Abstract
Context: Glycyrrhiza glabra L. (Febaceae) has been widely used in traditional medicine and scientifically explored for its anticonvulsant and memory improving potential.
Objective: The objective of this study is to investigate the effect of flavonoid rich fraction of G. glabra root extract against phenytoin-induced cognition deficit in pentylenetetrazol (PTZ) kindled mice.
Materials and methods: The ethyl acetate fraction was initially screened in different in vitro free radical scavenging assays. For in vivo studies, the kindled mice in different groups were given 15 d post-treatment with phenytoin (25 mg/kg; p.o.) per se or in combination with varying doses of the fraction (5, 10, and 15 mg/kg; p.o.). Seizure severity score and cognitive functions were accessed using Racine's scale and passive shock avoidance paradigm, respectively on every 5th d after a PTZ challenge dose (35 mg/kg; i.p.). At the end of study, the animals were scarified for cerebral biochemistry.
Results: The fraction showed marked antioxidant activity indicated by low IC50 values in DPPH (20.9 µg/mL), nitric oxide radical scavenging (195.2 µg/mL), and capacity of hydrogen peroxide scavenging (3.4 µg/mL) assays. Treatment with phenytoin per se and along with the flavonoid rich fraction showed significant reduction in seizure severity score as compared to vehicle control. The combined-treated groups also showed improved cognitive functions indicated by reduced number of mistakes and increased step-down latency in passive shock avoidance paradigm.
Conclusion: From the results, it can be concluded that the flavonoid rich fraction in combination with phenytoin reduces seizure severity and improve cognitive functions in PTZ-kindled mice.
Introduction
Epilepsy is the most common neurological disorders in which 30% of patients experience recurrent seizures with serious neurological complications. Epilepsy in patients is often associated with one or more types of psychiatric comorbid conditions like cognition deficit, depression, anxiety, psychosis, etc. (de Biase et al., Citation2014; Wiebe & Hesdorffer, Citation2007; Yemadje et al., Citation2011). These associated neurological impairments have been suggested to be due a close pathogenic association with neuronal epileptic circuits and diverse complexities of the brain. Similar to all available antiepileptic drugs, the most commonly used, phenytoin further worsens these neurological impairments. Several reasons have been suggested for potentiation of epileptic comorbid conditions by phenytoin like modulation of serotonergic pathway, decreased acetylcholinesterase activity in the hippocampus, abnormal changes in neuropeptides, and hippocampal injuries (Mei et al., Citation2006; Sudha et al., Citation1995; Tsutsumi et al., Citation1998). Cognitive impairment is a most common comorbidity that worsens in epileptic patients with the use of phenytoin. Therefore, there is a need of therapies that can ameliorate epileptic condition along with memory enhancement.
Several medicinal plants have been indicated in traditional medicine to treat epilepsy and have been extensively explored in experimental studies. Herbs contain multiple bioactive metabolites that together exert multiple mechanisms of action and are ideal for such diseases in which treatment of one worsens the other (Singh et al., Citation2012). Simultaneous use of certain herbs can mimic or magnify the effect of the principal drug (Singh et al., Citation2014a). Glycyrrhiza glabra L. (Febaceae) is an age-old traditional herb known around the globe for its medicinal values in management of various ailments. It is commonly known as licorice and is native to southern Europe and parts of Asia. It showed anticonvulsant effect in experimental animal models of convulsion, including maximal electroshock, pentylenetetrazol (PTZ) and lithium–pilocarpine (Ambawade et al., Citation2002; Chowdhury et al., Citation2013; Yazdi et al., Citation2011). Phytochemical studies conducted showed it to be rich in antioxidant components such as ascorbic acid and flavonoids such as glabridin and isoliquiritigenin (Maurya et al., Citation2009; Yu, Citation2008). In previous studies, it showed protective effect against dementia (Chakravarthi & Avadhani, Citation2013). The available literature showed that flavonoids increase seizures threshold and act as antiepileptic through multiple mechanisms (Devi et al., Citation2008; Singh et al., Citation2014b).
A literature survey reveals that the anticonvulsant flavonoids isolated from other plants which show ameliorative effect on epilepsy along with its associated memory impairments (Singh et al., Citation2014a; Spencer, Citation2007; Vauzour et al., Citation2008). Apart from epilepsy, flavonoids have also been reported to improve memory functions probably due to modulation of BDNF pathway, mTOR induced synaptic plasticity and long-term potention (LPT), cholinergic pathway, antioxidant potential, etc. (Katalinic et al., Citation2010; Spencer, Citation2007; Vauzour et al., Citation2008). Add-on treatment of curcumin with conventional antiepileptics potentiated their antiepileptic effect and prevented associated cognition deficit (Agarwal et al., Citation2011; Chimakurthy et al., Citation2008; Kiasalari et al., Citation2013; Reeta et al., Citation2011). However, curcumin has poor aqueous solubility with very low absorption index from the digestive tract, which hinders the clinical usefulness of curcumin (Moorthi & Kathiresan, Citation2013). Hence, the present study investigates the effect of flavonoid rich ethyl acetate fraction of G. glabra in combination with phenytoin for ameliorating epilepsy and phenytoin-induced cognition deficit in PTZ kindled mice.
Materials and methods
Drugs and chemicals
Pentylenetetrazol (dissolved in normal saline) was obtained from Sigma-Aldrich (St. Louis, MO). Ammonium thiocyanate, butylated hydroxyl anisole, thiobarbituric acid, butylated hydroxyl toluene, sulfanilamide, α-tocopherol, pyrocatechol, trichloroacetic acid, hydrogen peroxide, 5,5′-dithiobis-(2-nitrobenzoic acid), reduced glutathione (GSH), bovine serum albumin (BSA) from Loba Chemie (Mumbai, India). 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) from Himedia Chemicals (Mumbai, India). All other reagents employed in the study were of analytical grade. Phenytoin injection was obtained locally from Cadila Laboratories (Ahmedabad, India).
Plant material, extract preparation, and fractionation
The authenticated powder of G. glabra roots (500 g) was purchased from KK Industries, Jalandhar, Punjab, India (a GMP-certified unit producing 100% G. glabra root powder with Mfg. Lic. No. PB-AY-632, Bill no. 45772, Batch no. MLP001). The powder was subjected to extraction with methanol in a Soxhlet extractor for 16 h. The solvent was evaporated under reduced pressure using a rotavapor (Perfit, Ambala, India) and the yield was calculated. The ethyl acetate fraction was separated from the methanol extract according to the method described by Tian et al. (Citation2011), as earlier reports showed that maximum amount of flavonoids get separated using ethyl acetate (Chung et al., Citation2009; Mujwaha et al., Citation2010). Briefly, the extract was dispersed in water and was subjected to polarity-based liquid–liquid partitioning using petroleum ether in a separating funnel. The aqueous fraction was further fractionated with chloroform, followed by ethyl acetate. The ethyl acetate fraction was dried using a rotavapor and stored (Singh et al., Citation2014b).
Quantification of total flavonoids and phenolic components
The total flavonoid content was determined using aluminum chloride colorimetric assay (Kumar et al., Citation2008). The total flavonoid content of the extract and ethyl acetate fraction was calculated using regressed equation obtained via plotting the absorbance of standard quercetin on the y-axis and the concentration of quercetin on the x-axis. Results obtained were expressed as µg mg−1 of quercetin equivalent. The total phenolic compounds present in the different flavonoid-rich fractions were determined with the Folin–Ciocalteu reagent, according to the method suggested by Slinkard and Singleton (Citation1977). Total phenolic contents of the extracts were calculated using regressed equation obtained via plotting the absorbance of standard pyrocatechol on the y-axis and the concentration of pyrocatechol on the x-axis. Results obtained were expressed as µg mg−1 pyrocatechol equivalent.
In vitro antioxidant assay
DPPH free radical scavenging activity
The free radical scavenging activity of the ethyl acetate fractions was measured following the methodology described by Blois (Citation1958) with curcumin as a reference. The fraction and the curcumin were prepared in different concentration (5, 10, 15, 20, and 25 µg/mL) in methanol which was added to 1 mL of 0.1 mM solution of DPPH in methanol. The absorbance was measured at 517 nm after 30 min using a spectrophotometer. 0.1 mM DPPH (1 mL) and 1 mL of methanol were taken as a control. The experiment was performed in triplicate.
Nitric oxide radical scavenging activity
In this method, nitric oxide generated from sodium nitroprusside in aqueous solution at physiological pH, interacted with oxygen to produce nitrite ions which are measured using the Griess reaction (Green et al., Citation1982). The standard method was used to study nitric oxide radical scavenging activity of the ethyl acetate fraction with curcumin as a reference. Three mL of sodium nitroprusside in phosphate buffer was added in 2 mL of each ethyl acetate fraction and curcumin in different concentrations (10, 50, 100, 150, and 200 µg/mL). The solution was then incubated at 25 °C for 60 min. Thereafter, 5 mL of Griess reagent was added and the absorbance was measured using a spectrophotometer at 546 nm. A similar procedure was repeated using methanol as a blank. The experiment was performed in triplicate.
Hydrogen peroxide scavenging assay
The ability of the ethyl acetate fraction to scavenge hydrogen peroxide was determined by the standard method with curcumin as a reference standard (Blois, Citation1958). The experiment was performed in triplicate.
Pharmacological studies
Experimental animals
The present study was conducted on 6-month-old male Swiss albino mice weighing 20–30 g. Mice were obtained from Chaudhary Charan Singh, Haryana Agricultural University, Hisar. The animals were acclimatized under laboratory conditions for 5 d before the start of experiment. All the experimental work was carried out from 9.00 h and 16.00 h. The experimental protocol was duly approved by Institutional Animal Ethics Committee (IAEC), and care of the animals was carried out as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India.
Preparation of test samples and dose estimation
The anticonvulsant dose of alcoholic extract of G. glabra extract which afforded 100% protection to animals have been reported to be 100 mg/kg (Ambawade et al., Citation2002). Based on the yield of ethyl acetate fraction obtained from the extract and literature reports, the dose of the fraction was calculated and was extrapolated to select three doses, 5, 10, and 15 mg/kg. The fraction was reconstituted by dissolving in pure dimethylsulfoxide (DMSO) and then dispersing the resultant solution in distilled water (ratio 1:9), freshly before use and was injected intraperitoneally (i.p.). Vehicle control groups received equal volume of vehicle i.p. (injection volume 10 mL/kg).
PTZ kindling induction
The test procedure previously standardized and validated in our laboratory was used to induce chemical kindling (Singh et al., Citation2013). Randomly, 45 animals were given repeated injections of PTZ (35 mg/kg; i.p.) at 48 h ± 2 h interval. The animals were placed individually in isolated transparent chambers and their behavior was observed for 1 h after each PTZ injection. The intensity of convulsions was recorded according to a seven-point scale (modified Racine's scale): Stage 0: no response; Stage 1: hyperactivity, restlessness and vibrissae twitching; Stage 2: head nodding, head clonus and myoclonic jerks; Stage 3: unilateral or bilateral limb clonus; Stage 4: forelimb clonic seizures; Stage 5: generalized clonic seizures with falling; Stage 6: appearance of tonic extension; and Stage 7: death. The PTZ treatment was stopped when the animals showed Stage 5 after two successive PTZ injections and considered to be kindled. Naive group animals were injected with saline in a similar manner instead of PTZ. The regular PTZ schedule was not extended beyond day 33, i.e., 17 injections in any case.
Experimental protocol
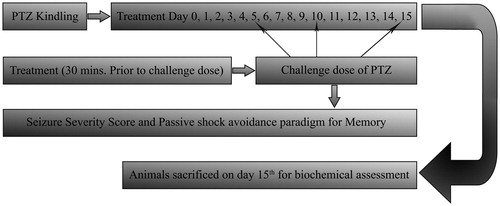
The day animals achieved a kindled state was considered as day 0. Successfully kindled animals (n = 30) were then divided into five different groups (n = 6). An additional naïve group (n = 6) of non-kindled animals was simultaneously subjected to the test. On day 1, the animals were subjected to a training session in passive shock avoidance paradigm in different groups, and received 15 d treatment as, Group 1: non-kindled (non-kindled) naive (vehicle 10 mL/kg; i.p.); Group 2: PTZ vehicle (kindled) control (vehicle 10 mL/kg; i.p.); Group 3: phenytoin (25 mg/kg; i.p.); Group 4: low-dose ethyl acetate fraction treated (5 mg/kg; i.p. extract after 30 min phenytoin 25 mg/kg; i.p.); Group 5: intermediate dose ethyl acetate fraction treated (10 mg/kg; i.p. extract after 30 min, Phenytoin 25 mg/kg; i.p.); and Group 6: high-dose ethyl acetate fraction treated (15 mg/kg; i.p. extract after 30 min, phenytoin 25 mg/kg; i.p.). Following 30 min of these treatments, the animals of Groups 2–6 received a challenging dose of PTZ (35 mg/kg; i.p.) on every 5th d, i.e., on days 5, 10, and 15, and the seizure severity was noted. After cessation of seizures, the animals were subjected to passive shock avoidance paradigm to record the number of mistakes and step down latency to check the memory impairment. Similar recordings were also carried out in non-kindled naïve animals, except they were injected with saline instead of PTZ. On the 15th day after behavioral assessment, all the animals were sacrificed for biochemical estimations ().
Passive shock avoidance paradigm
The test was used to study the memory function in mice (Jarvik & Kopp, Citation1967) using an automated step-down rodent memory evaluator (Rolex, Ambala, India). The apparatus consisted of a Plexiglas chamber (25 cm × 25 cm × 25 cm) with a grid floor (3 mm stainless steel rods at 8 mm apart) from which electric shock was delivered (20 V AC). The grid comprised a shock free zone (SFZ; 7 cm × 7 cm × 1.7 cm) on the centre. All the animals were trained to stay on the SFZ for at least 90 s on day 4. The animals were placed on the SFZ, the time it stepped down placing all paws on the grid floor; shock was delivered for 15 s. The training process was repeated until the animal learned to stay on the SFZ for at least 90 s. Retention was tested on the 5th, 10th, and 15th day of the treatment period. Each mouse was again placed on the SFZ, and the step down latency was measured (SDL; time (s) taken by the animal to step down from the central platform to the grid floor with all paws). SDL and numbers of mistakes were recorded, with an upper cut-off time of 120 s, for 5 min, respectively.
Biochemical parameters
At the end of experimentation on the 15th day, all the animals were sacrificed by cervical dislocation. The brains were removed and homogenized in phosphate buffer (pH 7.4, 10% w/v) using a homogenizer, centrifuged at 3000 rpm for 15 min and the collected supernatant was used to estimate thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH), catalase activity, and total protein by the methods described by Ohkawa et al. (Citation1979), Beutler et al. (Citation1963), Brannan et al. (Citation1981), and Lowry et al. (Citation1951), respectively.
Statistical analysis
The results were expressed as mean ± standard error (SEM). The significance for seizure severity score, number of mistakes, SDL was determined by two-way analysis of variance (ANOVA), and biochemical results using one-way ANOVA followed by Tukey's test post hoc test. The results were considered to be significant at p < 0.05.
Results
Qualitative and quantitative determination of flavonoids
The total flavonoid content of the ethyl acetate fraction of crude methanolic root extract of G. glabra was found to be 21.03 ± 0.06% µg mg−1 of quercetin equivalent and the total phenolic content of the fraction was 8 ± 0.31 µg mg−1 pyrocatechol equivalent.
In vitro antioxidant assay
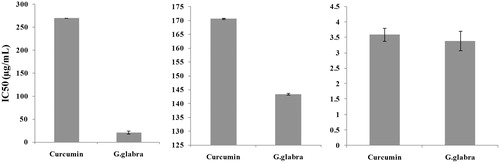
The IC50 value is the pre-eminent method for comparing and presenting the potencies of antioxidants. IC50 value is the concentration corresponding to 50% of maximum antioxidant activity. It was calculated using regressed equations for DPPH free radical scavenging activity (curcumin: y = 0.0229x − 10.869 and G. glabra: y = 1.4458x + 19.714), nitric oxide radical scavenging activity (curcumin: y = 0.0979x + 33.287 and G. glabra: y = 0.193x + 22.33), and capacity of hydrogen peroxide scavenging (curcumin: y = 0.168x + 49.396 and G. glabra: y = 0.4527x + 48.459). The IC50 values of curcumin and ethyl acetate fraction of G. glabra in different antioxidant assays are shown in .
Effect on PTZ-induced kindling
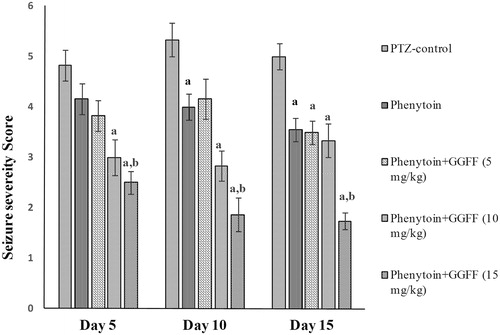
Treatment with phenytoin per se and G. glabra fractions in combination with phenytoin induced a significant 63.8% of total variation among the seizure score (F(4, 25) = 19.93, p < 0.0001). However, phenytoin per se was found to be effective only on days 10 and 15 in suppression of seizures, whereas combined treatment of phenytoin with the ethyl acetate fraction at the dose of 10 and 15 mg/kg showed significant (p < 0.05) dose-dependent decrease in seizure severity score starting from day 5 compared with PTZ-control. Furthermore combined treatment at the highest dose significantly (p < 0.05) ameliorated seizure severity on all days compared with both PTZ-control and phenytoin per se group ().
Effect on memory functions
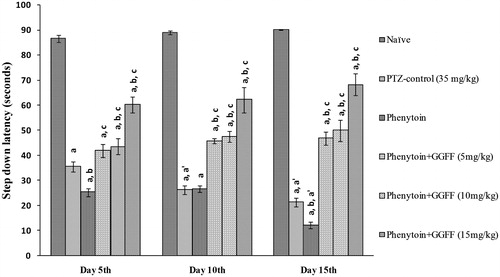
In passive shock avoidance paradigm, combined-treated group showed significant (p < 0.05) reduction in the number of mistakes (F(5, 30) = 38.09, p < 0.0001). PTZ-control and phenytoin per se groups showed impairment in retrieval of memory indicated by significant (p < 0.05) increased number of mistakes and decreased SDL on days 5, 10, and 15, after PTZ challenge when compared with non-kindled naïve animals (F(5, 30) = 602.5, p < 0.0001). Treatment with phenytoin per se further worsens the memory functions compared to PTZ-control animals. The number of mistakes increased and step down latency decreased, respectively, of phenytoin per se group on day 15th significantly compared with day 5th. Combined treatment of phenytoin with ethyl acetate fraction at the dose of 15 mg/kg significantly (p < 0.05) decreased the number of mistakes and increased SDL when compared with PTZ-control and phenytoin per se ( and ).
Figure 4. Effect of pharmacological interventions on number of mistakes. All values are represented as mean ± SEM; n = 6, ap < 0.05 as compared with naive, bp < 0.05 as compared with PTZ-control; cp < 0.05 as compared with phenytoin per se; a′p < 0.05 as compared with day 5. NM, no mistakes; GGFF, Glycyrrhiza glabra flavonoid fraction.
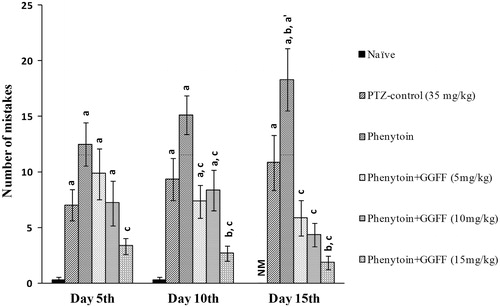
Figure 5. Effect of pharmacological interventions on step down latency. All values are represented as mean ± SEM; n = 6, ap < 0.05 as compared with naive, bp < 0.05 as compared with PTZ-control; cp < 0.05 as compared with phenytoin per se. a′p < 0.05 as compared with day 5. GGFF, Glycyrrhiza glabra flavonoid fraction.
Biochemical estimations
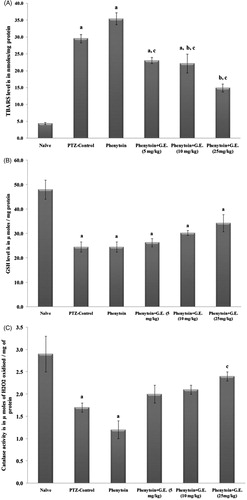
The brain homogenate of the PTZ-control group showed increased lipid peroxidation indicated by significant (F(5, 30) = 48.50, p < 0.0001) increased levels of TBARS as compared with naive. The homogenate of animals of these groups also showed significant decrease in the levels reduced glutathione (F(5, 30) = 12.14, p < 0.0001) and catalase activity (F(5, 30) = 7.533, p < 0.0001) when compared with naive. Phenytoin per se treatment further worsened the disorders as indicated by increased TBARS level and decreased levels of reduced glutathione and catalase activity. Treatment with phenytoin in combination with the fraction showed attenuation of these changes as compared with PTZ-control and phenytoin per se-treated groups ().
Figure 6. Effect of intervention of drugs on biochemical estimations. All values are represented as mean ± SEM; n = 6, ap < 0.05 as compared with the naive group; bp < 0.05 as compared with PTZ-control; cp < 0.05 as compared with phenytoin per se. (A) TBARS level in nmoles/mg protein; (B) GSH level in µ moles/mg protein; (C) catalase activity in µ moles of H2O2 oxidized/mg of protein. TBARS, thiobarbituric acid reactive substances; GSH, reduced glutathione; CAT, catalase; GGFF, Glycyrrhiza glabra flavonoid fraction.
Discussion
This is the first study to scientifically evaluate the combined effect of ethyl acetate fraction from the methanol root extract of G. glabra with phenytoin in PTZ kindling and associated cognitive deficit. The fraction treatment in combination with phenytoin reduces the seizure severity and ameliorated cognitive dysfunction of kindled animals. Treated animals also showed reduced biomarkers of oxidative stress in kindled animals.
Oxidative injury plays a vital role in the initiation and progression of epilepsy. Apart from epilepsy, the role of oxidative stress has also been reported in its associated co-morbidities like cognitive deficit, etc. The role of oxidative stress is established in the genesis of cognitive deficit associated with epilepsy via interaction with several neuronal pathways like CREB, PI3K, PKG, ERK1/2, etc. (Abdul & Butterfield, Citation2007; Clausen et al., Citation2010; Kolosova et al., Citation2006; Glade, Citation2010). The available conventional antiepileptic drugs further increases the oxidative stress (Gallagher et al., Citation2001; Hamed et al., Citation2004; Sudha et al., Citation2001; Turkdogan et al., Citation2002), hence worsens the epilepsy-associated comorbid conditions. Phenytoin is the most commonly prescribed antiepileptic drug due to low cost it is known to induce cognitive impairment. Available information revealed the detrimental effect of phenytoin on learning and memory with both acute and chronic treatment (Aldenkamp et al., Citation1994; Sudha et al., Citation1995; Vohora et al., Citation2000). Current treatment strategies are aimed on reducing oxidative stress to ameliorate tissue damage and to alter the clinical course of the disease (Costello & Delanty, Citation2004; Devi et al., Citation2008).
Flavonoids in this regard are the most important bioactive class of compounds reported in the suppression of epileptic seizures due to multiple mechanisms, including their antioxidant potential (Devi et al., Citation2008; Du et al., Citation2002; Griebel et al., Citation1999; Ishige et al., Citation2001; Kavvadias et al., Citation2004; Majewska et al., Citation2011). Hence, it was found worthwhile to combine the conventional antiepileptics with flavonoid component of traditional medicinal plants for better management of epileptic condition. Since G. glabra roots are rich in flavonoids (Denisova et al., Citation2006; Mostafa et al., Citation2014), a better approach to be used for G. glabra for not only cognitive impairments but also for providing seizure protection when combined with phenytoin.
In the preliminary phytochemical testing, the methanol extract showed the presence of flavonoids which has been earlier reported to be get separated using ethyl acetate solvent (Chung et al., Citation2009; Mujwaha et al., Citation2010). Moreover, ethyl acetate fraction from the G. glabra root extracts has been previously isolated and determined for the presence of flavonoids. The study comprising of GC-MS determination of flavonoid states that the fraction is rich in flavonoids mainly comprising of glabridin, 4-O-methylglabridin, and hispaglabridin B (Denisova et al., Citation2006). Hence, ethyl acetate fraction was used in the present study. Different parameters like DPPH, nitric oxide, and hydrogen peroxide free radical scavenging assays along with total flavonoids and phenolic content were estimated for the confirmation of antioxidant activity, as these components contribute directly to the antioxidant action (Gulcin et al., Citation2005). The fraction showed low IC50 value compared with curcumin in these tests, thus indicating better antioxidant potency of G. glabra ethyl acetate fraction over curcumin. The lower IC50 value is associated with greater free radical scavenging activity (Wang et al., Citation2013).
In the present study, the PTZ model was used to induce epileptic kindling and cognition deficit, as it is a well-established animal model which simulates clinical epilepsy (Golechha et al., Citation2010; Hosseini & Mirazi, Citation2015). PTZ kindling in rodents have been associated with neuronal damage in the hippocampus due to free radicals generation which results in memory loss (Pohle et al., Citation1997). In line to these observations, in the present study, kindled animals showed memory impairment indicated by increased number of mistakes and decreased SDL in passive shock avoidance paradigm. SDL and the number of mistakes committed show the retrieval of memory (Abdel-Hafez et al., Citation1998). The memory functions were further worsened by phenytoin per se treatment. These results are in line with the previous reports showing increased cognitive impairment with phenytoin (Aldenkamp et al., Citation1994; Iivanainen, Citation1998; Sudha et al., Citation1995; Vohora et al., Citation2000). Increased oxidative stress by conventional antiepileptic drugs has been proposed to be a possible cause of worsening of memory impairment (Reeta et al., Citation2009, Citation2010). The antiepileptic drugs like valproic acid, phenytoin, and carbamazepine induce abnormal production of reactive oxygen species (Nazıroglu & Yurekli, Citation2013). Combination of these drugs with antioxidants have been found to ameliorate epilepsy and associated neurological comorbidities (Frautschy et al., Citation2001; Reeta et al., Citation2010).
In the present study, the combined treatment of phenytoin with the ethyl acetate fraction at the dose of 10 and 15 mg/kg i.p. decreases seizure susceptibility compared with PTZ control, At the highest dose of ethyl acetate fraction, the combination ameliorated seizure threshold compared with both PTZ control and phenytoin per se. Combined treatment at the highest dose also improved memory functions compared with both PTZ-control and phenytoin on day 10th and 15th as indicated by decreased number of mistakes and increased SDL. These results are in-line with previous results in which PTZ-control and phenytoin both alter memory functions (Teixeira-Silva et al., Citation2008; Vohora et al., Citation2000).
The results of in vitro antioxidant activity were further supported by biochemical estimations. The brain homogenate of PTZ-control and phenytoin per se mice had decreased levels of GSH and catalase activity, while increased TBARS level. Our observations are in line with the earlier reports in which epilepsy and antiepileptic per se treatment was associated with increased TBARS level (Akbas et al., Citation2005; Mehla et al., Citation2010), decreased GSH levels (Gallagher et al., Citation2001; Mehla et al., Citation2010) and catalase activity (Bolayir et al., Citation2004; Sudha, Citation2001). A combined treatment with the phenytoin and flavonoid rich fraction resulted in amelioration of epilepsy associated cognition deficit. These findings support the notion that oxidative stress may be playing a role in epilepsy-associated cognition deficit.
Conclusion
The above findings indicate that the G. glabra roots contain bioactive flavonoids. The study also infers the combined treatment of phenytoin with the ethyl acetate fraction of G. glabra roots as a better option than phenytoin alone for the suppression of epilepsy and its associated cognition deficit in the mice model. More studies are required to develop it as an add-on therapy for comprehensive management of epilepsy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper. The authors are grateful to the Punjabi University, Patiala for providing support and funding for the present research project. The authors are also thankful to the All India Council for Technical Education, New Delhi for providing GPAT fellowship to Paramdeep Singh.
References
- Abdel-Hafez AA, Meselhy MR, Nakamura N, et al. (1998). Effects of paeoniflorin derivatives on scopolamine-induced amnesia using a passive avoidance task in mice; structure–activity relationship. Biol Pharm Bull 21:1174–9
- Abdul HM, Butterfield DA. (2007). Involvement of PI3K/PKG/ERK1/2 signaling pathways in cortical neurons to trigger protection by cotreatment of acetyl-l-carnitine and α-lipoic acid against HNE-mediated oxidative stress and neurotoxicity: Implications for Alzheimer's disease. Free Radical Biol Med 42:371–84
- Agarwal NB, Jain S, Agarwal NK, et al. (2011). Modulation of pentylenetetrazole-induced kindling and oxidative stress by curcumin in mice. Phytomedicine 18:756–9
- Akbas SH, Yegin A, Ozben T. (2005). Effect of pentylenetetrazol-induced epileptic seizure on the antioxidant enzyme activities, glutathione and lipid peroxidation levels in rat erythrocytes and liver tissues. Clin Biochem 38:1009–14
- Aldenkamp AP, Alpherts WC, Diepman L, et al. (1994). Cognitive side effects of phenytoin compared with carbamazepine in patients with localization-related epilepsy. Epilepsy Res 19:37–43
- Ambawade SD, Kasture VS, Kasture SB. (2002). Anticonvulsant activity of roots and rhizomes of Glycyrrhiza glabra. Indian J Pharmacol 34:251–5
- Beutler RG, Duron O, Kelly B. (1963). Improved method for determination of blood glutathione. J Lab Clin Med 61:882–90
- Blois MS. (1958). Antioxidant determinations by the use of a stable free radical. Nature 29:1199–200
- Bolayir E, Celik K, Tas A, et al. (2004). The effects of oxcarbazepine on oxidative stress in epileptic patients. Methods Find Exp Clin Pharmacol 26:345–8
- Brannan TS, Maker HS, Raes IP. (1981). Regional distribution of catalase in the adult rat brain. J Neurochem 36:307–9
- Chakravarthi KK, Avadhani R. (2013). Beneficial effect of aqueous root extract of Glycyrrhiza glabra on learning and memory using different behavioral models: An experimental study. J Nat Sci Biol Med 4:420–5
- Chimakurthy J, Murthy TEGK, Upadhyay L. (2008). Effect of curcumin on sub-therapeutic doses of AED’s and long term memory in mice induced GTC type of seizures in rats. Res J Pharm Technol 1:401–4
- Chowdhury B, Bhattamisra SK, Das MC. (2013). Anti-convulsant action and amelioration of oxidative stress by Glycyrrhiza glabra root extract in pentylenetetrazole-induced seizure in albino rats. Indian J Pharmacol 45:40–3
- Chung SK, Chen CY, Blumberg JB. (2009). Flavonoid-rich fraction from Sageretia theezans leaves scavenges reactive oxygen radical species and increases the resistance of low-density lipoprotein to oxidation. J Med Food 12:1310–15
- Clausen A, Doctrow S, Baudry M. (2010). Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging 31:425–33
- Costello DJ, Delanty N. (2004). Oxidative injury in epilepsy: Potential for antioxidant therapy?. Expert Rev Neurother 4:541–53
- de Biase S, Gigli GL, Valente M, Merlino G. (2014). Lacosamide for the treatment of epilepsy. Lacosamide for the treatment of epilepsy. Expert Opin Drug Metab Toxicol 10:459–68
- Denisova SB, Galkin EG, Murinov Yu I. (2006). Isolation and GC-MS determination of flavonoids from Glycyrrhiza glabra root. Chem Nat Compd 42:285–9
- Devi PU, Manocha A, Vohora D. (2008). Seizures, antiepileptics, antioxidants and oxidative stress: An insight for researchers. Expert Opin Pharmacother 9:3169–77
- Du XM, Sun NY, Takizawa N, et al. (2002). Sedative and anticonvulsant activity of goodyerin, a flavonol glycoside from Goodyera schlechtendaliana. Phytother Res 16:261–3
- Frautschy SA, Hu W, Kim P, et al. (2001). Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging 22:993–1005
- Gallagher EP, Sheehy KM. (2001). Effects of phenytoin on glutathione status and oxidative stress biomarker gene mRNA levels in cultured precision human liver slices. Toxicol Sci 59:118–26
- Glade Michael J. (2010). Oxidative stress and cognitive longevity. Nutrition 26:595–603
- Golechha M, Bhatia J, Arya DS. (2010). Hydroalcoholic extract of Emblica officinalis Gaertn. affords protection against PTZ-induced seizures, oxidative stress and cognitive impairment in rats. Indian J Exp Biol 48:474–8
- Green LC, Wagner DA, Glogowski J, et al. (1982). Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem 126:131–8
- Griebel G, Perrault G, Tan S, et al. (1999). Pharmacological studies on synthetic flavonoids: Comparision with diazepam. Neuropharmacology 38:965–77
- Gulcin I, Alici HA, Cesur M. (2005). Determination of in vitro antioxidant radical scavenging activities of propofol. Chem Pharm Bull (Tokyo) 53:281–5
- Hamed SA, Abdellah MM, El-Melegy N, et al. (2004). Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients. J Pharmacol Sci 96:465–73
- Hosseini A, Mirazi N. (2015). Alteration of pentylenetetrazole-induced seizure threshold by chronic administration of ginger (Zingiber officinale) extract in male mice. Pharm Biol 53:752–7
- Iivanainen M. (1998). Phenytoin: Effective but insidious therapy for epilepsy in people with intellectual disability. J Intellect Disabil Res 42:24–31
- Ishige K, Schubert D, Sagara Y. (2001). Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 30:433–46
- Jarvik ME, Kopp R. (1967). An improved one-trial passive avoidance learning situation. Psychol Rep 21:221–4
- Katalinic M, Rusak G, Domacinovic Barovic J, et al. (2010). Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur J Med Chem 45:186–92
- Kavvadias D, Sand P, Youdim KA, et al. (2004). The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses blood brain barrier and exhibits anticonvulsive effect. Br J Pharmacol 142:811–20
- Kiasalari Z, Roghani M, Khalili M, et al. (2013). Antiepileptogenic effect of curcumin on kainate-induced model of temporal lobe epilepsy. Pharm Biol 51:1572–8
- Kolosova NG, Shcheglova TV, Sergeeva SV, Loskutova LV. (2006). Long term antioxidant supplementation attenuates oxidative stress markers and cognitive deficits in senescent accelerated OXYS rats. Neurobiol Aging 27:1289–97
- Kumar S, Kumar D, Prakash O. (2008). Antioxidant free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm 58:215–20
- Lowry AH, Rosenbrough MJ, Farr AL, Randall RJ. (1951). Protein measurement with Folin–phenol reagent. J Biol Chem 193:265–75
- Majewska M, Skrzycki M, Podsiad M, Czeczot H. (2011). Evaluation of antioxidant potential of flavonoids: An in vitro study. Acta Pol Pharm 68:611–15
- Maurya SK, Raj K, Srivastava AK. (2009). Antidyslipidaemic activity of Glycyrrhiza glabra in high fructose diet induced dsyslipidaemic Syrian golden hamsters. Indian J Clin Biochem 24:404–40
- Mehla J, Reeta KH, Gupta P, Gupta YK. (2010). Protective effect of curcumin against seizures and cognitive impairment in a pentylenetetrazole-kindled epileptic rat model. Life Sci 87:596–603
- Mei PA, Montenegro MA, Guerreiro MM, Guerreiro CA. (2006). Pharmacovigilance in epileptic patients using antiepileptic drugs. Arq Neuropsiquiatr 64:198–201
- Moorthi C, Kathiresan K. (2013). Curcumin–piperine/curcumin–quercetin/curcumin–silibinin dual drug-loaded nanoparticulate combination therapy: A novel approach to target and treat multidrug-resistant cancers. J Med Hypo Ids 7:15–20
- Mostafa DM, Ammar NM, Abd El-Alim SH, El-anssary AA. (2014). Transdermal microemulsions of Glycyrrhiza glabra L.: Characterization, stability and evaluation of antioxidant potential. Drug Deliv 21:130–9
- Mujwaha AA, Mohammed MA, Ahmedc MH. (2010). First isolation of a flavonoid from Juniperus procera using ethyl acetate extract. Arabian J Chem 3:85–8
- Naziroglu M, Yurekli VA. (2013). Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: Focus on trace elements. Cell Mol Neurobiol 33:589–99
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Pohle W, Becker A, Grecksch G, et al. (1997). Piracetam prevents pentylenetetrazol kindling-induced neuronal loss and learning deficits. Seizure 6:467–74
- Reeta KH, Mehla J, Gupta YK. (2009). Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res 1301:52–60
- Reeta KH, Mehla J, Gupta YK. (2010). Curcumin ameliorates cognitive dysfunction and oxidative damage in phenobarbitone and carbamazepine administered rats. Eur J Pharmacol 644:106–12
- Reeta KH, Mehla J, Pahuja M, Gupta YK. (2011). Pharmacokinetic and pharmacodynamics interactions of valproate, phentoin, phenobarbitone and carbamazepine with curcumin in experimental models of epilepsy in rats. Pharmacol Biochem Behav 99:399–407
- Singh B, Singh D, Goel RK. (2012). Dual protective effect of Passiflora incarnata in epilepsy and its associated post-ictal depression. J Ethnopharmacol 139:273–9
- Singh D, Mishra A, Goel RK. (2013). Effect of saponin fraction from Ficus religiosa on memory deficit, and behavioral and biochemical impairments in pentylenetetrazol kindled mice. Epilepsy Behav 27:206–11
- Singh P, Singh D, Goel RK. (2014a). Ficus religiosa L. figs a potential herbal adjuvant to phenytoin for improved management of epilepsy and associated behavioral comorbidities. Epilepsy Behav 41:171–8
- Singh P, Singh D, Goel RK. (2014b). Phytoflavonoids: Antiepileptics for the future. Int J Pharm Pharm Sci 6:51–66
- Slinkard K, Singleton VL. (1977). Total phenol analyses: Automation and comparison with manual methods. Am J Enol Viticult 28:49–55
- Spencer JP. (2007). The interactions of flavonoids within neuronal signaling pathways. Genes Nutr 2:257–73
- Sudha K, Rao AV, Rao A. (2001). Oxidative stress and antioxidants in epilepsy. Clin Chim Acta 303:19–24
- Sudha S, Lakshmana MK, Pradhan N. (1995). Chronic phenytoin induced impairment of learning and memory with associated changes in brain acetylcholine esterase activity and monoamine levels. Pharmacol Biochem Behav 52:119–24
- Teixeira-Silva F, Santos FN, Sarasqueta DFO, et al. (2008). Benzodiazepine-like effects of the alcohol extract from Erythrina velutina leaves: Memory, anxiety, and epilepsy. Pharm Biol 46:321–8
- Tian S, Shi Y, Zhou X, et al. (2011). Total polyphenolic (flavonoids) content and antioxidant capacity of different Ziziphora clinopodioides Lam. extracts. Pharmacogn Mag 7:65–8
- Tsutsumi S, Akaike M, Ohno H, Kato N. (1998). Learning/memory impairments in rat offspring prenatally exposed to phenytoin. Neurotoxicol Teratol 20:123–32
- Turkdogan D, Toplan S, Karakoc Y. (2002). Lipid peroxidation and antioxidative enzyme activities in childhood epilepsy. J Child Neurol 17:673–86
- Vauzour D, Vafeiadou K, Mateos AR, et al. (2008). The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr 3:115–26
- Vohora D, Pal SN, Pillai KK. (2000). Protection from phenytoin-induced cognitive deficit by Bacopa monniera, a reputed Indian nootropic plant. J Ethnopharmacol 71:383–90
- Wang CZ, Yuan HH, Bao XL, Lan MB. (2013). In vitro antioxidant and cytotoxic properties of ethanol extract of Alpinia oxyphylla fruits. Pharm Biol 51:1419–25
- Wiebe S, Hesdorffer D. (2007). Epilepsy: Being ill in more ways than one. Epilepsy Curr 7:145–8
- Yazdi A, Sardari S, Sayyah M, Hassanpour Ezzati M. (2011). Evaluation of the anticonvulsant activity of the leaves of Glycyrrhiza glabra var. glandulifera grown in Iran, as a possible renewable source for anticonvulsant compounds. Iran J Pharm Res 10:75–82
- Yemadje LP, Houinato D, Quet F, et al. (2011). Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia 52:1376–81
- Yu XQ. (2008). In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice). Life Sci 82:68–78