Abstract
Context: Dichapetalum filicaule Breteler (Dichapetalaceae) is a rare species occurring only in Côte d’Ivoire and Ghana. Although research on several species of the genus has produced interesting bioactive compounds, particularly the Dichapetalins, a novel class of triterpenoids with antineoplastic properties, there is virtually no information on the ethnobotanical uses and chemical constituents of D. filicaule.
Objective: The phytochemical and anthelminthic activities of the constituents of D. filicaule were investigated.
Materials and methods: Chemical constituents of the petroleum ether, chloroform–acetone, and methanol root extracts of D. filicaule were isolated by column chromatography and characterized by their physico-chemical properties, 1-D and 2-D NMR spectroscopy and mass spectrometry. In vitro anthelminthic activity of the extracts and compounds against the human hookworm, Necator americanus, Stiles 1902 (Nematoda: Ancylostomatidae) was determined within a concentration range of 2500–250 μg/ml using the Egg Hatch Inhibition (EHI) Assay. The hookworm species were identified using a published polymerase chain reaction (PCR) method.
Results: A new dichapetalin, dichapetalin X (1), together with the known dichapetalin A (2), pomolic acid (3), glycerol monostearate (4), D:A-friedooleanan-3β-ol (5), and D:A-friedooleanan-3-one (6) were isolated. Compounds 1, 2, and 4 exhibited EHI with IC50 values of 523.2, 162.4, and 306.0 μg/ml, respectively, against the hookworm. The positive control albendazole gave an IC50 value of 93.27 μg/ml.
Discussion and conclusion: This is the first report of the phytochemical investigation of D. filicaule. The study has yielded a new dichapetalin and also demonstrated the potential anthelminthic properties of the constituents.
Introduction
Dichapetalum filicaule Breteler (Dichapetalaceae) (Breteler, Citation1970) is one of the less common species of the genus Dichapetalum Thouars. It occurs only in the West African countries of Côte d’Ivoire and Ghana and is more widespread in the former than in the latter. In Ghana, the plant is located at both the Boi-Tano and the Draw River Forest Reserves in the Western Region, and Yamoransa in the Central Region (Bongers et al., Citation2005; McCullough et al., Citation2005). Although extensive research has been carried out on several species of this genus for their toxic fluorinated carboxylic acids, as well as the recent focus on isolation of dichapetalins, there is virtually no information in the literature concerning the ethnobotanical uses, medicinal applications or the chemical constituents of D. filicaule. Moreover, only a few species have so far been investigated specifically for the presence or otherwise of dichapetalins. Further investigation of other species for these compounds is therefore of chemotaxonomic importance.
Since the isolation of dichapetalin A and other dichapetalins from D. madagascariensis, six other species of Dichapetalum have been investigated. The dichapetalins represent a unique class of triterpenoids in which a 2-phenylpyrano moiety is annellated to ring A of a 13, 30-cyclodammarane-type triterpenoid. Their main distinguishing feature is the nature of the side chain at C-17.
Nine dichapetalins, A–H, and M have been isolated and characterized from the Ghanaian collection of the roots of D. madagascariensis (Achenbach et al., Citation1995; Addae-Mensah et al., Citation1996; Osei-Safo et al., Citation2008). A Philippino collection of D. gelonioides has also yielded dichapetalins I, J, K, and L, in addition to dichapetalin A (Fang et al., Citation2006). Long et al. (Citation2013) also screened D. mombuttense (collected from the Democratic Republic of Congo), D. leucosia (Madagascar), D. zenkeri, D. eickii, and D. ruhlandii (Kenya) for the presence of dichapetalins. Six new dichapetalins, named dichapetalins N–S were isolated from D. mombuttense, D. zenkeri, and D. leucosia. The known dichapetalins A and M were isolated from D. eickii while D. ruhlandii yielded dichapetalin A only. Also accompanied in these isolations were dichapetalins B, C, I, and L. For the first time, the parent compound, dichapetalin A which has been identified in all previous investigations was absent from D. leucosia. A recent re-investigation of D. gelonioides from China gave 14 new dichapetalins–dichapetalins T–W and derivatives of dichapetalins E, G, M, P, V, W, and Q in addition to dichapetalins A and K (Jing et al., Citation2014). Closely related to the dichapetalins are the acutissimatriterpenes isolated from Phyllanthus acutissima (Euphorbiaceae) (Tuchinda et al., Citation2008). Their unique structural feature is the methylenedioxy unit at the phenyl ring and particularly the side chain at C-17 exhibiting a spiro-lactone moiety substituted at C-25 with a methoxy group.
The dichapetalins are not the only triterpenoid compounds isolated from the Dichapetalaceae. Others are D:A-friedooleanan-3β-ol (5) and D:A-friedooleanan-3-one (6), 2β-hydroxy-3-oxo-D:A-friedooleanan-29-oic acid, betulonic acid, betulinic acid, canophyllol, canophyllal, β-sitosterol, stigmasterol, zeylanol, and 28-hydroxyzeylanol. A derivative of abietic acid as well as esters of (E)-ferulic acid and some fatty acids have also been reported from the genus (Addae-Mensah et al., Citation2007; Darbah, Citation1994).
Biological activity of the dichapetalins has focused mainly on cytotoxicity measurements in various cancer cell lines or brine shrimp assays. Dichapetalins A and M have shown promising cytotoxic and anti-proliferative activity results with 21 cancer cell lines (Achenbach et al., Citation1995; Addae-Mensah et al., Citation1996; Fang et al., Citation2006; Long et al., Citation2013). Similarly, Tuchinda et al. (Citation2008) also recorded selective activity for the acutissimatriterpenes against a panel of six cancer cell lines. The recently isolated dichapetalins by Jing et al. (Citation2014) have exhibited additional biological activities including antifeedant, nematicidal, antifungal and nitric oxide (NO), and acetylcholinesterase (AChE) inhibitory activities.
Recent recognition of the subtle but significant impact of helminths on health and education, together with their co-infection with malaria and HIV/AIDS, has spurred international efforts to control morbidity due to helminth infections. The treatment of helminth infections is dangerously over-reliant on only a few mainly synthetic drugs and these are under-researched. The dearth of existing treatments and the inevitable development of drug resistance, therefore, demand the need to develop new promising compounds for treating these neglected tropical diseases. Historical evidence clearly indicates that in the search for drugs for treating neglected and/or infectious tropical diseases, the plant kingdom has been a more productive source of such drugs than purely synthetic sources (Hotez et al., Citation2008; Moran et al., Citation2009). In drug discovery programs, it is common for drugs showing activity for one disease to be screened for their potential against other diseases. Hence, it will not be out of order for the dichapetalins which have shown potential antitumor activity and cytotoxicity, to be screened for possible activity against pathogens which are known to be the causative agents for various neglected tropical diseases. These include helminth infections such as the human hookworm, Necator americanus, Stiles 1902 (Nematoda: Ancylostomatidae) which is reported to be the predominant species in sub-Saharan Africa including Ghana (de Gruijter et al., Citation2005; Humphries et al., Citation2013; Treger et al., Citation2014; Verweij et al., Citation2007). Records have shown that albendazole and mebendazole which are currently the primary drugs for hookworm infection have started exhibiting lower efficacies of 72 and 15%, respectively, in single-dose treatment (Keiser & Utzinger, Citation2008). The discovery and development of new anthelminthic drugs, especially from natural products, is, therefore, imperative. Furthermore, Mass Drug Administrations (MDA) for the control of lymphatic filariasis and onchocerciasis in humans make use of broad spectrum anthelmintics, including albendazole and ivermectin (Ottesen, Citation2000; Seketeli et al., Citation2002). As a result, there is a potential for drug resistance to arise in other human nematode parasites which will also be exposed to these drugs (Albonico et al., Citation2004; Geerts & Gryseels, Citation2000).
In the present study, a preliminary investigation of the crude polar extracts of the leaves, stem, and roots of the plants by TLC revealed that only the roots contained dichapetalins (Long et al., Citation2013). Thus the roots were chosen as the plant part for study. We report the isolation of a new dichapetalin, named dichapetalin X (1), together with the known dichapetalin A (2), pomolic acid (3), glycerol monostearate (4), D:A-friedooleanan-3β-ol (5), and D:A-friedooleanan-3-one (6) from the petroleum ether, chloroform–acetone, and methanol extracts of the roots of D. filicaule. The in vitro anthelminthic activity of the compounds was determined using the Egg Hatch Inhibition Assay, the EHI (ovicidal) method.
Materials and methods
General experimental procedures
TLC was performed on aluminum foil slides pre-coated with silica gel (thickness 0.2 mm, type Kiesegel 60 F254, Merck, Rogers, AR) using petrol/CHCl3/Me2CO (7:5:5); detection: I2 vapor, anisaldehyde spray reagent, the Liebermann–Buchard reagent and the H2SO4 spray reagent. Column chromatography was carried out on silica gel 60 (Fluka Analytical, Bellefonte, PA). Melting points (uncorrected) were determined on a Stuart Scientific Melting Point Apparatus (Sigma-Aldrich, St. Louis, MO). IR spectra were recorded in KBr discs on a Shimadzu IR-408 spectrophotometer (Shimadzu Scientific Instruments, Kyoto, Japan). 1H NMR was run at 600 MHz and 13C NMR at 150 MHz in CDCl3 with TMS as the internal standard, on a Brüker Avance 600 Spectrophotometer (Brüker Inc., Fremont, CA). EIMS were obtained at 70 eV using a JEOL JMS-GCMate II instrument with direct probe inlet. HR ESI-TOF-MS of the Na complex of compound 1 was measured with a Brüker microTOF (Brüker Inc., Fremont, CA) using positive electrospray ionization. The optical rotation was determined in CHCl3 on a Bellingham Stanley Polarimeter (ADP220, PE02037) (Bellingham & Stanley Ltd, Kent, UK).
Plant material
Whole roots of D. filicaule were collected from Yamoransa (Central Region, Ghana) in September, 2012 and identified by Mr. J. Y. Amponsah of the University of Ghana National Herbarium, Botany Department, Legon. A voucher specimen (DF001) is deposited at the Herbarium. The plant material was pulverized after air drying for 7 weeks.
Extraction
The air-dried pulverized whole root of D. filicaule (2.5 kg) was exhaustively extracted with 6.5 l petroleum ether for 96 h. The extract, on concentration under reduced pressure, gave a total of 15.6 g of orange-red syrup. Successive cold percolation with about 6 l CHCl3 (96 h × 3) followed by percolation with 6 l Me2CO (96 h × 3) yielded, respectively, 9.2 g (dark green) and 3.5 g (green) crude extracts. Fresh pulverized roots (700 g) were then added to the 2.5 kg extracted plant material. This was exhaustively percolated with 6 l Me2CO for the same period to yield 5.1 g of a green mass. The CHCl3 and Me2CO extracts were combined due to their similar TLC profiles. This gave a total mass of 17.8 g of CHCl3/Me2CO extract. The extraction was continued with 6 l of MeOH (96 h × 3) to yield 9.1 g of a dark-red sticky crude extract.
Isolation
Trituration and purification by column chromatography of the petrol extract (15.6 g) on silica gel, eluting with petrol/EtOAc mixtures of increasing polarity afforded three solids; friedelan-3β-ol 5 (32.0 mg); friedelan-3-one 6 (40.8 mg), and a mixture of the two compounds (15.4 mg). The combined CHCl3 and Me2CO extracts (17.8 g) were chromatographed on silica gel (500 g), eluting with petrol/EtOAc mixtures of increasing polarity to give 10 fractions (1–10). Fractions 1 and 2 yielded more of compounds 6 (38.0 mg) and 5 (15.5 mg), respectively. Compound 4 (16.7 mg) was obtained from fraction 5 after recrystallization from petroleum ether. Fraction 6 yielded compound 2 (8.0 mg) while fractions 7 and 8 gave 40 mg of compound 1 after recrystallization from petroleum ether. The chromatographic separation of the methanol extract (9.1 g) gave nine fractions, eluting with petrol/EtOAc mixtures of 10:3, 10:6, 1:1, and 1:3; 100% EtOAc, 100% EtOH, and 100% MeOH, respectively. Fraction 1 precipitated a mixture of 5 and 6, fraction 4 gave 2 (2 mg), and fraction 5 gave 1 (3 mg). The remaining fractions were combined with fractions obtained from the CHCl3/Me2CO extract based on their TLC profiles. Further purification of the combined fractions afforded 3 (8 mg), 4 (8 mg), 2 (2 mg), and 1 (2 mg) from fractions 3, 4, 5, and 6, respectively.
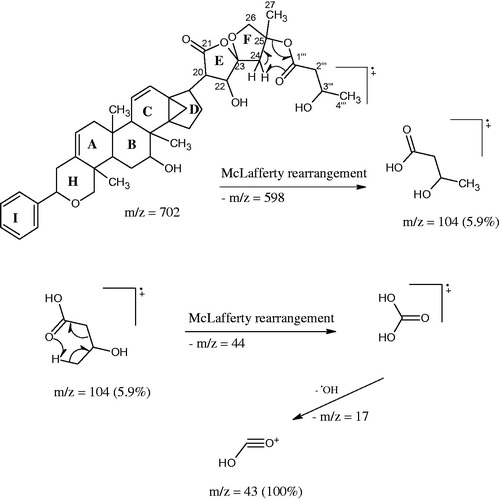
Dichapetalin X (1): Compound 1 was obtained from fractions 7 and 8 and recrystallized in petroleum ether as white crystals (40 mg); m.p. 246–248 °C; = + 11.4° (c 0.22, CHCl3); IR in KBr; νmax cm−1: 3577, 3368 (O–H hydrogen bonded), 2973, 2921, 1782 (>C=O lactone), 1743 (>C=O ester), 1602 (C=C), 699 (C6H5 bending). 1H NMR and 13C NMR, see . HR ESI-TOF-MS of the Na complex NaC42H54O9 is 725.36632 (calcd: 725.36600); EIMS: m/z (rel. int.): (M + -104) m/z 598 (3.3%), 502 (3.0%), 398 (22.5%), 200 (5.8%), 199 (15.8%), 187 (6.2%), 185 (11.5%), 105 (46.2%), 104 (5.9%), 91 (54.9%), 83 (14.8%), 60 (36.6%), 44 (37.4%), and 43 (100%).
Table 1. 1H and 13C NMR data of dichapetalin X (1).
Dichapetalin A (2): White crystals; m.p. 214–216 °C (from petroleum ether) (lit. value: 212–213 °C, Osei-Safo et al., Citation2008); anisaldehyde: purple; IR νmax (KBr) 3573, 3535, 3369, 2957, 2932, 1713, 1654, 1449, 1191, 1071, 1050, 767, and 701 cm−1; EIMS m/z (rel. int.) (%) 584 (M+, 6.7), 507 (7.2), 384 (8.5), 200 (10.9), 199 (5.2), 185 (10.6), 105 (100), 91 (43). Co-TLC with authentic sample in petrol/CHCl3/Me2CO (7:5:5), Rf = 0.82; petrol/EtOAc (1:1), Rf = 0.46; toluene/EtOH (10:2), Rf = 0.61; cyclohexene/EtOAc (1:1.5), Rf = 0.58.
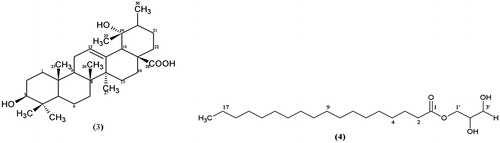
Pomolic acid (3): White crystals; m.p. 270–272 °C (from absolute EtOH) (lit value: 276–278 °C, Zhu et al., Citation2012); anisaldehyde: purple; IR νmax (KBr) 3569, 3413, 2875, 1687, 1461, and 1388 cm−1; 1H NMR (in C5D5N): see ; 13C NMR (in C5D5N): δ 39.4 (C-1), 28.2 (C-2), 78.2 (C-3), 40.4 (C-4), 55.9 (C-5), 19.0 (C-6), 33.6 (C-7), 39.0 (C-8), 48.3 (C-9), 37.4 (C-10), 24.1 (C-11), 128.1 (C-12), 137.9 (C-13), 42.4 (C-14), 29.4 (C-15), 26.4 (C-16), 47.8 (C-17), 54.7 (C-18), 72.7 (C-19), 42.2 (C-20), 27.0 (C-21), 38.5 (C-22), 28.8 (C-23), 16.8 (C-24), 15.6 (C-25), 17.3 (C-26), 24.7 (C-27), 180.7 (C-28), 27.2 (C-29), and 16.5 (C-30).
Table 2. Some 13C and 1H NMR (in C5D5N) signals of pomolic acid (3).
Glycerol monostearate (4): White crystals; m.p. 75–76 °C (from petrol) (lit value: 81.5 °C, Cornish, Citation1968); H2SO4 spray: black; IR νmax (KBr) 3309, 2911, 2850, 1740, 1731, 1470, and 1180 cm−1; 1H and 13C NMR: see ; EIMS m/z (rel. int.) (%) 358 (M+, 3), 327 (16.7), 285 (16.7), 267 (48.5), 239 (25), 154 (16.7), 134 (85.6), 112 (47.0), 98 (100), 84 (87.1), 74 (97.7), 57 (99.2), 43 (98.5), and 29 (37.9).
Table 3. 13C and 1H NMR data of glycerol monostearate (4).
Friedelan-3β-ol (5): White crystals; m.p. 274–276 °C (from EtOH) (lit value: 278–280 °C, Boonyaratavej & Petsom, Citation1991); anisaldehyde: purple; IR νmax (KBr) 3619, 3474, 2911, 2870, 1448, and 1385 cm−1.
Friedelan-3-one (6): White crystals; m.p. 248–250 °C (from EtOH) (lit value: 246–248 °C) (Ee et al., Citation2006); anisaldehyde: yellow, darkens with time; IR νmax (KBr) 2971, 2926, 2869, 1715, 1463, and 1389 cm−1.
Anthelminthic activity tests
Approval for the conduct of this study was obtained from the Institutional Review Board of Noguchi Memorial Institute for Medical Research of the University of Ghana (CPN 006/12-13). Field isolates of human hookworm (Necator americanus) were obtained following the collection of fecal samples from 231 consenting volunteers of two rural communities on the Kintampo – Tamale highway: Bawa Akuraa (N 08.23888°, W 001.63234°) and Jato Akuraa (N 08.25099°, W 001.61732°) in the Kintampo North Municipality in the Brong Ahafo Region of Ghana. The human hookworm was identified as Necator amaericanus; Stiles 1902 (Nematoda: Ancylostomatidae) by the PCR method. The participants comprised 146 females (63.2%) and 85 males (36.8%) aged 4–95 years, who upon oral adult and parental consent submitted stool samples. All persons with infections were accordingly treated.
The Kato–Katz fecal smear technique was employed to detect the presence of hookworm eggs. Fecal samples with more than 1200 eggs (or 300 eggs per g) were pooled for egg extraction. A density float method was employed to purify the eggs and the mean number of eggs per milliliter was calculated.
The in vitro anthelminthic activity test was conducted in 96-well flat bottom MicrotestTM Tissue culture plates (Becton Dickinson Labware Europe, BD Biosciences, Bedford, MA). The egg hatch inhibition (EHI) assay was set up by plating the purified eggs suspension (approximately 50 eggs per well) in a 96-well plate. Stock solutions (5 mg/ml) of each of the crude extracts in DMSO was prepared while the following concentrations of the isolated compounds were prepared: glycerol monostearate (4) (2 mg/ml), dichapetalin A (2) (1 mg/ml), and dichapetalin X (1) (2 mg/ml). Six dilute concentrations (dilution factor = 0.5) of the stock solution of each test sample was prepared in DMSO and added to the wells. Duplicate tests were performed. Albendazole and water were used as a positive and a negative standard, and a control, respectively. A stock solution of 2.5 mg/ml was prepared for the positive control. The concentration range was selected after some initial trials. For each treatment group, the lowest concentration that gave 100% Egg Hatch Inhibition (EHI) was chosen and this was then serially diluted to obtain a concentration-dependent activity. The plates were incubated at ambient temperature (≈30 °C) for 24 h after which a drop of 2% Lugol’s iodine was added to stop hatching of the eggs.
The number (#) of hatched and unhatched eggs was counted by light microscopy and percent egg hatch inhibition values were calculated as
The test results were analyzed using Probit and IC50 values with standard error of means (SEM) were computed with the GraphPad Prism 5 software (GraphPad Inc., San Diego, CA). p < 0.05 was considered significant when compared among the various treatment groups (one-way ANOVA). Dunnett’s multiple comparison test was used to determine the differences between means.
PCR method for hookworm identification
Genomic DNA of hookworm was isolated from five batches of pooled larvae obtained from Baermann cultures and used for hookworm speciation using a slightly modified direct PCR instead of the nested method of Monti et al. (Citation1998). Briefly, 50 µl single reaction containing 1 × PCR buffer, 1.5 mM MgCl2, 10 mM dNTP mix, 1 U of Taq polymerase (Sigma, St. Louis, MO), 10 µM each of oligonucleotide universal primer NC2 (TTAGTTTCTTTTCCTCCGCT) and primers, NA (CGTTAACATTGTATACCTGTACATAC) for N. americanus amplification or AD (TGCGAACTTCGCGTTCGCTGAGC) for A. duodenale amplification. PCR cycling conditions were an initial melt at 94 °C for 5 min, 40 cycles of 94 °C for 1 min, 45 °C for 1 min, and 72 °C for 1 min and ended with a final extension of 72 °C for 5 min. Negative controls (no DNA) and positive controls of N. americanus and A. duodenale (known DNA) were run alongside each reaction. The PCR products were electrophoresed in an ethidium bromide-stained 2% agarose gel and visualized under UV light. The sizes were estimated using 1 kb plus DNA ladder (Invitrogen, Grand Island, NY). The expected band sizes were 870 base pairs (bp) for N. americanus and 690 bp for A. duodenale.
Results and discussion
Characterization of compounds
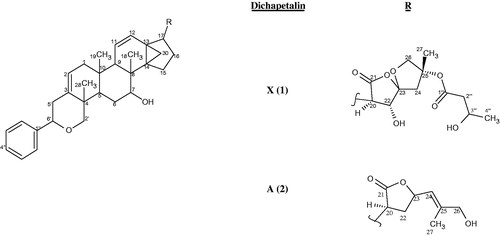
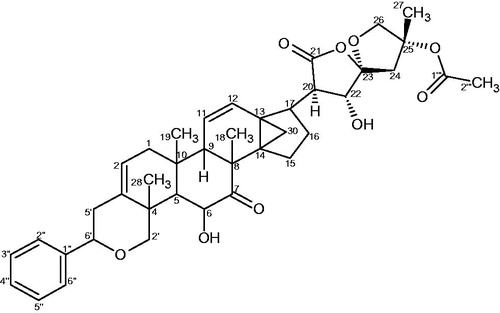
The chloroform–acetone extract of the roots of D. filicaule on chromatographic separation afforded compound 1 and the previously isolated dichapetalin A (2) (), which was identified by co-TLC with an authentic sample in several solvent systems and comparison of its physico-chemical data with published data (Achenbach et al., Citation1995; Addae-Mensah et al., Citation1996). The IR spectrum of 1 showed the presence of ester and lactone carbonyls as well as a OH group. The HR ESI-TOF MS of the Na complex ion of 1 gave a molecular ion peak at m/z 725.36632. The calculated mass for NaC42H54O9 is 725.36600, indicating a molecular mass of 702 for the parent compound.
The EIMS gave a significant peak at m/z 598 (3.3%) [M+-104] as a result of a McLafferty rearrangement of the side chain attached to the spiroketal group with a loss of m/z 104 from the molecular ion which should have appeared at m/z 702. Two orientations are possible for the McLafferty rearrangement; one in which the hydrogen is abstracted from the C-27 methyl to form an exo alkene on the tetrahydrofuran ring and the other in which hydrogen is abstracted from the methylene of the tetrahydrofuran ring to form an endo alkene of a methyl substituted dihydrofuran (). The 1H and 13C NMR data of 1 () exhibited the typical structural features of a dichapetalin skeleton (Achenbach et al., Citation1995; Addae-Mensah et al., Citation1996; Long et al., Citation2013; Osei-Safo et al., Citation2008) particularly for dichapetalins A and M. Chemical shifts and multiplicities of the triterpenoid skeleton were comparable with those found for dichapetalin A; those for the side chain correlated well with the values of dichapetalin M with the only exception being the presence of a β-hydroxybutyrate instead of the acetate moiety in M. Since all the NMR data agree very well with those of dichapetalin A and dichapetalin M, the relative configuration of 1 should correspond with the respective parts of A and M. The absolute configuration of dichapetalin A has already been determined by X-ray crystallography (Weckert et al., Citation1996). Therefore, the stereochemistry at the various chiral centers of 1 must be similar to that of dichapetalin A for the main skeleton and dichapetalin M for the side chain at C-25.
Compared with dichapetalins A and M, compound 1 bears structural similarities with dichapetalin A at the phenylpyranotriterpenoid moiety but with a different side chain at C-17. The presence of a spiroketal moiety in 1 as against a lactone moiety unambiguously distinguishes 1 from dichapetalin A (2). Similarly, the absence of oxygenation at C-6 in the phenylpyranotriterpenoid moiety of 1, and the presence of a β-hydroxybutyrate instead of an acetoxy on the spiroketal group at C-25 clearly differentiates 1 from dichapetalin M (). These findings make 1 a new dichapetalin, bringing to 24, the total number of dichapetalins isolated from the family. Thus, 1 has been named dichapetalin X, after dichapetalin W (Jing et al., Citation2014).
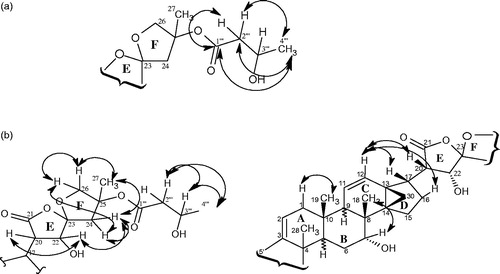
Coupling constants derived from the 1H NMR spectrum and NOESY connectivities were used in confirming the relative configuration at some stereocenters of compound 1. The most important HMBCs and NOEs are shown in and , and , respectively. The isolation of dichapetalin A in the present investigation is of strong chemotaxonomic significance. With the exception of D. leucosia, dichapetalin A has occurred with all dichapetalin isolations from the Dichapetalum species so far investigated.
Compound 3 () was identified from its spectral data and by comparison with reports corresponding to compounds in the literature as the rare ursane-type pentacyclic triterpenoid, pomolic acid. Pomolic acid (3) is the 19-hydroxy derivative of the more common ursolic acid and has previously been isolated from other plant families including Rosaceae (Ju et al., Citation2003), Sapotaceae (Lee et al., Citation2005a), Oleaceae (Saimaru et al., Citation2007; Yoo et al., Citation2013), Staphyleaceae (Cheng et al., Citation2010), and the blueberry fruit (Neto, Citation2011). Natural and semisynthetic ursane-type triterpenoids are believed to have the potential as candidates for the design of multi-target bioactive compounds, with focus on their anticancer effects (Salvador et al., Citation2012) and pomolic acid is reported to induce apoptosis in some cancer cell lines (Vasconcelos et al., Citation2007; Yoo et al., Citation2013; Youn et al., Citation2012). It has also been identified as an anti-HIV agent (EC50 1.4 μg/ml, T.I. 16.6) (Kashiwada et al., Citation1998) that possesses the ability to elicit endothelium-dependent relaxation in rat aortic rings.
This is the first report of the isolation of pomolic acid and for that matter, an ursane-type triterpenoid from the Dichapetalaceae and this occurrence is of significant chemotaxonomic importance. Triterpenoids so far isolated from the Dichapetalaceae are the dammarane-type – dichapetalins (Achenbach et al., Citation1995; Addae-Mensah et al., Citation1996; Fang et al., Citation2006; Long et al., Citation2013; Osei-Safo et al., Citation2008); friedelane-type – friedooleananes, zeylanol, canophyllal, and canophyllol (Addae-Mensah et al., Citation2007; Darbah, Citation1994; Fang et al., Citation2006) and the lupane-type – betulinic acid and betulonic acid (Addae-Mensah et al., Citation2007; Fang et al., Citation2006). Pomolic acid isolation has reportedly occurred usually with the oleanane-type and the lupane-type triterpenoids (Saimaru et al., Citation2007; Neto, Citation2011; Yoo et al., Citation2013).
Compound 4 () was obtained as white crystals with melting point of 75–76 °C. The MS showed an M+ at m/z 358 corresponding to the MF C21H42O4 and a base beak at m/z 98 (100%). Most of the peaks were due to fragmentations of an alkane side chain. The presence of an ester carbonyl was inferred by both the IR data (1740, 1731, and 1180 cm−1) and the 13C NMR signal at δC 174.34. In the 1H NMR spectrum, a triplet observed at δH 0.80 (3H, t, J = 7 Hz) accounted for one methyl group while a multiplet at δH 1.23–1.35 (28H, m) was assigned to hydrogen atoms of 14 methylene units, corroborating the fragmentation pattern observed in the MS. The corresponding 13C NMR signals for these assignments occurred at δC 14.10 (C-18) and δC 22.68–31.91 (C-4 to C-17). Signals at δC 34.16 and 24.91 were attributed to the carbons C-2 and C-3, respectively, due to the electron withdrawing effect of the carbonyl group. The methylene protons H-3 were split by the four protons of H-2 and H-4 to give a signal at δH 1.63 (2H, quintet, J = 7 Hz) while the signal for H-2 was observed at δH 2.35 (2H, t, J = 7 Hz). Signals at δC 70.00, 65.00, and 63.00, which were missing in the 13C NMR spectrum due to extremely low intensity but clearly visible in the HSQC, together with the COSY and O–H stretching vibration of alcohol (3309 cm−1) in the IR spectrum enabled the assignment of carbons C-2′, C-1′, and C-3′ of the glycerol ().
Based on the spectral data, compound 4 was identified as the glycerol monoester of stearic acid. It is a food additive used as an emulsifying, thickening, anti-caking and preservative agent; an emulsifying agent for oils, waxes and solvents; a protective coating for hygroscopic powders; a solidifier and control release agent in pharmaceuticals; and a resin lubricant. It is also used in cosmetics and hair care products. Although this is the first time this compound is being reported in the Dichapetalaceae, it has however been isolated in the marine brown algae Sargaassum sagamianum (Chang et al., Citation2008), the plants Saururus chinensis (Lee et al., Citation2005b)), and Hyoscyamus niger (Ma et al., Citation2002). It is reported to have recorded an IC50 value = 100 µM towards cultured LNCaP human prostate cancer cells (Ma et al., Citation2002).
Compounds 5 and 6 were identified as D:A-friedooleanan-3β-ol (friedelan-3β-ol) and D:A-friedooleanan-3-one (friedelan-3-one), respectively, on the basis of comparative IR spectra, co-TLC, and mixed melting point with authentic samples previously isolated from D. madagascariense and D. barteri (Addae-Mensah et al., Citation2007; Darbah, Citation1994).
Results of anthelminthic activity test
The petroleum ether (PE) and methanol (M) crude extracts of D. filicaule showed 100% egg hatch inhibition (EHI) at concentrations of 2500 μg/ml while the same concentration of the chloroform-acetone extract (CA) gave 98.7% inhibition. The PE after a 2-fold dilution to 1250 μg/ml exhibited 96.8% EHI. Among the pure isolated compounds, dichapetalin A was the most potent, giving 100% EHI at a concentration of 500 μg/ml while glycerol monostearate and dichapetalin X recorded EHI values of 91.1 and 48.8%, respectively, at the same concentration. The potency of dichapetalin A was further revealed by a 90.2% EHI at 250 μg/ml (). For the standard anthelminthic drug, albendazole was tested as a positive control and the highest concentration that completely inhibited egg hatch was 1250 μg/ml, a concentration at which PE showed 96.8% inhibition. Lower concentrations of the dichapetalins and glycerol monostearate than those of the standard were required to achieve complete egg hatch inhibition. However, two-fold serial dilutions did not significantly affect the hatch rate of albendzole as compared with the crude extracts and isolated compounds ().
Table 4. In vitro average % EHI values at different concentrations of extracts, pure compounds, and albendazole.
All the crude extracts, PE, CA and M showed IC50 values in the increasing order 856.0, 1023, and 1236, respectively, μg/ml. The pure compounds also gave IC50 values of 162.4, 306.0, and 523.2 μg/ml, respectively, for dichapetalin A, glycerol monostearate, and dichapetalin X (). Thus, both the extracts and isolated compound exhibited lower potency in comparison to the albendazole (IC50 value = 93.27 μg/ml). The most promising anthelminthic compound is dichapetalin A. However, a one-way ANOVA followed by Dunnett’s multiple comparison test showed that there was no significant difference in the anthelminthic activity of all extracts, compounds and the standard (p = 0.2107, F7,48 = 1.442).
Table 5. IC50 values (± SEM) for extracts (M, CA, and PE), pure compounds (Dichapetalin X, Dichapetalin A and Glycerol monostearate), and albendazole.
Result of PCR method for hookworm identification
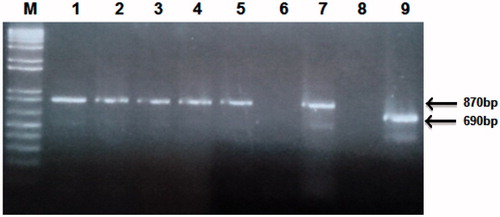
All the five pooled larvae were identified as N. americanus and no A. duodenale was identified ().
Figure 6. PCR identification of hookworm species. Lane M is the molecular weight marker, lanes 1–5 are genomic DNA of N. americanus eggs, lane 7 is the N. americanus positive control corresponding to 870 base pairs, lanes 6 and 8 are the two negative controls for the two species and lane 9 is the A. duodenale, positive control corresponding to 690 base pairs.
Conclusion
The phytochemical investigation of D. filicaule led to the isolation of a new dammarane-type triterpenoid, named dichapetalin X and the known dichapetalin A. Occurring for the first time in the Dichapetalaceae is the rare ursane-type triterpenoid, pomolic acid and the food additive glycerol monostearate. The friedelane-type triterpenoids, D:A-friedooleanan-3β-ol and D:A-friedooleanan-3-one, which have been found in several Dichapetalum species were also isolated. Dichapetalins A and X as well as glycerol monostearate were evaluated for their anthelminthic activity against the human hookworm and they exhibited egg hatching inhibition activities with IC50 values of 162.4, 523.2, and 306.0 μg/ml, respectively. These activities were lower than that of the positive control, albendazole (IC50 value = 93.27 μg/ml). Although these activities are lower than that of albendazole (IC50 value = 93.27 μg/ml), dichapetalin A could be investigated further for potential anthelminthic activity.
Acknowledgements
Thanks are due to the Office of Research, Innovation and Development (ORID) of the University of Ghana for a research grant, the people of Bawa Akuraa and Jato Akuraa in Ghana for consenting to provide samples for the anthelminthic assay and Dr. Patrick Amoateng (Department of Pharmacology and Toxicology, School of Pharmacy, College of Health Sciences, University of Ghana) for his assistance with the statistical analysis.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Achenbach H, Asunka SA, Waibel R, et al. (1995). Dichapetalin A, a novel plant constituent from Dichapetalum madagascariense. Nat Prod Lett 7:93–100
- Addae-Mensah I, Adu-Kumi S, Waibel R, et al. (2007). A novel D:A-friedooleanane triterpenoid and other constituents of the stem bark of Dichapetalum barteri Engl. ARKIVOC IX:1–9
- Addae-Mensah I, Waibel R, Asunka SA, et al. (1996). The Dichapetalins – A new class of triterpenes. Phytochemistry 43:649–56
- Albonico M, Engels D, Savioli L. (2004). Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: A pressing public health agenda for helminth control. Int J Parasitol 34:1205–10
- Bongers F, Parren MPE, Traore D., eds. (2005). Forest Climbing Plants of West Africa; Diversity, Ecology and Management. Wallingford, UK: CAB International Publishing
- Boonyaratavej S, Petsom A. (1991). Chemical constituents of the roots of Bridelia tomentosa BL. J Sci Soc Thai 17:61–9
- Breteler FJ. (1970). The African Dichapetalaceae W; Three new species from West Africa. Acta Bot Neerl 19:1–15
- Chang HW, Jang KH, Lee D, et al. (2008). Monoglycerides from the brown alga Sargassum sagamianum: Isolation, synthesis, and biological activity. Bioorg Med Chem Lett 18:3589–92
- Cheng J-J, Zhang L-J, Cheng H-L, et al. (2010). Cytotoxic hexacyclic triterpene acids from Euscaphis japonica. J Nat Prod 3:1655–8
- Cornish R. (1968). Studies of glyceryl monostearate. J Soc Cosmet Chem 19:109–17
- Darbah VF. (1994). Chemical constituents and biological activity of the stem bark of D. madagascariense [M.Phil (Chemistry) thesis]. University of Ghana
- de Gruijter JM, van Lieshout L, Gasser RB, et al. (2005). Polymerase chain reaction-based differential diagnosis of Ancylostoma duodenale and Necator americanus infections in humans in northern Ghana. Trop Med Int Health 10:574–80
- Ee GCL, Kua ASM, Lim CK, et al. (2006). Inophyllin A, a new pyranoxanthone from Calophyllum inophyllum (Guttiferae). Nat Prod Res 20:485–91
- Fang L, Ito A, Chai H-B, et al. (2006). Cytotoxic constituents from the stem bark of D. gelonioides collected in the Philippines. J Nat Prod 69:332–7
- Geerts S, Gryseels B. (2000). Drug resistance in human helminths: Current situation and lessons from livestock. Clin Microbiol Rev 13:207–22
- Hotez PJ, Brindley PJ, Bethony JM, et al. (2008). Helminth infections: The great neglected tropical diseases. J Clin Invest 118:131–21
- Humphries D, Simms BT, Davey D, et al. (2013). Hookworm infection among school age children in Kintampo North Municipality, Ghana: Nutritional risk factors and response to albendazole treatment. Am J Trop Med Hyg 89:540–8
- Ju J-H, Zhou L, Lin G, et al. (2003). Studies on constituents of triterpene acids from Eriobotrya japonica and their anti-inflammatory and antitussive effects. Chin Pharm J 38:752--7
- Jing S-X, Luo S-H, Li C-H, et al. (2014). Biologically active dichapetalins from Dichapetalum gelonioides. J Nat Prod 77:882–93
- Kashiwada Y, Wang H-K, Nagao T, et al. (1998). Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J Nat Prod 61:1090–5
- Keiser J, Utzinger J. (2008). Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. J Am Med Assoc 299:1937–48
- Lee T-H, Juang S-H, Hsu F-L, Wu C-Y. (2005a). Triterpene acids from the leaves of Planchonella duclitan (Blanco) Bakhuizan. J Chin Chem Soc 52:1275–80
- Lee WS, Kim MJ, Beck YI, et al. (2005b). Lp-PLA2 inhibitory activities of fatty acid glycerols isolated from Saururus chinensis roots. Bioorg Med Chem Lett 15:3573–5
- Long C, Aussagues Y, Molinier N, et al. (2013). Dichapetalins from Dichapetalum species and their cytotoxic properties. Phytochemistry 94:184–91
- Ma CY, Liu WK, Che CT. (2002). Lignan amides and non-alkaloidal components of Hyoscyamus niger seeds. J Nat Prod 65:206–9
- McCullough J, Decher J, Gubakpelle D. (2005). A Biological Assessment of the Terrestrial Ecosystems of the Draw River, Boi-Tano, Nimri and Krokosua Hill Forest Reserves of Ghana. RAP Bull Biol Asses 36. Washington, DC: Conservation International
- Monti JR, Chilton NB, Qian BZ, Gasser RB. (1998). Specific amplification of Necator americanus or Ancylostoma duodenale DNA by PCR using markers in ITS-1 rDNA, and its implications. Mol Cell Probes 12:71–8
- Moran M, Guzman J, Ropars A-L, et al. (2009). Neglected disease research and development: How much are we really spending? PLoS Med 6:e1000030
- Neto CC. (2011). Ursolic acid and other pentacyclic triterpenoids: Activities and occurrences in berries. In: Stoner GD, Seeram NP, eds. Berries and Cancer Prevention. New York: Springer Science + Business Media, 41–9
- Osei-Safo D, Chama MA, Addae-Mensah I, et al. (2008). Dichapetalin M from Dichapetalum madagascariense. Phytochem Lett 1:147–50
- Ottesen EA. (2000). The global programme to eliminate lymphatic filariasis. Trop Med Int Health 5:591–94
- Saimaru H, Orihara Y, Tansakul P, et al. (2007). Production of triterpene acids by cell suspension cultures of Olea europaea. Chem Pharm Bull 55:784–8
- Salvador JAR, Moreira VM, Gonçalves BMF, et al. (2012). Ursane-type pentacyclic triterpenoids as useful platforms to discover anticancer drugs. Nat Prod Rep 29:1463–79
- Seketeli A, Adeoye G, Eyamba A, et al. (2002). The achievements and challenges of the African Programme for Onchocerciasis Control (APOC). Ann Trop Med Parasitol 96:S15–28
- Treger RS, Otchere J, Keil MF, et al. (2014). In vitro screening of compounds against laboratory and field isolates of human hookworm reveals quantitative differences in anthelminthic susceptibility. Am J Trop Med Hyg 90:71–4
- Tuchinda P, Kornsakulkarn J, Pohmakotr M, et al. (2008). Dichapetalin-type triterpenoids and lignans from the aerial parts of Phyllanthus acutissima. J Nat Prod 71:655–63
- Vasconcelos FC, Gattass CR, Rumjanek VM, Maia RC. (2007). Pomolic acid-induced apoptosis in cells from patients with chronic myeloid leukemia exhibiting different drug resistance profile. Invest New Drugs 25:525–33
- Verweij JJ, Brienen EA, Ziem J, et al. (2007). Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am J Trop Med Hyg 77:685–90
- Weckert E, Hümmer K, Addae-Mensah I, et al. (1996). The absolute configuration of Dichapetalin A. Phytochemistry 43:657–60
- Yoo KH, Park J-H, Lee DK, et al. (2013). Pomolic acid induces apoptosis in SK-OV-3 human ovarian adenocarcinoma cells through the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways. Oncol Lett 5:386–90
- Youn SH, Lee JS, Lee S, et al. (2012). Anticancer properties of pomolic acid-induced AMP-activated protein kinase activation in MCF-7 human breast cancer cells. Biol Pharm Bull 35:105–10
- Zhu C-C, Gao L, Zhoa Z-X, Lin C-Z. (2012). Triterpenes from Callicarpa integerrima Champ. Acta Pharma Sin 47:77–83