smooth membranes, and exhibited a diffuse homogeneous green fluorescence (). In contrast, TTF-treated cells were smaller and had typical morphological features of apoptosis such as shrunken nuclei as well as condensed chromatin (). In addition to morphological evidence, the ultrastructure changes of the cell also provide evidence of apoptotic effect of TTF. Compared with the untreated cells (), the TTF-treated cells () showed decreased microvilli, a minished cytoplasm with increased electron density and physalodes, and marked karyopyknosis in the nucleolus.
Figure 2. Fluorescence imaging. AO staining was performed in SGC-7901 cells untreated (A) or treated (B) with TTF from C. nudicaule for 48 h at 8 μg/mL.
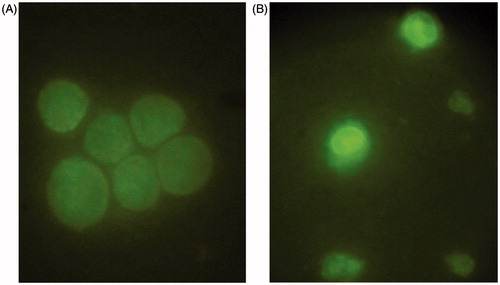
Figure 3. Ultrastructure imaging of SGC-7901 cells. TEM illustrates the effect of apoptosis on SGC-7901 cells: (A) was untreated; (B) was treated with TTF from C. nudicaule for 48 h at 8 μg/mL. Apoptotic cells exhibited decreased microvilli, minished cytoplasm with increased electron density and physalides, and evident karyopyknosis in nucleolus.
The induction of DNA ladders
Further evidence of TTF-induced apoptosis in the SGC-7901 cell can be seen through DNA fragmentation analysis. TTF was able to induce DNA fragmentation into multiples of approximately 180 bp. Typical apoptotic DNA ladders were also observed ().
Arrest of cell cycle
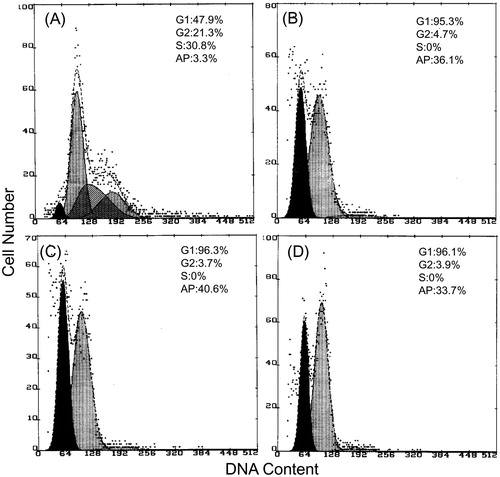
Finally, the apoptosis induced by TTF was examined by a flow cytometry (FCM) analysis of the cell cycle. The results showed that TTF induced a G1 phase cell cycle arrest in SGC-7901 cells (). Compared with the untreated cells, the percentage of all TTF-treated cells in the G1 phase increased distinctly and then decreased in the S phase. Furthermore, a marked apoptotic peak was observed in the sub-G1 phase. The apoptotic percentages of TTF-treated cells at the sub-G1 phase were 36.1% at 4 μg/mL, 40.6% at 8 μg/mL, and 33.7% at 16 μg/mL, respectively, which were higher than the percentage displayed by the untreated control cells.
Discussion and conclusion
In this study, the flavone TTF, one of the two compounds isolated from C. nudicaule, was found to significantly inhibit the growth of the SGC-7901 human stomach cancer cell line. This inhibitory effect was due to the ability of TTF to induce apoptosis.
Many anticancer drugs have been discovered in various plants (He et al., Citation2012; Mishra & Tiwari, Citation2011; Yingkun et al., Citation2010), which indicated that natural products and their synthetic derivatives were significant sources of anticancer drugs. Flavonoid compounds found in a large number of plants were known to have anticancer activity (Erhart et al., Citation2005; Sonoda et al., Citation2004). In particular, several studies also showed that flavonoid compounds obtained from medicinal plants, such as apigenin, Astragalus complanatus flavonoids, and Lysimachia clethroides Duby flavonoids induced apoptosis in a human cancer cell line (Das et al., Citation2010; Hu et al., Citation2009; Liu et al., Citation2010). Chrysosplenium nudicaule is rich in flavonoids (Shi et al., Citation2011) and previous study showed flavonoid compound TTF isolated from C. nudicaule could inhibit the growth of the tumor cells from the blood system (Wang et al., Citation2005). However, C. nudicaule, as a folk-medicinal plant, has been traditionally used as a remedy for diseases of the digestive system in China's Tibet. Thus, we speculated flavonoid compounds from C. nudicaule may have the ability of inhibiting the growth of gastrointestinal tumors. In the current report, we investigated the potential application of two flavonoid compounds (TTF and DTFG) to treat gastric cancer using SGC-7901 human stomach cancer cell line. As expected, our results showed that TTF presented strong inhibition to the growth of stomach cancer cells. However, the other flavonoid (DTFG) produced no suppression of the growth of the cancer cell, which suggested chemical group may impact the activation of flavonoid compound. The results of accumulated researches showed that the presence of an additional hydroxyl group at 4′ of the B ring or 5 of A ring was important for antitumor activity of flavonoids (Anso et al., Citation2010; Lopez-posadas et al., Citation2008), which was also found in our research work. Accordingly, it was suggested that potential antitumor effects can be similarly revealed in other cancer cell lines.
It has long been known that the ability to induce apoptosis in cancer cells is a key criterion for screening anticancer drugs (Hickman, Citation1996). TTF was determined to possess this characteristic when apoptosis was detected in SGC-7901 cells treated with TTF. Given that chromatin concentration may arise in the mitotic phase of both apoptotic and normal cells, fluorescence microscope coupled with AO stain was also used in order to distinguish these two cells (Mirakian et al., Citation2002; Negoescu et al., Citation1998). AO stain was used because it can pass through the cell membrane and stain the DNA of the cell nucleus green, and in non-apoptotic cells, the yellow-green fluorescence is evenly distributed in the nuclei. In contrast, AO staining in apoptotic cells appears as dense hyperchromatic yellow-green dye stains because of karyopyknosis or splintering. With the help of AO stain, we were able to observe a typical apoptotic reaction upon TTF treatment of SGC-7901 cells. The main biochemical characteristic of apoptotic cells is the activation of the endogenous Ca2 + - and Mg2 + -dependent endonuclease by which a chromosome cleaves two nucleosomes that form integral multiple 180–200 bp DNA segments. This DNA ladder can be visualized in agarose gel electrophoresis (Bortner et al., Citation1995; Li et al., Citation2012). However, if a cell dies through necrosis, a DNA ladder is not formed. In the current study, we found that a typical DNA ladder appeared in the agarose gel, which indicated that TTF could induce the apoptosis of gastric cancer cells.
Another commonly used marker for determining the effectiveness of a potential anticancer drug is to measure its impact on the cell cycle. Most anticancer drugs function by causing some component damage to tumor cells, which led to cell-cycle arrest and apoptosis (Huang et al., Citation2005). In the current report, FCM analysis was conducted to determine the cell cycle. The results suggested that TTF might induce cell apoptosis by preventing the cells in the G1 phase from entering the S phase, which indicated the growth of cells stopped.
Flavonoids were generally recognized as antioxidants, which were found to induce cancer cell apoptosis through caspase activation (Shanker et al., Citation2007) or the downregulation of antiapoptotic proteins Bcl-2 and Bcl-x(L) (Auyeung & Ko, Citation2010). However, the anticancer activity and precise mechanism of TTF in vivo remain unknown, and further studies are necessary to address these issues.
In conclusion, the compound TTF, one of flavones isolated from C. nudicaule, displayed definite anticancer activity, and was able to induce apoptosis in the SGC-7901 human stomach cancer cell line. This information can prove useful in future applications of TTF as a treatment for stomach cancer.
Acknowledgements
The authors thank Dr. Juan Li for her assistance in getting gel pictures of DNA, Huifang Zhang for observing the ultrastructure of the cells and Dr. Xun Liu of Lanzhou University in China for drawing the chemical structure.
Declaration of interest
This work was supported by the National Natural Science Foundation of China (31360604).
References
- Anso E, Zuazo A, Irigoyen M, et al. (2010). Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochem Pharmacol 79:1600–9
- Arisawa M, Bai H, Shimizu S, et al. (1992). Isolation and identification of a cytotoxic principle from Chrysosplenium grayanum Maxim. (Saxifragaceae) and its antitumor activities. Chem Pharm Bull (Tokyo) 40:3274–6
- Arisawa M, Hayashi T, Shimizu M, et al. (1991). Isolation and cytotoxicity of two new flavonoids from Chrysosplenium grayanum and related flavonols. J Nat Prod 54:898–901
- Auyeung KK, Ko JK. (2010). Novel herbal flavonoids promote apoptosis but differentially induce cell cycle arrest in human colon cancer cell. Invest New Drugs 28:1–13
- Beijers AJ, Jongen JL, Vreugdenhil G. (2012). Chemotherapy-induced neurotoxicity: The value of neuroprotective strategies. Neth J Med 70:18–25
- Bortner CD, Oldenburg NB, Cidlowski JA. (1995). The role of DNA fragmentation in apoptosis. Trends Cell Biol 5:21–6
- Choedon T, Dolma D, Kumar V. (2011). Pro-apoptotic and anticancer properties of Thapring-A Tibetan herbal formulation. J Ethnopharmacol 137:320–6
- Das A, Banik NL, Ray SK. (2010). Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 116:164–76
- Erhart LM, Lankat-buttgereit B, Schmidt H, et al. (2005). Flavone initiates a hierarchical activation of the caspase-cascade in colon cancer cells. Apoptosis 10:611–17
- He W, Wang B, Zhuang Y, et al. (2012). Berberine inhibits growth and induces G1 arrest and apoptosis in human cholangiocarcinoma QBC939 cells. J Pharmacol Sci 119:341–8
- Hickman JA. (1996). Apoptosis and chemotherapy resistance. Eur J Cancer 32A:921–6
- Hu YW, Liu CY, Du CM, et al. (2009). Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by flavonoids from Astragalus complanatus. J Ethnopharmacol 123:293–301
- Huang X, Halicka HD, Traganos F, et al. (2005). Cytometric assessment of DNA damage in relation to cell cycle phase and apoptosis. Cell Prolif 38:223–43
- Jeong SJ, Koh W, Kim B, Kim SH. (2011). Are there new therapeutic options for treating lung cancer based on herbal medicines and their metabolites? J Ethnopharmacol 138:652–61
- Li FF, Yi S, Wen L, et al. (2014). Oridonin induces NPM mutant protein translocation and apoptosis in NPM1c + acute myeloid leukemia cells in vitro. Acta Pharmacol Sin 35:806–13
- Li Y, Liu L, Niu Y, et al. (2012). Modified apple polysaccharide prevents against tumorigenesis in a mouse model of colitis-associated colon cancer: Role of galectin-3 and apoptosis in cancer prevention. Eur J Nutr 51:107–17
- Liu YL, Tang LH, Liang ZQ, et al. (2010). Growth inhibitory and apoptosis inducing by effects of total flavonoids from Lysimachia clethroides Duby in human chronic myeloid leukemia K562 cells. J Ethnopharmacol 131:1–9
- Lopez-posadas R, Ballester I, Abadia-molina AC, et al. (2008). Effect of flavonoids on rat splenocytes, a structure-activity relationship study. Biochem Pharmacol 76:495–506
- Lou LH, Jing DD, Lai YX, et al. (2012). 15-PGDH is reduced and induces apoptosis and cell cycle arrest in gastric carcinoma. World J Gastroenterol 18:1028–37
- Lu C, Zhou LY, Xu HJ, et al. (2014). RIP3 overexpression sensitizes human breast cancer cells to parthenolide in vitro via intracellular ROS accumulation. Acta Pharmacol Sin 35:929–36
- Ma XM, Luo YP, Yu HJ, Cui Y. (2006). Ethanolic extracts of Sophora moorcroftiana seeds induce apoptosis of human stomach cancer cell line SGC-7901 in vitro. Afr J Biotech 5:1669–74
- Meng Y, Xu Z, Wu F, et al. (2014). Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in nude mice. Fertil Steril 102:871–7 e3
- Mirakian R, Nye K, Palazzo FF, et al. (2002). Methods for detecting apoptosis in thyroid diseases. J Immunol Methods 265:161–75
- Mishra BB, Tiwari VK. (2011). Natural products: An evolving role in future drug discovery. Eur J Med Chem 46:4769–807
- Negoescu A, Guillermet C, Lorimier P, et al. (1998). Importance of DNA fragmentation in apoptosis with regard to TUNEL specificity. Biomed Pharmacother 52:252–8
- Shanker M, Gopalan B, Patel S, et al. (2007). Vitamin E succinate in combination with mda-7 results in enhanced human ovarian tumor cell killing through modulation of extrinsic and intrinsic apoptotic pathways. Cancer Lett 254:217–26
- Shi Y, Hu Z, Wu C, et al. (2011). A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nat Genet 43:1215–18
- Sonoda M, Nishiyama T, Matsukawa Y, Moriyasu M. (2004). Cytotoxic activities of flavonoids from two Scutellaria plants in Chinese medicine. J Ethnopharmacol 91:65–8
- Wang YP, Yang YS, Yang LX, et al. (2005). K562 Apoptosis induced by flavone from Tibetan medicine Chrysosplenium nudicaule Bunge and its molecular mechanism. Pract J Cancer 20:374–6
- Wangchuk P, Keller PA, Pyne SG, et al. (2011). Evaluation of an ethnopharmacologically selected Bhutanese medicinal plants for their major classes of phytochemicals and biological activities. J Ethnopharmacol 137:730–42
- Wu ZY, Pan JT. (1992). Flora of China. Beijing: Science Press; Vol. 34
- Yang YS, Shi GF, Lu RH, Shen T. (2003). Studies on the flavonols compounds from Chrysosplenium nudicaule Bunge. Chin Tradit Herbal Drugs 34:98–100
- Yingkun N, Lvsong Z, Huimin Y. (2010). Shikonin inhibits the proliferation and induces the apoptosis of human HepG2 cells. Can J Physiol Pharmacol 88:1138–46
- Zong YY, Dang HH, Luo GF, et al. (2000). Antitumor screening research from 110 Tibetan medicines. J Pharm Pract 18:290–1