Abstract
Context: TGF-β plays a central role in hypertrophic scar (HS) formation and development.
Objective: This study investigated the role of a TGF-β antagonist peptide in inhibiting fibrotic behavior of human HS-derived fibroblasts (HSFs).
Materials and methods: HSFs were seeded at a density of 3.1 × 104/cm2 and were subjected to treatment of peptide antagonist (30 μM) or TGF-β receptor inhibitor LY2109761 (10 μM) or without treatment followed by the analyses of quantitative PCR, Elisa, in vitro wounding and fibroblast-populated collagen lattice (FPCL) assays.
Results: qPCR and Elisa analyses showed that the peptide could, respectively, reduce the gene (at 48 h) and protein (at 72 h) expression levels of collagen I (86 ± 4.8%; 56.6 ± 7.3%), collagen III (73 ± 10.7%; 43.7 ± 7.2%), fibronectin (90 ± 8.9%; 21.1 ± 2.8%), and TGF-β1 (85 ± 9.3%; 25.0 ± 9.4%) as opposed to the non-treated group (p < 0.05), as the LY2109761 group similarly did. Cell proliferation was also significantly inhibited at day 5 (CCK-8 assay) by both peptide and LY2109761 treatments compared with the non-treated group (p < 0.05). The peptide also significantly inhibited cell migration as opposed to blank control at 24 h (43 ± 6.7% versus 60 ± 2.1%, p < 0.05) and at 48 h (63.9 ± 3.1% versus 95 ± 4.1%, p < 0.05). Similar to LY2109761, the peptide antagonist significantly reduced HS FPCL contraction compared with the non-treated group with significant differences in surface area at 48 h (0.71 ± 0.06 cm2 versus 0.51 ± 0.06 cm2, p < 0.05) and at 72 h (0.65 ± 0.02 cm2 versus 0.42 ± 0.01 cm2, p < 0.05).
Conclusion: The TGF-β antagonist peptide may serve as an important drug for HS prevention and reduction given the obvious benefits of good biosafety, low cost, and easy manufacture and delivery.
Introduction
Transforming growth factor-β (TGF-β) probably is the most potent fibrosis leading factor among all growth factors and is closely associated with scar formation (Liu et al., Citation2004). Overexpressions of TGF-β and its receptors have been discovered in hypertrophic scar and keloid tissues (Chin et al., Citation2001; Lee et al., Citation1999; Schmid et al., Citation1998) as well as their derived fibroblasts (Bettinger et al., Citation1996; Smith et al., Citation1999; Wang et al., Citation2000) when compared with normal skin and normal fibroblasts. By contrast, fetal skin contains minimal or undetectable TGF-β and its receptors (Broker et al., Citation1999; Hsu et al., Citation2001). Moreover, fetal skin wound also contains significantly lower amount of TGF-β compared with adult wound (Broker et al., Citation1999; Hsu et al., Citation2001), which is considered as one of the important mechanisms of fetal scarless wound healing.
Because of this, manipulation of wound TGF-β function is a well-received strategy for reducing wound scaring (Liu et al., Citation2004). These approaches include gene manipulation such as TGF-β1 antisense oligonucleartide (Choi et al., Citation1996), or overexpression of truncated TGF-β receptor II (Liu et al., Citation2005) and protein manipulation like TGF-β neutralizing antibody (Shah et al., Citation1992) and recombinant TGF-β3 (Shah et al., Citation1995). Different from these strategies, Huang et al. (Citation1997) developed a TGF-β peptide antagonist, a pepantagonist that was able to compete with the ligands for the binding to TGF-β receptor and thus block TGF-β function. More importantly, this peptide antagonist was applied to the treatment of pig burn injured wound and its efficacy on scar reduction was also proven (Singer et al., Citation2009), indicating the potential of this antagonist in clinical therapy of hypertrophic scar.
However, there is no report so far to demonstrate its scar reduction effect on hypertrophic scar (HS) in human tissue or cell models. We thus would like to investigate the role of this antagonist in potential inhibition of HS as well as the possible mechanisms using HS-derived fibroblasts (HSFs) as a model with particular focus on the aspects of cell proliferation, migration, contraction, and gene/protein expression of scar related molecules.
Materials and methods
Patients, tissue samples, and chemical agents
Total six HS tissues in an active stage were harvested from six patients (age ranged 20–55, three males and three females), who did not receive any treatment previously before surgical treatment. The anatomical locations of hypertrophic scar included chest (three samples), arms (two samples), and neck (one sample). Protocols for the handling of human tissue and cells were approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine, and all tissue samples were donated by the patients for research purpose only with written informed consent. Primary fibroblasts derived from six tissue samples were mixed and frozen. Three frozen samples were randomly selected from the frozen pooled samples, and cells were independently thawed and cultured for each experiment.
Cell culture
Hypertrophic scar tissues derived from a surgical excision procedure were first rinsed three times in 2.5% chloramphenicol solution, and then the tissues were thoroughly washed three times with phosphate-buffered saline (PBS), each lasting 5 min. Afterwards, the tissues were sectioned into 2 × 4 mm2 pieces and subjected to the treatment with 0.2% dispase (Roche Diagnostics, Indianapolis, IN, dissolved in DMEM containing 10% fetal bovine serum, FBS) overnight at 4 °C. After the treatment, the dermis was mechanically separated from the epidermis using a forceps. Then the dermis was further cut into small fragments aseptically followed by enzyme digestion with 0.2% collagenase II (SERVA, Heidelberg, Germany, dissolved in DMEM containing 10% FBS) for 3.5 h at 37 °C on a rotator. After the digestion, the cell suspension was washed in DMEM culture medium containing 10% FBS, penicillin (100 U/ml) and streptomycin (0.1 mg/ml) followed by centrifugation at 1500 rpm for 5 min. The pellet was then resuspended in DMEM culture medium and seeded onto 10 cm culture dish (BD, Falcon, TX) in regular density (1.0 × 106 per dish) for culture in a humidified 5% CO2 at 37 °C. When cells became confluence, they were detached with 0.25% trypsin-EDTA (Gibco, Grand Island, NY) and subcultured at the same density. All experiments were performed with the cells between 3 and 5 passages.
Experimental design
The peptide antagonist was kindly provided by Dr. Jung Huang from University of St. Louis School of Medicine. The agent used in the study is pegylated-TGF-β antagonist, which is a peptide consisting of amino acids corresponding to the 41st to 65th residues of human TGF-β with its functional confirmation in a previous study (Huang et al., Citation1997). To perform the study, the peptide powder was first dissolved at the concentration of 3 mM using sterile PBS, and then small amount of 1 N NaOH was added to assist the dissolution, but the pH should not exceed 9.5. Then the peptide stock solution was stored at −20 °C before use. According to the literature (Huang et al., Citation1997), we added the antagonist stock solution to DMEM culture medium to make the final concentration at 30 µM.
Cells treated with the peptide antagonist were considered as the experimental group, whereas no drug treatment served as a blank control group. As a positive control, a novel dual inhibitor for both TGF-β receptors I and II, LY2109761 (Selleck, Houston, TX), was used. Briefly, the inhibitor was first dissolved in DMSO to make the stock solution at the concentration of 4.53 mM and stored at −80 °C before use. To perform the experiment, the LY2109761 stock solution was added to DMEM culture medium to make final concentration to 10 µM as previously described (Melisi et al., Citation2008).
RNA extraction and quantitative PCR (qPCR)
HSF suspension was adjusted to 1 × 106 cells/ml, and then 0.25 ml of the suspension along with another 3 ml of DMEM culture medium containing 10% FBS, penicillin (100 U/ml) and streptomycin (0.1 mg/ml) was added to 3.5 cm culture dish with a density of 3.1 × 104/cm2. After 24 h, a new medium was changed and the cells were cultured for 48 h in the absence and presence of peptides or LY2109761. Afterwards, HSFs were harvested and total RNA was extracted using Trizol Reagent (Invitrogen, Carlsbad, CA). RNA purity was evaluated by the ratio of A260/A280 and only RNA products with a ratio of 1.8–2.0 were used for qPCR analysis.
cDNA was converted from 2 μg total RNA with AMV reverse transcriptase (Promega, Madison, WI) in a 20 μl reaction solution composed of 4 μl 5 × buffer, 4 μl MgCl2, 1 μl oligo-(dT), 2 μl dNTP, 0.5 μl RNase inhibitor, and 0.5 μl AMV reverse transcriptase, using ddH2O to meet the final volume. To convert cDNA, the mixture was incubated at 30 °C for 10 min, 42 °C for 60 min, 95 °C for 5 min, and 5 °C for 5 min.
qPCR was performed using a Power SYBRGreen PCR master mix (2×) (Applied Biosystems, Foster City, CA) in a real-time thermal cycler (Stratagene Mx3000PTM qPCR System, La Jolla, CA). The mixture was first incubated at 95 °C for 10 min followed by 40 cycles (30 s at 95 °C, 30 s at annealing temperature listed in and 45 s at 72 °C) and terminated by 5 min extension at 72 °C and stored at 4 °C until analysis. The amplified cDNA products were normalized to internal control of GAPDH. The primers for qPCR analysis are listed in . Each assay was performed in triplicate and experiments were repeated in three cell samples.
Table 1. Primers used in quantitative PCR analysis.
Enzyme-linked immunosorbent assay (ELISA)
As mentioned above, HSF suspension was adjusted to 1 × 106/ml, and then 0.25 ml of cell suspension along with another 3 ml of DMEM culture medium containing 10% FBS, penicillin (100 U/ml) and streptomycin (0.1 mg/ml) was added to the 3.5 cm culture dish. After 24 h, culture medium was replaced with serum-free DMEM with or without the peptide or LY2109761 and cultured for another 72 h. Afterwards, culture media were collected and subjected to Elisa analysis with the kits special for collagens I and III (Southern Biotech; Birmingham City, AL), for fibronectin (Sigma, San Francisco, CA) and for TGF-β1, (R&D, San Jose, CA). The absorbance measurement was performed at a wavelength of 450 nm using a microreader (Thermo, Waltham, MA). The assay was performed in triplicates and repeated in three cell samples.
Cell proliferation assay
Cell counting kit-8 (CCK-8 kit, Doijndo, Kumamoto, Japan) was used to evaluate cell proliferation according to instructions of the manufacturer. Cells in the logarithmic phase were prepared as single-cell suspension for inoculation onto 96-well plates. Cell density was adjusted to 2 × 104/ml, and to each well, 100 μl of the cell suspension in DMEM culture medium containing 10% FBS, penicillin (100 U/ml) and streptomycin (0.1 mg/ml) were added. Then the wells were divided into three groups, peptide-containing medium, LY2109761-containing medium, and DMEM medium only were added to the three groups upon cell adherence. Each tested sample contained six parallel wells. Cells were cultured for days 1, 2, 3, 4, and 5 before analysis. At the end of desired incubation time, 10 μl of CCK-8 solution were added to each well followed by incubation at 37 °C for 4 h. Then media were collected and subjected to absorbance measurement on a microplate reader (Molecular Devices, Sunnyvale, CA) at 450 nm wave length. The assay was performed in six parallel wells and repeated in three cell samples.
Scratch wound assay
As previously described (Liang et al., Citation2007), an in vitro scratch wound assay was used to evaluate cell migration. Briefly, cells (2 × 105/well) were grown in 6-well culture plates until confluence, then a scratch (in vitro wounding) was created on each confluent monolayer using a 200 μl pipette tip (PipetTipFinder, LLC, Knoxville, TN) perpendicular to the bottom of the dish. This generated a “wound” about 0.45–0.50 mm in width. The cultures were refreshed with new medium and were maintained under incubation. Digital photographs of each wound were taken under a microscope (Nikon, Tokyo, Japan) immediately after the scraping, 24 h and 48 h post-scraping. Wound closure (cell migration) was investigated by measuring the area between the opposite edges of the wound using the commercial software Image pro-plus version 6.0 (Media Cybernetics, Silver Spring, MD) and public domain image processing program. Data (means ± SD, n = 3) were presented as the percentage of the initial wound (distance at time zero) using the following formula: cell migration rate (%) = (Gap0h – Gap24 h)/Gap0h × 100%. In each sample, three views were randomly photographed to obtain the mean, and final mean rate plus standard deviation was derived from the means of three cell samples.
Fibroblast-populated collagen lattice (FPCL) contractility
FPCL contractility assay was proceeded as previously described (Bell et al., Citation1979). Briefly, collagen lattices were polymerized in 24-well tissue culture plate. To proceed, 400 μl of rat tail tendon collagen solution (2 mg/ml, Shenyou Biotechnology Co., Ltd, Zhejiang, China) were mixed with 24 μl 0.1 mol/L NaOH, and then 46 μl 10 × PBS was added into the mixture. Afterwards, 1.52 ml of cell suspension (containing 6.08 × 104 cells) were added to the collagen solution, gently mixed and added into the 24-well plate (500 μl per well). Collagen lattices were allowed to gel for 30 min under the normal temperature, followed by gentle addition of 1 ml of culture medium to each well. The cell-contained gels were cultured for 24 h, and then were detached from culture plate as the floating lattices to allow for self-contraction in the wells. Digital images of the floating lattices were captured at the time intervals of 24, 48, and 72 h. To quantify contracture, FPCL surface areas of three time points were determined using image software (Macropath 5, PRO, Guangzhou, China), and sequential area calculation was normalized to the areas measured immediately after their release.
Statistical analysis
Results are presented as mean ± standard deviation. The differences among multiple groups were analyzed using the one-way ANOVA test and statistical significance between two groups of interest was tested with a post-hoc statistical method along with ANOVA test. p < 0.05 was considered statistically significant. SPSS software (version 19.0, SPSS Inc., Chicago, IL) was applied in this statistical analysis.
Results
TGF-β peptide antagonist inhibited the gene expression of collagen I, collagen III, fibronectin, and TGF-β1
HSFs were incubated with and without the peptide and LY2109761 for 48 h, then the anti-TGF-β effect of the peptide was evaluated with qPCR to examine the gene expression of collagens I and III, fibronectin, and TGF-β1 in both control groups and the experimental group. As shown in , the gene expression levels of collagens I and III, fibronectin, and TGF-β1 were significantly downregulated with the reduction levels of 86 ± 4.8% (p < 0.05, ), 73 ± 10.7% (p < 0.05, ), 90 ± 8.9% (p < 0.05, ), and 85 ± 9.3% (p < 0.05, ), in the peptide group, when compared with the blank control group. As a positive control of anti-TGF-β effect, treatment with LY2109761 also significantly downregulated the gene expression of these molecules compared with the blank control (p < 0.05, ). ANOVA analysis showed there were significant differences in the expression levels of all molecules among three groups (p < 0.05). There were no significant differences between peptide and LY2109761 groups for collagen III (p > 0.05, ), fibronectin (p > 0.05, ), and TGF-β1 (p > 0.05, ). However, collagen I expression level was significantly lower in the peptide group than in the LY2109761 group (p < 0.05, ).
Figure 1. TGF-β peptide antagonist inhibited the gene expression of collagen I (COL I, A), collagen III (COL III, B), fibronectin (FN, C) and TGF-β1 (D) in HS fibroblasts. qPCR was employed to analyze the expression of various genes of the cells that were treated with peptide antagonist (peptide, 30 μM) and TGF-β type I/II receptor kinase inhibitor (LY2109761, 10 μM) or without treatment (control) for 48 h. The gene expression levels were presented relative to the control level. *p < 0.05 between control and LY2109761 groups; #p < 0.05 between control and peptide groups; &p < 0.05 between LY2109761 and peptide groups.
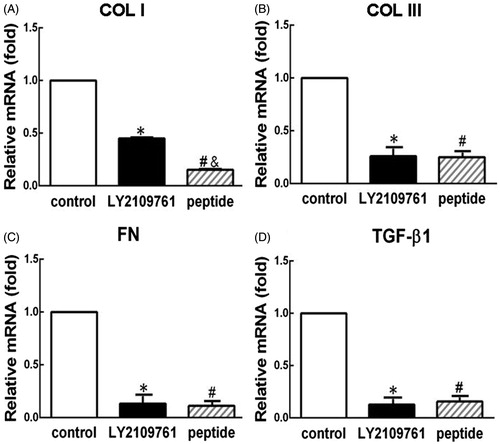
TGF-β peptide antagonist suppressed the protein production of collagen I and collagen III, fibronectin, and TGF-β1
HSFs were incubated with and without the peptide and LY2109761 for 72 h, then the anti-TGF-β effect of the peptides was evaluated with ELISA to measure the protein production of related molecules. As shown in , compared with the blank control group, treatment of the peptide could significantly reduce the protein production of collagen I (56.6 ± 7.3%, , p < 0.05), and collagen III (43.7 ± 7.2%, , p < 0.05), fibronectin (21.1 ± 2.8%, , p < 0.05), and TGF-β1 (25.0 ± 9.4%, , p < 0.05). ANOVA analysis showed there were significant differences in the expression levels of all four molecules among three groups (p < 0.05). Nevertheless, no significant difference in the protein productions of collagen I, collagen III, and TGF-β1 could be observed between the LY2109761-treated group and the peptide-treated group (p > 0.05, ). But the production of fibronectin was significantly lower in the LY2109761-treated group than in the peptide-treated group with significant difference (p < 0.05, ).
Figure 2. TGF-β peptide antagonist inhibited the protein production of collagen I (COL I, A), collagen III (COL III, B), fibronectin (FN, C), and TGF-β1 (D) in HS fibroblasts. Elisa was employed to analyze the protein production of various molecules from the cells that were treated with peptide antagonist (peptide, 30 μM) and TGF-β type I/II receptor kinase inhibitor (LY2109761, 10 μM) or without treatment (control) for 72 h. Fold changes relative to control were presented as the means of protein production levels among different groups. *p < 0.05 between control and LY2109761 groups; #p < 0.05 between control and peptide groups; &p < 0.05 between LY2109761 and peptide groups.
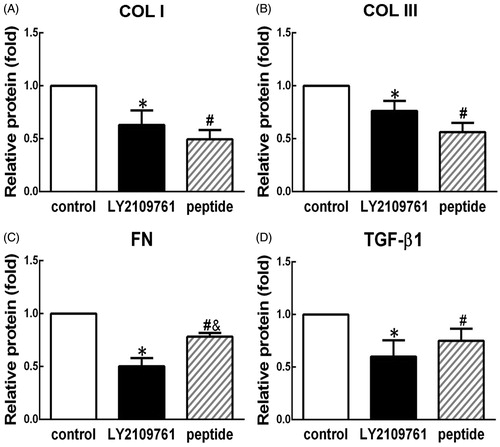
TGF-β peptide antagonist suppressed cell proliferation
CCK-8 assay was used to evaluate the effect of the peptide on cell proliferation. As shown in , both peptide antagonist and LY2109761 could inhibit cell proliferation at day 5 compared with the blank control with significant difference among three groups (p < 0.05), and no significant difference was found between LY2109761 and peptide groups (p > 0.05). There were no significant differences in cell proliferation rate among three groups in the first 4 d.
Figure 3. TGF-β peptide antagonist inhibited proliferation of HS fibroblasts. CCK-8 assay was employed to measure proliferation rate in the cells treated with peptide antagonist (peptide, 30 μM) and TGF-β type I/II receptor kinase inhibitor (LY2109761, 10 μM) or without treatment (control) at the time points of days 1, 2, 3, 4, and 5. *p < 0.05 between control and LY2109761 groups; #p < 0.05 between control and peptide groups.
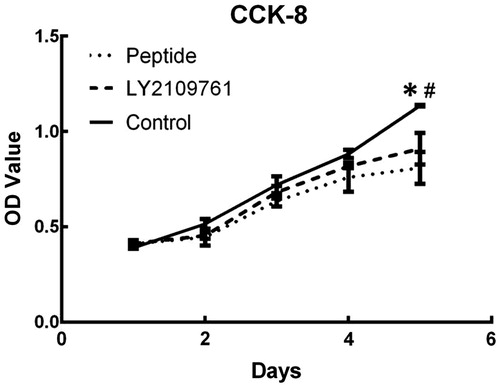
TGF-β peptide antagonist inhibited cell migration
As shown in , treatment of the peptide could significantly inhibit the migration of HSFs. At 24 h, HSFs of the control group migrated 60 ± 2.1% of the scratched area, whereas the peptide-treated HSFs migrated 43 ± 6.7% of the area (p < 0.05, ). However, the LY2109761-treated HSFs migrated only 29.9 ± 5.9% of the area, which was significantly different from the peptide group and the blank control group (p < 0.05, ). At the time point of 48 h, blank control HSFs migrated 95 ± 4.1% of the area, whereas the HSFs of the peptide group and the LY2109761 group, respectively, migrated 63.9 ± 3.1% and 53.2 ± 2.2% area (p < 0.05, ), and the migration rate was significant lower in LY2109761 than in other two groups (p < 0.05, ). ANOVA analysis revealed significant difference in the migration rate among three groups at both 24 and 48 h time points (p < 0.05).
Figure 4. TGF-β peptide antagonist inhibited migration of HS fibroblasts. In vitro scratch wound assay was employed to measure cell migration rate, which was semi-quantified with Image pro-plus version 6.0 (Media Cybernetics, Silver Spring, MD). (A) Gross view of scratched and migrated areas at 24 h and 48 h for the cells treated with peptide antagonist (PEB, 30 μM) or TGF-β type I/II receptor kinase inhibitor (LY2109761, 10 μM) or without treatment (CTRL). (B) Semi-quantification of migration rate at 24 h post-wounding. (C) Semi-quantification of migration rate at 48 h post-wounding. *p < 0.05 between control and LY2109761 groups; #p < 0.05 between control and peptide groups; &p < 0.05 between LY2109761 and peptide groups.
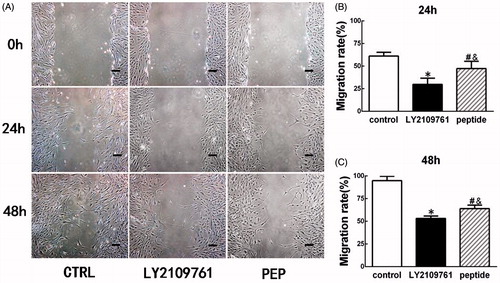
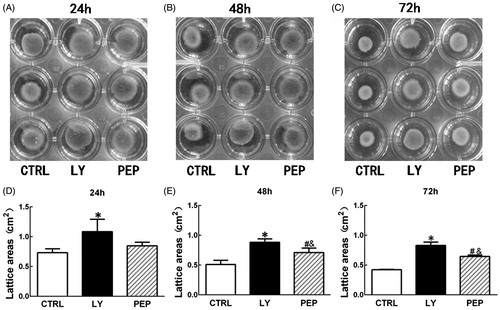
TGF-β peptide antagonist attenuated the contraction of FPCL
As shown in , the inhibitory effect of the peptide antagonist on HSF contractility was evaluated using a FPCL model. At 24 h, the average areas of the control, LY2109761, and the peptide groups were 0.73 ± 0.01, 1.09 ± 0.17, and 0.85 ± 0.05 cm2, respectively, there was significant difference in area among three groups (p < 0.05, ). At this time point, there was no significant difference between the blank control group and the peptide group (p > 0.05, ), although the contraction was significantly lower in LY2109761 than in other two groups (p < 0.05, ). At 48 h, the collagen gels contracted more and their average areas were 0.51 ± 0.06, 0.88 ± 0.05, and 0.71 ± 0.06 cm2, respectively, for blank control, LY2109761, and the peptide groups and there was significant difference among three groups (p < 0.05, ). At this time point, significant difference was observed between the blank control group and the peptide group (p < 0.05) and the contraction rate was significantly lower in the LY2109761 group than in other two groups (p < 0.05, ). At 72 h, the average areas of the blank control, LY2109761 and the peptide groups were 0.42 ± 0.01, 0.83 ± 0.05, and 0.65 ± 0.02 cm2, respectively, with respective area reduction of 79.3 ± 0.2%, 58.5 ± 2.3%, and 67.5 ± 1.0%, and there was significant difference among three groups (p < 0.05, ). At this time point, the contraction rate in the LY2109761 group was significantly lower than that in the peptide group (p < 0.05) and than that in the blank control group (p < 0.05). Meanwhile, the contraction rate of the peptide group was also significantly lower than that of the blank control (p < 0.05).
Figure 5. TGF-β peptide antagonist attenuated the contraction of HS fibroblast-populated collagen lattice (FPCL). FPCL contraction assay was employed to measure the contraction, and the contraction rate was semi-quantified with image software (Macropath 5, PRO, Guangzhou, China). (A–C) Gross view of FPCL contraction at the time points of 24 h, 48 h, and 72 h. (D–F) Semi-quantification of contraction rate of the cells treated with peptide antagonist (PEP, 30 μM) and TGF-β type I/II receptor kinase inhibitor (LY, 10 μM) or without treatment (CTRL). *p < 0.05 between control and LY2109761 groups; #p < 0.05 between control and peptide groups; &p < 0.05 between LY2109761 and peptide groups.
Discussion
Hypertrophic scar is a common disease after tissue injury, especially after burn injury. Clinically, it results in tissue hypertrophy and severe contracture, leading to functional disability and facial organ disfigurement (John et al., Citation2004; Newell, Citation2000; Robert et al., Citation1999; Valente, Citation2004). Pathologically, it is characterized with overproduction and deposition of extracellular matrices, cell overgrowth and irregular distribution, enhanced angiogenesis, and enhanced transformation of fibroblasts to myofibroblast (Ehrlich et al., Citation1994; Lee et al., Citation2004; Niessen et al., Citation1999; Verhaegen et al., Citation2009; Wolfram et al., Citation2009).
The mechanism for hypertrophic scar development is complicated and is likely to be involved in multi aspects of the pathological process. Previous literature revealed that TGF-β might play a leading role in its formation and development, as it involves multiple pathological processes of HS (Beanes et al., Citation2004; Smith et al., Citation1999; Wang et al., Citation2000). It has been shown that TGF-β could significantly enhance HSF proliferation (Wang et al., Citation2000), matrix production (Narayanan et al., Citation1989; Whitby & Ferguson, Citation1991; Younai et al., Citation1994), contractility (Desmouliere et al., Citation2005) and enhance the gene expression of collagen (Younai et al., Citation1994), fibronectin (Ignotz et al., Citation1987) and could induce autocrine production of itself (Kaplan et al., Citation1982). TGF-β also enhanced TIMP (tissue inhibitor of metalloproteinase) expression, an inhibitor of metalloproteinase (MMP), and thus caused reduced matrix degradation (Ignotz et al., Citation1987; Roberts et al., Citation1988).
Therefore, interference of TGF-β function has gained significant attention in order to prevent HS formation and development. Despite that tremendous effort has been made in experimental research, therapeutic application of TGF-β antagonist as a drug to address clinical scarring remains limited. In reality, technical and regulation hurdles are the limiting steps towards clinical translation of anti-TGF-β therapy. For example, the use of TGF-β1 anti-sense oligonucleotide for scar prevention has been published more than 15 years (Choi et al., Citation1996), yet, low efficiency of cell penetration rendered its application difficult. Regarding anti-TGF-β-based gene therapy, although proved efficacy (Yamamoto et al., Citation1996), they are difficult to use clinically simply because the biosafety concern remains an issue for gene therapy.
In contrast, protein-based therapy is relatively safe with no obvious side effect due to the short half-life. Shah et al. (Citation1992) reported long time ago that TGF-β neutralizing antibody could effectively prevent scar formation in an animal model, whereas Ferguson et al. (Citation2009) reported the successful clinical trials of recombinant TGF-β3 for scar prevention, indicating the technical feasibility and well-controlled safety of protein based anti-TGF-β therapy. It is noted that both antibody and recombinant protein-based therapy are apparently costly.
Peptide is relatively new approach that is likely to achieve a therapeutic goal similar to full protein, because the designed peptide represents the functional part of the protein, such as key binding site between ligand and receptor (Crawford et al., Citation2002; Colas, Citation2008; Li et al., Citation2011). More importantly, rapid action, low cost, and relatively high stability make it an attractive approach for protein-based therapy.
Interestingly enough, a TGF-β peptide antagonist, peptantagonists, seems to well fit the purpose (Huang et al., Citation1997). Huang et al. (Citation1997) reported that three chemically synthesized peptantagonists could inhibit the binding of radiolabeled TGF-β1, TGF-β2, and TGF-β3 to TGF-β receptors and reduce the expression of PAI-1 (plasminogen activator inhibitor 1), which was induced by TGF-β in mink lung epithelial cells.
Moreover, this peptide antagonist has also been applied to treat acute burn injury and effectively reduced hypertrophic scar formation in a porcine model (Singer et al., Citation2009). Their study demonstrated that this TGF-β peptantagonist could accelerate reepithelialization, reduce scar formation, and reduce scar contraction when compared with a control vehicle. These results are consistent with the evidence from a rat incisional wound model, which demonstrated reduced scar formation after intradermal injection of TGF-β neutralizing antibody (Shah et al., Citation1994). At least, in porcine model, it was approved that this peptide is likely to be applied for hypertrophic scar treatment in human.
We further collaborated with Dr. Jung Huang from St Louis University to explore the effect of this peptide on inhibiting fibrotic behavior of human HSFs. Previously, LY2109761, a TGF-β type I/II receptor kinase inhibitor, has been widely reported for its anti-TGF-β function in various studies, including attenuation of radiation-induced pulmonary murine fibrosis (Flechsig et al., Citation2012), and reversion of the anti-apoptotic effect of TGF-β1 in myelo-monocytic leukemic cells (Xu et al., Citation2008). Therefore, LY2109761 was used as a proper control to demonstrate whether the peptide antagonist could exert similar anti-TGF-β functions.
According to the literature (Huang et al., Citation1997), the concentration of 30 μM was employed for testing the peptide mediated functions. Similarly, the concentration of 10 μM was used for LY2109761-mediated functions based on the literature report (Zhang et al., Citation2011). As shown in , a short-time culture period already revealed the inhibitory effect of the peptide on HSF proliferation with significant difference from the control group (p < 0.05), but no significant difference from the LY2109761 group.
Scar contracture is a typical character of hypertrophic scar and it was also closely associated with TGF-β overexpression (Desmouliere et al., Citation2005). Collagen lattice contraction is a typical tool to assay fibroblast mediated contractility (Bell et al., Citation1979), and thus was employed in this study as well. As shown in , no apparent inhibitor effect on collagen contraction could be observed when compared to blank control group at 24 h, although the inhibitor already showed significant inhibition. With prolonged culture time, significant difference in contraction rate between the blank control group and the peptide-treated group was observed at both 48 and 72 h (p < 0.05). In addition, peptide-mediated contraction inhibitory effect also approaches to that of LY2109761 at 72 h. Although not tested, this reduced contractility was highly likely due to the reduced transformation of fibroblasts to myofibroblasts as other means of anti-TGF-β revealed the same mechanism (Levinson et al., Citation2001; Zhang et al., Citation2009).
We also demonstrated the inhibitory effect on HSF activity mediated by the peptide antagnonist. As shown in , the cell migration was significantly inhibited in the peptide-treated group at both 24 and 48 h, although the kinase inhibitors seemed to have a more potent inhibitory effect. Overexpressions of collagens I and III, fibronectin, and TGF-β1 were usual characters of HSFs (Narayanan et al., Citation1989; Niessen et al., Citation1999; Younai et al., Citation1994). In this study, we were able to show that the gene expression levels of all four molecules were significantly downregulated in the peptide-treated group compared to those of blank control group as shown in . Moreover, Elisa analysis also provided the supporting evidence of reduced protein production of all these four molecules. Particularly, reduced TGF-β1 expression indicates a disrupted autocrine regulation of TGF-β, which is often observed in HS or keloid fibroblasts (Kaplan et al., Citation1982).
Overall, using in vitro culture as a model, we demonstrated for the first time that this pepantagonist indeed exhibited potent inhibitory effect on the fibrotic phenotype of human HSFs including inhibited cell proliferation, migration, and contractility and reduced expression and production of scar related molecules. It was also noted that receptor inhibitor seemed to have more potent effect than the peptide in inhibiting cell migration and contraction. Additionally, whether the increased concentration of the peptide will further enhance its inhibitory effect remains to be investigated.
Conclusion
The current study demonstrated that a TGF-β peptide antagonist was able to inhibit the fibrotic phenotype of human hypertrophic scar fibroblasts in vitro including cell proliferation and migration, collagen lattice contraction, and expression of scar related molecules. The results of this study provide solid evidence to support the potential therapeutic function of this peptide antagonist as a special scar treatment drug. With the advantages of low cost in preparation and high efficiency in blocking function and easy to store and transport, the peptide-based anti-TGF-β therapy is well expected for its clinical translation in scar therapy.
Acknowledgements
The authors thank Dr. Jung Huang at University of St Louis School of Medicine for his kind gift of TGF-β peptide antagonists.
Declaration of interest
The authors report that they have no conflicts of interest. This research was supported by grants from the Natural Science Foundation of China (81272109).
References
- Beanes SR, Dang C, Soo C, Ting K. (2004). Skin repair and scar formation: The central role of TGF-beta. Expert Rev Mol Med 5:1–11
- Bell E, Ivarsson B, Merrill C. (1979). Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 76:1274–8
- Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. (1996). The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg 98:827–33
- Broker BJ; Chakrabarti R, Blynman T, et al. (1999). Comparison of growth factor expression in fetal and adult fibroblasts. Arch Otolaryngol Head Neck Surg 125:676–80
- Chin GS, Liu W, Peled Z, et al. (2001). Differential expression of transforming growth factor-beta receptors I and II and activation of smad3 in keloid fibroblasts. Plast Reconstr Surg 108:423–9
- Choi BM, Kwak HJ, Jun CD, et al. (1996). Control of scarring in adult wounds using antisense transforming growth factor-beta 1oligodeoxynucleotides. Immunol Cell Biol 74:144–50
- Colas P. (2008). The eleven-year switch of peptide aptamers. J Biol 7:2
- Crawford M, Woodman R, Ferrigno PK. (2002). Peptide aptamers: Tools for biology and drug discovery. Brief Funct Genomics Proteomic 2:72–9
- Desmouliere A, Chaponnier C, Gabbiani G. (2005). Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13:7–12
- Ehrlich HP, Diegelmann RF, Cohen K, et al. (1994). Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol 145:105–13
- Ferguson MW, Duncan J, Bond J, et al. (2009). Prophylactic administration of avotermin for improvement of skin scarring: Three double-blind, placebo-controlled, phase I/II studies. Lancet 373:1264–74
- Flechsig P, Dadrich M, Bickelhaupt S, et al. (2012). LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res 18:3616–27
- Hsu M, Peled ZM, Chin GS, et al. (2001). Ontogeny of expression of transforming growth factor TGF-beta 1, 3 and TGF-beta receptors I and II in fetal rat fibroblasts and skin. Plast Reconstr Surg 107:1787–94
- Huang SS, Liu QJ, Johnson FE, et al. (1997).Transforming growth factor beta peptide antagonists and their conversion to partial agonists. J Biol Chem 272:27155–9
- Ignotz R, Endo T, Massague J. (1987). Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem 262:6443–6
- John W. Lawrence JW, Fauerbach JA, Heinberg L. (2004). Visible vs hidden scars and their relation to body esteem. J Burn Care Rehabil 25:25–32
- Kaplan P, Anderson M, Ozanne B. (1982). Transforming growth factor(s) production enables cells to grow in the absence of serum: An autocrine system. Cell Biol 79:485–9
- Lee JY, Yang CC, Chao SC, Wong TW. (2004). Histopathological differential diagnosis of keloid and hypertrophic Scar. Am J Dermatopathol 26:379–84
- Lee TY, Chin GS, Kim WJH, et al. (1999). Expression of transforming growth factor beta 1, 2 and 3 proteins in keloids. Am J Pathol 43:179–84
- Levinson H, Peled Z, Liu W, et al. (2001). Fetal rat amniotic fluid: Transforming growth factor β and fibroblast collagen lattice contraction. J surg Res 100:205–10
- Liang CC, Park AY, Guan JL. (2007). In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–33
- Li J, Tan S, Chen X, et al. (2011). Peptide aptamers with biological and therapeutic applications. Curr Med Chem 18:4215–22
- Liu W, Chua C, Wu XL, et al. (2005). Inhibiting scar formation in rat wounds by adenovirus-mediated overexpression of truncated TGF-beta receptor II. Plast Reconstr Surg 115:860–70
- Liu W, Wang DR, Cao YL. (2004). TGF-β: A fibrotic factor in wound scarring and a potential target for anti-scarring gene therapy. Curr Gene Ther 4:123–36
- Melisi D, Ishiyama S, Sclabas GM, et al. (2008). LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther 7:829–40
- Narayanan AS, Page RC, Swanson J. (1989). Collagen synthesis by human fibroblasts: Aegulation by transforming growth factor-fl in the presence of other inflammatory mediators. Biochem J 260:463–9
- Newell R. (2000). Psychological difficulties amongst plastic surgery ex-patients following surgery to the face: A survey. Br J Plast Surg 53:386–92
- Niessen FB, Spauwen PH, Schalkwijk J, Kon M. (1999). On the nature of hypertrophic scars and keloids: A review. Plast Reconstr Surg 104:1435–58
- Roberts AB, Thompson NL, Heine U, et al. (1988). Transforming growth factor-beta: Possible roles in carcinogenesis. Br J Cancer 57:594–600
- Robert R, Meyer W, Bishop S, et al. (1999). Disfiguring burn scars and adolescent self-esteem. Burns 25:581–5
- Schmid P, Itin P, Cherry G, et al. (1998). Enhanced expression of transforming growth factor-β3 type I and type II receptors in wound granulation tissue and hypertrophic scar. Am J Pathol 152:485–93
- Shah M, Foreman DM, Ferguson MW. (1992). Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet 339:213–14
- Shah M, Foreman DM, Ferguson MW. (1994). Neutralising antibody to TGF-β1,2 reduces cutaneous scarring in adult rodents. J Cell Sci 107:1137–57
- Shah M, Foreman DM, Ferguson MW. (1995). Neutralisation of TGF-β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J Cell Sci 108:985–1002
- Singer AJ, Huang SS, Huang JS, et al. (2009). A novel TGF-beta antagonist speeds reepithelialization and reduces scarring of partial thickness porcine burns. J Burn Care Res 30:329–34
- Smith P, Mosiello G, Deluca L, et al. (1999). TGF-beta2 activates proliferative scar fibroblasts. J Surg Res 82:319–23
- Valente SM. (2004). Visual disfigurement and depression. Plast Surg Nurs 24:140–6
- Verhaegen PD, van Zuijlen PP, Pennings NM, et al. (2009). Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair Regen 17:649–56
- Wang RJ, Ghahary Z, Shen Q, et al. (2000). Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-β1 mRNA and protein than normal skin and cells. Wound Repair Regen 8:128–37
- Whitby D, Ferguson MW. (1991). The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development 112:651–68
- Wolfram D, Tzankov A, Pulzl P, Piza-Katzer H. (2009). Hypertrophic scars and keloids – A review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg 35:171–81
- Xu Y, Tabe Y, Jin L, et al. (2008). TGF-beta receptor kinase inhibitor LY2109761 reverses the anti-apoptotic effects of TGF-beta1 in myelo-monocytic leukaemic cells co-cultured with stromal cells. Br J Haematol 142:192–201
- Yamamoto H, Ueno H, Ooshima A, Takeshita A. (1996). Adenovirus-mediated transfer of a truncated transforming growth factor-beta 2 receptors completely and specifically abolishes diverse signaling by TGF-β in vascular wall cells in primary culture. J Biol Chem 271:16253–9
- Younai S, Nichter LS, Wellisz T, et al. (1994). Modulation of collagen synthesis by transforming growth factor-beta in keloid and hypertrophic scar fibroblasts. Ann Plast Surg 33:148–54
- Zhang M, Kleber S, Rohrich M, et al. (2011). Blockade of TGF-beta signaling by the TGF betaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res 71:7155–67
- Zhang Z, Garron TM, Li XJ, et al. (2009). Recombinant human decorin inhibits TGF-β1-induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns 35:527–37
